Herpes simplex virus type 2 (HSV2) is a member of the alpha Herpesviridae subfamily associated with various central nervous system (CNS) manifestations.Reference Berger and Houff1 Variable clinical presentations, delay in diagnosis, and ongoing structural changes in the brain posttreatment can contribute to the morbidity and mortality of HSV encephalitis. We report a case of HSV2 encephalitis with multifocal brain hemorrhage, associated vasogenic edema, and discrete lesions masquerading as a metastatic process.
A 36-year-old healthy immunocompetent male developed new intermittent frontal headaches aggravated with postural changes. This was associated with nausea and vomiting and in the context of 30-pound weight loss over 3 months.
He presented to a community hospital where a computed tomography (CT) head showed right cerebellar and right frontal intraparenchymal hemorrhages with surrounding edema. CT-angiography ruled out any intracranial vascular abnormalities. MRI brain showed bilateral inferior cerebellar intraparenchymal hemorrhages with mass effect and a right frontal hematoma, thought to be suggestive of hypertensive encephalopathy or amyloid angiopathy. Workup for vasculitis and secondary causes of hypertension were negative. It was concluded that the intraparenchymal hemorrhages were secondary to hypertension. The patient was discharged on two antihypertensive medications and a short course of dexamethasone.
Over the next 2 months, the patient deteriorated and was readmitted to the community hospital after acute onset right facial droop. An initial CT head showed three hemorrhagic foci in the inferior posterior left frontal lobe, left subinsular region, and left temporal lobe, with associated vasogenic edema and midline shift. A repeat CT head showed expansion of the intraparenchymal hematomas with worsening midline shift and subfalcine herniation. As such, the patient was urgently transferred to our hospital for a decompressive craniectomy.
The patient underwent a left-sided decompressive hemicraniectomy for evacuation of the frontotemporal hemorrhage. A CT head post-decompressive craniectomy demonstrated residual left frontal intraparenchymal hematoma with extensive adjacent edema, mass effect, and herniation. Small hypoattenuating lesions were noted in the posterior right frontal lobe and left cerebellar hemisphere with moderate surrounding edema concerning for metastatic disease (Figure 1 A-B). An MRI showed a residual left frontal hematoma with extensive surrounding edema, mass effect, and herniation with ill-defined T2 FLAIR-enhancing lesions in the left cerebellar hemisphere, posterior left temporal lobe, medial right temporal lobe, and lateral right frontal lobe, suggestive of metastases (Figure 1C-D).

Figure 1: CT head post-decompressive craniectomy demonstrating residual left frontal intraparenchymal hematoma with extensive edema and mass effect, with small hypodense lesions in the posterior right frontal lobe and left cerebellar hemisphere (A–B) with surrounding edema. Ill-defined T2 FLAIR enhancing lesions in the left cerebellar hemisphere (C) and lateral right frontal lobe (D) were seen on MRI.
There were no vascular abnormalities on CT angiography. A primary malignancy was not detected. Limited CSF analysis demonstrated elevated protein with leukocytes on gram stain, negative cultures, negative fungal analysis and negative syphilis, HSV1/2 and VZV serologies. Systemic vasculitic workup was negative. Pathological examination of resected tissue revealed no evidence of neoplasm and cerebral amyloid angiopathy. There was marked inflammation with a few blood vessels exhibiting marked transmural infiltration of inflammatory cells with prominent vessel wall destruction and fibrinoid necrosis consistent with necrotizing vasculitis (Figure 2 A–E). Thrombosed blood vessels were also found. Viral immunohistochemistry showed numerous HSV2-positive cells (Figure 2 F).
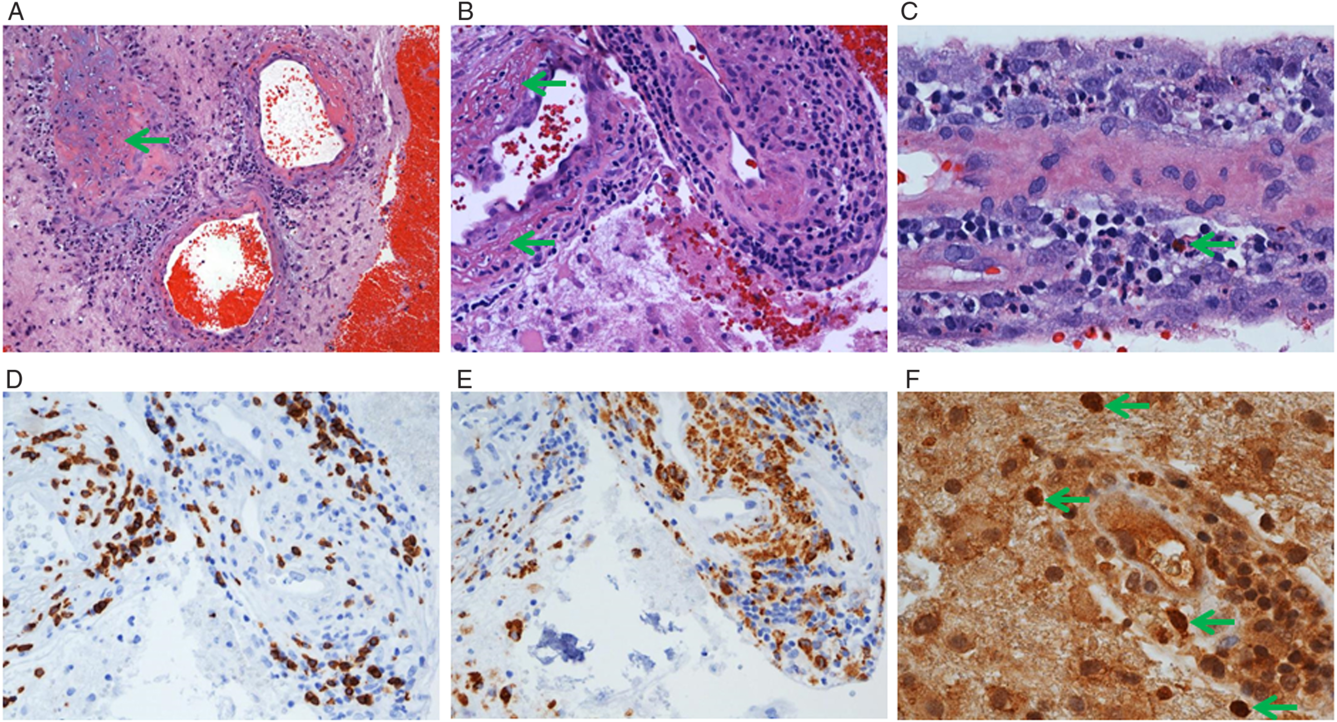
Figure 2: Surgical resection from the left frontal lobe shows acute hemorrhage (A, right) and cerebral tissue containing inflamed blood vessels with occasional thrombosis (A, arrow), marked fibrinoid necrosis (B, arrows), and transmural infiltration of inflammatory cells including neutrophils (A and C), lymphocytes (B), macrophages, and eosinophils (C, arrow). There are abundant CD8 + T-cells in the vessel walls (D), and CD68+ macrophages in the vessel walls and adjacent cerebral tissue (E). HSV2 immunostaining revealed scattered positive nuclei often containing positive inclusions (F, arrows). Of note, immunostaining for HSV1 and CMV was negative. Original magnification, x100 (A), 200 (B, D, E), and x400 (C, F).
The patient was treated with acyclovir with addition of steroids and hypertonic saline for worsening edema and increased intracranial pressure. Due to ongoing ventilator dependency and unchanged clinical status, care was withdrawn and the patient died.
HSV2 is a ubiquitous infection acquired through direct contact with mucosal surfaces or skin breakdown. It is thought to gain access to the CNS via neuronal translocation and commonly manifests as meningitis in immunocompetent individuals.Reference Berger and Houff1 Cerebrovascular complications including ischemic strokeReference Guerrero, Dababneh, Hedna, Johnson, Peters and Waters2–Reference Zis, Stritsou, Panagiotis and Tavernarakis4 and intracerebral hemorrhageReference Gaye and Grimaud5–Reference Zepper, Wunderlich, Forschler, Nadas, Hemmer and Sellner7 are thought to arise from direct viral invasion into cerebral vessel walls leading to inflammatory vessel wall remodeling and secondary stenosis and thrombosis.Reference Zhang, Sumida, Margolesky, Tornes, Ramos and Koch3 Upregulation of matrix metalloproteinase-9 further leads to vessel wall breakdown, increasing the propensity for hemorrhage and eventual necrosis.Reference Zepper, Wunderlich, Forschler, Nadas, Hemmer and Sellner7
To our knowledge, this is the first case of HSV2 giving rise to multifocal hemorrhages with prominent edema and discrete lesions masquerading as a metastatic process. Previous case reports have detailed single and/or multifocal ischemic infarctsReference Guerrero, Dababneh, Hedna, Johnson, Peters and Waters2–Reference Zis, Stritsou, Panagiotis and Tavernarakis4 and multifocal ischemic infarcts with a single intracerebral hematoma,Reference Gaye and Grimaud5–Reference Zepper, Wunderlich, Forschler, Nadas, Hemmer and Sellner7 thought to be secondary to HSV2-induced vasculopathy.
Despite the overarching symptom of headache, CSF studies were not pursued on prior admissions, as an infectious process was not suspected due to the lack of systemic infectious signs. Moreover, the focus was on the presence of the intraparenchymal hemorrhages, which were thought to be hypertensive related. Although HSV2 generally gives rise to meningitis in an immunocompetent individual, the meninges are not the only component of the CNS that can be infected with HSV2, therefore contributing to the atypical clinical presentations of HSV2.Reference Berger and Houff1,Reference Zepper, Wunderlich, Forschler, Nadas, Hemmer and Sellner7 In the clinical case outlined above, HSV2 involvement would likely not have been detected without the significant clinical deterioration warranting neurosurgical intervention, which allowed for a tissue biopsy.
As outlined above, the pathological report on the tissue specimen revealed marked inflammation of blood vessels exhibiting transmural infiltration of inflammatory cells with prominent vessel wall destruction and fibrinoid necrosis consistent with necrotizing vasculitis. As such, causes of necrotizing vasculitis that manifest with intracranial hemorrhage were sought and ultimately led to positive immunohistochemical staining for HSV2.
Although it is tempting to speculate that a patchy meningitic process may have created multifocal areas of disruption to the neurovasculature unit, which, in turn, caused discrete areas of inflammation affecting nearby cerebral blood vessels, leading to vasogenic edema, inflammatory vascular remodeling, brain parenchyma inflammation, and ultimately hemorrhage and tissue necrosis, this would not explain why cerebral HSV2 infections do not give rise to a greater incidence of cerebrovascular complications. Indeed, a better understanding of the pathophysiology of the cerebrovascular complications from HSV2 involvement is warranted to explain how intracranial hemorrhage can ensue.
Unfortunately, both the delay in diagnosis and therapy ultimately contributed to the poor outcome in the patient, as HSV infections of the central nervous system require prompt treatment with acyclovir to reduce mortality. However, in the context of multifocal intracerebral hemorrhage, HSV involvement is rarely a consideration.
Conflict of Interest
The authors have no conflicts of interest or disclosures to report.
Author Contributions
GM was involved in drafting/revising the manuscript, design/conceptualization of the study, analysis/interpretation of data. J-QL was involved in acquisition of data, analysis/interpretation of data, revising the manuscript. DDSB was involved in analysis/interpretation of data. KP was involved in supervising the study, revising the manuscript, analysis/interpretation of data.




