Experimental tonic pain in a non-clinical sample is a model that resembles clinical chronic pain and serves as a useful tool to examine underpinning brain mechanisms (Huber, Bartling, Pachur, Woikowsky-Biedau, & Lautenbacher, Reference Huber, Bartling, Pachur, Woikowsky-Biedau and Lautenbacher2006; Nir, Sinai, Moont, Harari, & Yarnitsky, Reference Nir, Sinai, Moont, Harari and Yarnitsky2012). A plethora of electroencephalography (EEG) studies reported decreases of EEG alpha oscillations at around 10 Hz to tonic pain (Chang, Arendt-Nielsen, & Chen, Reference Chang, Arendt-Nielsen and Chen2002a, Reference Chang, Arendt-Nielsen and Chen2002b; Chen & Rappelsberger, Reference Chen and Rappelsberger1994; Dowman, Rissacher, & Schuckers, Reference Dowman, Rissacher and Schuckers2008; Nir et al., Reference Nir, Sinai, Moont, Harari and Yarnitsky2012; Peng, Hu, Zhang, & Hu, Reference Peng, Hu, Zhang and Hu2014; Shao, Shen, Yu, Wilder-Smith, & Li, Reference Shao, Shen, Yu, Wilder-Smith and Li2012), whereas other investigators obtained increases in the magnitude of gamma oscillations (30–100 Hz) (Dowman et al., Reference Dowman, Rissacher and Schuckers2008; Peng et al., Reference Peng, Hu, Zhang and Hu2014; Schulz et al., Reference Schulz, May, Postorino, Tiemann, Nickel, Witkovsky and Ploner2015; Veerasarn & Stohler, Reference Veerasarn and Stohler1992). However, little research effort has been devoted to study how individual differences in personality traits modulate EEG oscillations during tonic pain experience.
Among the neurophysiological-based personality theories that could potentially play an important role in pain experience is the revised reinforcement sensitivity theory (rRST) of personality (Corr, Reference Corr2008; Corr & McNaughton, Reference Corr and McNaughton2012; Gray & McNaughton, Reference Gray and McNaughton2000; McNaughton & Corr, Reference McNaughton, Corr and Corr2008) that represents a reconceptualization of the RST originally formulated by Gray (Reference Gray1982, Reference Gray1990). The behavioral inhibition system (BIS) and behavioral approach system (BAS) represent two neurophysiological brain systems that regulate how individuals respond to signals of potential punishment and/or reward. Specifically, the BIS is seen to be activated by signals of punishment and non-reward, novel stimuli, or unconditioned/conditioned fear stimuli. Its activation facilitates behavioral orienting through novel stimuli with interruption and inhibition of the ongoing behavior. The BAS is an appetitive–motivational system that is thought to respond to signals of reward and non-punishment. The BAS is activated by reward consumption and conditioned signals of reward or non-punishment and the associated approach behavior and positive emotions. In the rRST, the BAS is reconceptualized as a multidimensional system (Corr, Reference Corr2016). The two systems work independently, although they can interact. While Gray’s original conception of RST mentioned a less defined fight-flight system (FFS), rRST developed this system further into a fight-flight-freeze system (FFFS). The original conception of BIS dealt with responses to aversive stimuli, but in the rRST, the FFFS was primarily responsible for this role, while the BIS serves primarily to detect and resolve the conflict between BAS and FFFS. The FFFS encompasses functional behavioral responses to threat, including fighting the threat, fleeing in active avoidance, or freezing to avoid attracting the attention of the predator. Pain and other aversive treat stimuli can require either avoidance or cautious approach. Simple avoidance is thought to be controlled by the FFFS. A cautious approach, induced by approach-avoidance conflict, is thought to initiate a conflict resolution process in the BIS. This resolution process could increase behavioral inhibition, negative bias, arousal, attention, and risk assessment (McNaughton & Corr, Reference McNaughton and Corr2004).
Recent neuroimaging research has highlighted that BAS, but not BIS sensitivity of the Carver and White BIS/BAS scale (Carver & White, Reference Carver and White1994), is positively associated with μ-opioid receptor availability in frontal cortex, cingulate cortex, insula amygdala, ventral, striatum, and brainstem, indicating that endogenous opioid system underlies BAS (Karjalainen et al., Reference Karjalainen, Tuominen, Manninen, Kalliokoski, Nuutila, Jääskeläinen and Nummenmaa2016). Although abovementioned studies indicate that pain responses may represent a form of responding to negative stimuli, the underlying brain mechanisms of the immediate effects of pain and their relation with motivational personality traits have been poorly understood. This is mainly due to the fact that, in motivational and physiological pain research, Carver and White BIS/BAS scale (Carver & White, Reference Carver and White1994) is the most extensively used personality questionnaire, which is limited to only two measures of BIS and BAS. In addition, the BAS scale has no clear theoretical justification for its subdivision in three components, that is, drive, reward responsiveness, and fun seeking (for review, see Harmon-Jones, Harmon-Jones, & Price, Reference Harmon-Jones, Harmon-Jones and Price2013). However, the major problem with this questionnaire is the lack of separation of FFFS and BIS (Corr, Reference Corr2016), which may account for inconsistent findings when relating BIS scale to placebo and nocebo effects (Corsi & Colloca, Reference Corsi and Colloca2017). For example, Peciña et al. (Reference Peciña, Azhar, Love, Lu, Fredrickson, Stohler and Zubieta2013) found that neuroticism was a negative predictor, while ego-resilience and agreeableness were positive predictors of pain reduction magnitude. Coen et al. (Reference Coen, Kano, Farmer, Kumari, Giampietro, Brammer and Aziz2011) found no influence of neuroticism on pain perception, whereas they found a positive correlation between brain activity and neuroticism during pain anticipation in regions associated with emotional and cognitive pain processing, including the parahippocampus, insula, thalamus, and anterior cingulate cortex (ACC). In contrast, these regions showed a negative correlation with neuroticism during pain perception. Further, neuroticism was also negatively correlated to ventral ACC activity when shocks were expected (Kumari, Das, Wilson, Goswami, & Sharma, Reference Kumari, Das, Wilson, Goswami and Sharma2007). Overall, BIS and FFFS-related personality traits were found positively correlated with ACC and posterior cingulate cortex (PCC) reactivity in response to negative events, and negatively with PCC activity while anticipating a negative event (for review, see Kennis, Rademaker, & Geuze, Reference Kennis, Rademaker and Geuze2013).
Consistent with theoretical and empirical considerations of the rRST, a new questionnaire has been proposed, namely the Reinforcement Sensitivity Theory of Personality Questionnaire (RST-PQ; Corr & Cooper, Reference Corr and Cooper2016), developed on the basis of qualitative responses to defensive and approach scenarios. The RST-PQ highlighted a robust six-factor structure: two unitary defensive factors, the FFFS related to fear and the BIS related to anxiety, and four BAS facets (Reward Interest, RI; Goal-Drive Persistence, GDP, Reward Reactivity, RR; Impulsivity, Imp). The RST-PQ allows the separation of GDP, RI, and RR from Imp sub-factors of the BAS, making possible to test the unique predictive power of each sub-factor. Reward theory distinguishes between the anticipation of reward, closely linked to the motivation to obtain the reward, and the actual hedonic experience of reward (“wanting” vs. “liking”) (Berridge, Reference Berridge1996; Berridge, Robinson, & Aldridge, Reference Berridge, Robinson and Aldridge2009). Whereas the “liking” component is associated with striatal opioids (Peciña & Berridge, Reference Peciña and Berridge2000), “wanting” seems to be related to dopaminergic neurotransmission in the ventral striatum (Wyvell & Berridge, Reference Wyvell and Berridge2000). Individuals differ with respect to their sensitivity to reward and reward-predicting cues (Beaver et al., Reference Beaver, Lawrence, van Ditzhuijzen, Davis, Woods and Calder2006; Cohen, Young, Baek, Kessler, & Ranganath, Reference Cohen, Young, Baek, Kessler and Ranganath2005; Shoaib, Spanagel, Stohr, & Shippenberg, Reference Shoaib, Spanagel, Stohr and Shippenberg1995) and, in particular, “wanting” personality traits as assertiveness and reward sensitivity (i.e., drive and interest to achieve a reward) are associated with dopaminergic neurotransmission (DeYoung, Reference DeYoung2010; Yacubian et al., Reference Yacubian, Sommer, Schroeder, Gläscher, Braus and Büchel2007). The magnitude of opioid as well as dopamine release in the ventral striatum is related to the amount of pain relief (Scott et al., Reference Scott, Stohler, Egnatuk, Wang, Koeppe and Zubieta2007, Reference Scott, Stohler, Egnatuk, Wang, Koeppe and Zubieta2008; Zubieta et al., Reference Zubieta, Bueller, Jackson, Scott, Xu, Koeppe and Stohler2005), while change in the actual enjoyment of reward once it is achieved (“liking”) seems to be more closely related to opioidergic than to dopaminergic neurotransmission (Drago, Caccamo, Continella, & Scapagnini, Reference Drago, Caccamo, Continella and Scapagnini1984; Schweinhardt, Seminowicz, Jaeger, Duncan, & Bushnell, Reference Schweinhardt, Seminowicz, Jaeger, Duncan and Bushnell2009; Shimizu et al., Reference Shimizu, Iwata, Morioka, Masuyama, Fukuda and Nomoto2004). In this vein, since RI and GDP components of the BAS are conceptualized to serve the early stages of approach behavior (wanting or reward anticipation), these traits can be seen as the approach components linked to the dopaminergic activity. Additionally, as RR and Imp facets of the BAS are thought to serve the emotional excitement to reward, these traits are likely to depend from the function of the endogenous opioid system, which is mainly activated by the final biological reinforcer (Karjalainen et al., Reference Karjalainen, Tuominen, Manninen, Kalliokoski, Nuutila, Jääskeläinen and Nummenmaa2016; Peciña et al., Reference Peciña, Azhar, Love, Lu, Fredrickson, Stohler and Zubieta2013).
In the RST-PQ, the BIS and FFFS measures are postulated to have different functional properties and distinct neuropsychopharmacological bases (Corr & McNaughton, Reference Corr and McNaughton2012; McNaughton & Corr, Reference McNaughton, Corr and Corr2008) and separate sources of aversion (Perkins, Kemp, & Corr, Reference Perkins, Kemp and Corr2007). However, we do not currently have specific biological markers that can be used to distinguish these sources in humans. According to McNaughton and Corr (Reference McNaughton, Corr and Corr2008), these two dimensions account for the differentiation between different defensive behaviors and involve somewhat different neural networks, especially with active versus passive avoidance. Serotonergic and noradrenergic fibers that essentially mediate global threat sensitivity are seen to modulate all the structures controlling defense (for more details, see Corr, DeYoung, & McNaughton, Reference Corr, DeYoung and McNaughton2013).
EEG research on RST-related personality primarily attempted to link lateral frontal cortex, especially on the right, with avoidance-related processing mainly using Carver and White (Reference Carver and White1994) BIS/BAS scales to resting EEG alpha activity but inconsistent findings were found (for review, see Kennis, Rademaker, & Geuze, Reference Kennis, Rademaker and Geuze2013; Wacker, Chavanon, & Stemmler, Reference Wacker, Chavanon and Stemmler2010). Original studies which related the BIS subscale with right frontal activity did not consider that Carver and White (Reference Carver and White1994) BIS scale was developed with only one general avoidance system in mind. However, research has linked right frontal activity to BIS-related states of response inhibition, and regulatory control. Enhanced activity in the right inferior frontal gyrus, following transcranial direct current stimulation, did produce greater response inhibition in a stop-signal task (Jacobson, Javitt, & Lavidor, Reference Jacobson, Javitt and Lavidor2011; Stramaccia et al., Reference Stramaccia, Penolazzi, Sartori, Braga, Mondini and Galfano2015) and, conversely, lesions of the right prefrontal cortex led to poor inhibition in a stop-signal task (Aron, Fletcher, Bullmore, Sahakian, & Robbins, Reference Aron, Fletcher, Bullmore, Sahakian and Robbins2003). Kelley and Schmeichel (Reference Kelley and Schmeichel2016) found the right frontal cortex involved in the inhibition of both approach and avoidance behavior, a key function of the BIS. Research using large samples of resting data has demonstrated greater BIS-anxiety related to the greater relative right frontal activity (De Pascalis, Fracasso, & Corr, Reference De Pascalis, Fracasso and Corr2017; Neal & Gable, Reference Neal and Gable2016, Reference Neal and Gable2017). Knyazev et al., using resting EEG data, also found that the relative prevalence of parietal alpha power and reduction in delta power were associated with higher BIS/N individuals, whereas relative prevalence of delta oscillations, mostly in the frontal region, predicted higher BAS impulsive individual (Knyazev, Reference Knyazev2006; Knyazev & Slobodskaya, Reference Knyazev and Slobodskaya2003). Other authors reported a negative association between theta power and neuroticism (Chi et al., Reference Chi, Park, Lim, Park, Lee, Lee and Kim2005). A large literature suggests that enhanced theta activity in response to emotional stimuli is an index of perceived motivational salience, that is, the significance of a stimulus to the individual (Güntekin & Başar, Reference Güntekin and Başar2014; Knyazev, Reference Knyazev2007; Knyazev, Slobodskoj-Plusnin, & Bocharov, Reference Knyazev, Slobodskoj-Plusnin and Bocharov2009). Frontal theta is generated in the ACC, which is crucial in the evaluation of stimulus salience in order to drive behavior (Bush, Luu, & Posner, Reference Bush, Luu and Posner2000; Pizzagalli, Oakes, & Davidson, Reference Pizzagalli, Oakes and Davidson2003). Andersen, Moore, Venables and Corr (Reference Andersen, Moore, Venables and Corr2009) found theta band power especially responsive to anxious ruminative thinking and consistent with the model of recursive processing between the hippocampus and neocortex during goal-conflict resolution proposed by Gray and McNaughton (Reference Gray and McNaughton2000). Higher theta power reactivity to response execution during goal conflict in higher BIS participants was later reported by Moore, Mills, Marshman and Corr (Reference Moore, Mills, Marshman and Corr2012) in a continuous monitoring task. Right frontal theta power has been found to increase in both conflict- and loss-induced theta power and associated with higher neuroticism scores (Neo & McNaughton, Reference Neo and McNaughton2011). Very recently, we obtained heart-rate variability and conventional EEG band power measures during cold pain (CP) and placebo analgesia to identify RST-PQ traits that predict placebo analgesic responding. We found that a linear compound of HR slowing and higher EEG delta activity during placebo analgesia explained a substantial proportion of the variance in placebo analgesic responses, wherein RI had a significant mediating effect. These findings parallel our previous observations of reduced current density in the primary somatosensory cortex in higher total BAS and RI participants (De Pascalis & Scacchia, Reference De Pascalis and Scacchia2017a, Reference De Pascalis and Scacchia2017b). Other studies have outlined delta oscillations as a correlate of basic homeostatic and motivational processes as those involved in the detection of motivationally salient stimuli of reward and defensive mechanisms associated with pain and anxiety (Knyazev, Reference Knyazev2007, Reference Knyazev2012; Knyazev et al., Reference Knyazev, Slobodskoj-Plusnin and Bocharov2009) and behavioral inhibition (Harmony, Reference Harmony2013; Kamarajan et al., Reference Kamarajan, Porjesz, Jones, Choi, Chorlian, Padmanabhapillai and Begleiter2004; Knyazev, Reference Knyazev2007; Putman, Reference Putman2011).
To date, research using primarily Carver and White (Reference Carver and White1994) BIS scale has not identified a neurocognitive correlate of this trait in humans (Kennis et al., Reference Kennis, Rademaker and Geuze2013; Wacker et al., Reference Wacker, Chavanon and Stemmler2010). Thus, on the basis of the abovementioned EEG-pain findings, the aims of the present study were to detect, among EEG delta, theta, alpha, beta, and gamma bands, the brain rhythm sensitive to tonic CP and to highlight those sensitive to individual differences in pain and distress sensations. A further aim was to evaluate the link of both BIS and FFFS, as measured by the RST-PQ, with pain and distress sensations and to identify EEG band regional rhythms that can differentiate the defensive systems in terms of the BIS and FFFS. In line with Gray and McNaughton (Reference Gray and McNaughton2000) view, during pain, we expected a positive link between EEG theta activity and BIS scores as well as between delta activity and BIS scores. Finally, assuming that higher FFFS scorers should be prone to avoid painful stimulation, or to pay less attention to painful stimulation, we expected a reduced activity within the high-frequency EEG bands in these individuals. Finally, considering that BAS trait and its facets are activated by reward, we did not expect a link between pain sensation or EEG activity and these personality measures.
1. Methods
1.1 Participants
A total of 60 right-handed women (M = 23.8, SD = 2.1 years, range 19–32 years) student volunteers participated in the study, 4 of them were excluded for large EEG artifacts and 1 for presenting outliers, leaving 55 participants available for data analyses. We tested our hypotheses only in women since a body of literature clearly suggests that men and women differ in their responses to pain, with women being more sensitive (Bartley & Fillingim, Reference Bartley and Fillingim2013; Berkley, Reference Berkley1997). Further, to avoid individual differences in pain sensitivity due to menstrual pain, participants who were in a menstrual period were invited for the EEG recordings between the 5th and 11th day after the onset of menses. The study was approved by the institutional review board of the Department of Psychology according to Helsinki Declaration. Participants signed approved informed consent forms.
1.2 Questionnaires
The handedness of participant was assessed using the Italian version of the Edinburgh Inventory Questionnaire (Oldfield, Reference Oldfield1971). Participants also completed the RST-PQ (Corr & Cooper, Reference Corr and Cooper2016). This tool measures three major systems: FFFS (related to fear); BIS (related to neuroticism/anxiety); BAS. The BAS, namely the total BAS, is a composite of four subscales Reward Reactivity (BAS-RR), Impulsivity (BAS-IMP), Goal-Drive Persistence (BAS-GDP), and BAS-Reward Interest (BAS-RI). Just before starting the EEG recordings, participants completed the State Anxiety Inventory (Spielberger, Gorsuch, Lushene, Vagg, & Jacobs, Reference Spielberger, Gorsuch, Lushene, Vagg and Jacobs1988) to measure state anxiety.
1.3 Experimental procedure
Participants came to the laboratory and consented to participate. They first completed the handedness and personality questionnaires and, then, an electro cap for EEG recordings was fitted. Participants completed an averaged time of about 13 min of EEG recordings during two treatments with eyes open (see Figure 1).
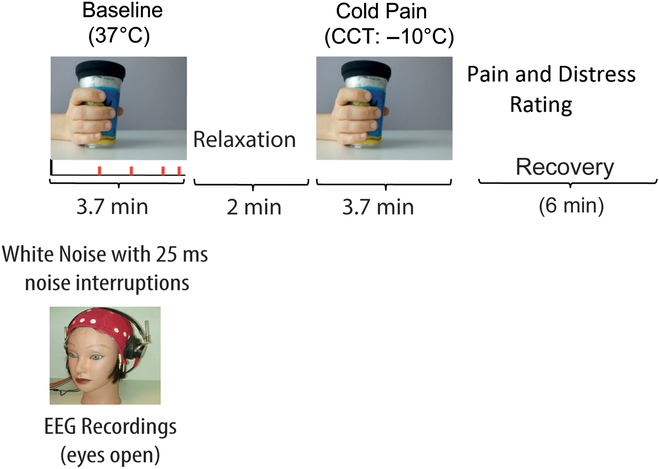
Figure 1. Schematic diagram depicting experimental treatments and procedure. Left quadrant in the panel shows a Baseline condition during which participants had to detect interruptions of a continuous white noise (Baseline). Right quadrant in the panel shows enduring CP induced by the CCT. Treatments were administered in counterbalanced order across participants. Following CP treatment, participants rated the intensity of experienced pain and distress sensation.
CP was induced by administration of the cold cup test (CCT; Chen, Chang, & Arendt-Nielsen, Reference Chen, Chang and Arendt-Nielsen2000; De Pascalis & Scacchia, Reference De Pascalis and Scacchia2019). The CCT can be considered as a variation of the ice-water cold-pressor test. This CCT proved to be convenient and consistent across the testing conditions. Participants received two treatments of 3.7 min each: (i) no-pain active Baseline, requiring to hold, in the right hand, a cup at about 37°C while listening, via binaural headphone, a continuous broadband white noise (70 dB, 0–44 kHz) wherein there were eight short interruptions (25 ms, rise time = 5 ms), randomly distributed within the Baseline time; and (ii) CCT, requiring a natural holding in the right hand of a tin-plastic chilled cold cup at −10°C. During the Baseline condition, the participant was required to pay attention to the ongoing white noise and to count the number of any possible changes in sound interruption, if any. As soon as the Baseline period was over, participants had to report verbally the number of white noise interruptions they detected. This Baseline condition was attempted to minimize the variability in arousal, attention, and vigilance both between and within participants by the auditory task. According to previous research (Dowman et al., Reference Dowman, Rissacher and Schuckers2008; Shao et al., Reference Shao, Shen, Yu, Wilder-Smith and Li2012), the active non-pain condition should represent a better Baseline condition in contrast to the CP condition than the classic passive no-task control condition. Just at the end of CP treatment, the participant was required to rate the perceived pain and distress scores (NPS and NDS) on two separate 11-point (0–100) numerical rating scales (NRSs) (Jensen, Karoly, & Braver, Reference Jensen, Karoly and Braver1986). The NRS for the perceived pain intensity was as follows: 0 = no pain, 10 = barely noticeable pain, 50 = mild pain, and 100 = maximum pain tolerable. The NRS for the perceived pain distress was as follows: 0 = neutral, 1 = barely distressing, 5 = distressing, and 10 = worst distressing imaginable. CP and Baseline treatments were administered in counterbalanced order across participants. In the cases in which Baseline preceded CP treatment, a 2-min relaxation period was given between treatments, in the opposite cases, the relaxation period was of 6 min.
1.4 EEG recording and processing
Participants were seated in a semi-reclined position inside a quiet dimly lit room. They were fitted with a pure-tin electrode electro-cap (Electro-Caps, Eaton, OH, USA) using an electrode placement based on the 10–20 system with a ground electrode mounted between FPz and Fz. Scalp EEG was recorded from 30 scalp sites (Fp1, Fp2, F7, F8, F3, F4, FT7, FT8, T3, T4, FC3, FC4, C3, C4, CP3, CP4, TP7, TP8, T5, T6, P3, P4, O1, O2, Fz, FCz, Cz, CPz, Pz, Oz) and referenced online to digitally linked ears [(A1 + A2)/2].
Bipolar horizontal and vertical electrooculograms (EOG) were recorded respectively from the epicanthus of each right and left eye, and from supra- and infra-orbital positions of the right eye using standard tin electrodes. Electrode impedances were kept under 5 kΩ, with homologous sites kept within 1 kΩ of one another. Data were collected using a 40-channels Neuroscan NuAmp amplifier unit (El Paso, TX, USA) settled in DC mode with a gain of 200 (100 for eye channels) and a band-pass of 0.01–75 Hz (Butterworth zero phase filter with 24 dB/octave roll off), notch filtered at 50 Hz (range 5 Hz), and digitized at 1000 Hz. EEG time series were then re-referenced to a common average reference and segmented into consecutive 2-s intervals. In order to eliminate artifacts, all data were offline visually inspected and hand-corrected for eyeblink artifacts using Brain Vision Analyzer 2.1 software. Eye-movement artifacts were removed by extracting 1–3 out of 30 independent components (IC; using Infomax algorithm, Brain Products; Vision Analyzer 2.01, Gilching, Germany) that clearly represented vertical and horizontal eye movements and had been identified by visual (topographic) inspection of the independent component analysis (ICA) maps and comparisons with the EEG and EOG time series (Delorme, Sejnowski, & Makeig, Reference Delorme, Sejnowski and Makeig2007; Olbrich, Jödicke, Sander, Himmerich, & Hegerl, Reference Olbrich, Jödicke, Sander, Himmerich and Hegerl2011). Due to the influence of ICA correction on coherence measures (Castellanos & Makarov, Reference Castellanos and Makarov2006; Olbrich et al., Reference Olbrich, Jödicke, Sander, Himmerich and Hegerl2011), only ICs without visible neural activity were discarded. Any segment that still contained muscle, movement, sweating, or eye-movement artifacts, as revealed by a visual inspection by two experienced clinical raters, was excluded from further analysis (no subject had more than 15% artifacts). A frequency resolution of 0.5-Hz steps for assessment of the different EEG bands was obtained. These 2-s EEG epochs were exported for further analysis.
1.5 EEG quantification
The data were parsed into 2-s epochs through a Hamming window, which was specified to diminish the signal on 10% at the borders of the epoch, to prevent spurious estimates of spectral power. After a visual re-examination for muscle, eye, movement, sweat, and technical artifacts, artifact-free epoch data were identified and, to facilitate later processing, downsampled to 256 Hz. An average of 90.7 (SD = 5.8) and 88.2 (SD = 7.1) of usable non-overlapping epochs were obtained, respectively, for Baseline and Pain conditions in each participant. Fast Fourier transform algorithm was used to perform EEG frequency analysis, with 2-s interval on the EEG signal, in all scalp locations. The bands inspected were the traditional delta (0.5–3.75 Hz), theta (4–7.75 Hz), alpha (8–12.75 Hz), beta (13–35.75 Hz), and gamma (36–45 Hz), and power values were averaged across epochs. Since the CP has a strong negative valence, which is known to increase over the course of time (Streff, Kuehl, Michaux, & Anton, Reference Streff, Kuehl, Michaux and Anton2010), power spectra were computed 30 s after that painful stimulation had started. The same starting time of 30 s was used for EEG analysis during the Baseline. Power values were natural-logarithm (ln) transformed to normalize the data (Gasser, Bächer, & Möcks, Reference Gasser, Bächer and Möcks1982). These values were then used to calculate EEG band power changes from Baseline by subtracting EEG band values during Baseline from those obtained during Pain. Based on previous pain study reports (Apkarian, Bushnell, Treede, & Zubieta, Reference Apkarian, Bushnell, Treede and Zubieta2005; Dowman et al., Reference Dowman, Rissacher and Schuckers2008; Koessler et al., Reference Koessler, Maillard, Benhadid, Vignal, Felblinger, Vespignani and Braun2009; Okamoto et al., Reference Okamoto, Dan, Sakamoto, Takeo, Shimizu, Kohno and Kohyama2004), we selected for statistical analyses the following 15 scalp recording sites: F7, F3, Fz, F4, F8, T3, C3, Cz, C4, T4, T5, P3, Pz, P4, and T6.
1.6 Statistical analyses
To evaluate the relation of pain with personality measures of interest, we first calculated the zero-order correlation of NPS, NDS, state anxiety, and RST-PQ traits. Significance of these correlations was assessed using the bias-corrected bootstrap method, which is effective in controlling for type 1 errors associated with multiple comparisons (Efron & Efron, Reference Efron and Efron1982; Efron & Gong, Reference Efron and Gong1983). For each correlation, we also computed the 95% confidence intervals (CI) using bootstrap resampling (5000 samples, bias-corrected confidence limits). All significant coefficients with an associated CI that did not include zero were considered statistically significant (P < .05).
To evaluate the effect of pain and personality on EEG activity, separate ANCOVAs were applied (SAS-9.4 system) using each of the associated pain and personality measures as a covariate. Condition (Baseline, CP) and Topography served as within subject’s factors. Within Topography, sagittal plane (anterior-frontal [F7, F3, Fz, F4, F8], temporo-central [T3, C3, Cz, C4, T4] and temporo-parietal [T5, P3, Pz, P4, T6] regions) and coronal plane (left-1 [F7, T3, T5], left-2 [F3, C3, P3], midline [Fz, Cz, Pz], right-1 [F4, C4, P4], right-2 [F8, T4, T6] regions) were repeated-measures factors. An α level of .05 was used for all analyses. Huynh-Feldt adjustments were used when the assumption of sphericity was violated. To control for false-positive errors, significance levels were corrected using the false discovery rate (FDR) method (“proc multitest,” SAS-9.4; Benjamini & Hochberg, Reference Benjamini and Hochberg1995). Only for graphical illustrations, and to understand the direction of changes of significant main and/or interaction effects involving NPS or personality traits of interest, we applied separate median splits on these self-report measures. Participants were considered as belonging to either group “high” (Hi) or “low” (Lo) when their scores on the pain and personality measures were above or below the median. Pain and personality scores falling on the median were excluded.
2. Results
2.1 Correlations among RST-PQ personality traits and NPS and NDS measures
Pearson correlation coefficients (bias-corrected bootstrap method) among RST-PQ personality traits, state-anxiety, NPS, and NDS ratings together with descriptive statistics are reported in Table 1. Correlations among personality measures confirm the pattern of originally reported associations (Corr & Cooper, Reference Corr and Cooper2016), while no significant correlations were found between RST-PQ traits as well as state anxiety and pain sensation measures.
Table 1. Pearson correlation coefficients and descriptive statistics for rRST personality traits and numerical pain and distress score (NPS and NDS). Bootstrapped 95% CI is reported in parentheses (N = 55)

BIS, behavioral inhibition system; FFFS, fight-flight-freeze system; T-BAS, total score for behavioral approach system; GDP, goal-drive persistence; RI: reward reactivity; Imp: impulsivity.
Notes: Personality Measures: Reinforcement Sensitivity Theory Personality Questionnaire (RST-PQ; Corr & Cooper, Reference Corr and Cooper2016).
NPS and NDS: 0–100 Numeric Rating Scale (Jensen, Karoly, & Braver, Reference Jensen, Karoly and Braver1986). STAI-Y1: State Anxiety (Spielberger et al., Reference Spielberger, Gorsuch, Lushene, Vagg and Jacobs1988).
*P < .05; **P < .01; ***P < .001; †P < .0001.
Bold entries indicate significant values.
2.2 Pain perception, BIS, FFFS, and relevant EEG band power measures
For each of the EEG delta, theta, alpha, beta, and gamma band power measures, separate ANCOVAs were performed by separately using each of the self-reported measures of interest as a covariate (i.e., pain and distress ratings and RST-PQ traits). Results of the ANCOVAs are given in Table 2. Additionally, for graphical illustrations, and to display the direction of significant changes, post-hoc t-tests were performed based on a median split of NPS, BIS, and FFFS scores (see Figures 2–5). The number of individuals falling on the median was: 7 for NPS (N = 26 Hi-NPSs and N = 22 Lo-NPSs); 5 for BIS (27 Hi-BIS, 23 Lo-BIS); and 6 for FFFS (24 Hi-FFFS, 25 Lo-FFFS).

Figure 2. Topographic patterns of significant ANCOVA effects (individual pain score (NPS) as a covariate) on beta power for (a) High-Pain vs. Low-Pain scorers; (b) Interaction of NPS with Topography and Condition (Baseline, CP). Independent t-test topographies compared High-Pain vs. Low-Pain scorers.

Figure 3. Delta power topographic patterns of a significant ANCOVA interaction of BIS trait (covariate) with Topography (Sagittal, Coronal plane) and Condition (Baseline, CP). Independent t-test topographies compared Low BIS vs. High BIS scorers.

Figure 4. Topographic patterns of significant ANCOVA effects on delta power for (a) High FFFS vs. Low FFFS scorers; (b) Interaction of FFFS with Topography and Condition (Baseline, CP). Independent t-test topographies compared High FFFS vs. Low FFFS scorers.

Figure 5. Gamma power topographic patterns of a significant ANCOVA interaction of FFFS trait (covariate) with Topography (Sagittal, Coronal plane) and Condition (Baseline, CP). Independent t-test topographies compared High FFFS vs. Low FFFS scorers.
Table 2. F-, P- and ![]() $\eta_p^2$-values for the main and interaction effects in the analyses of covariance with the factor Condition (Pain vs. Baseline), Coronal and Sagittal topography in the 2 × 3 × 5 factorial design for EEG band power measures
$\eta_p^2$-values for the main and interaction effects in the analyses of covariance with the factor Condition (Pain vs. Baseline), Coronal and Sagittal topography in the 2 × 3 × 5 factorial design for EEG band power measures

P values are corrected using FDR method. Bold entries indicate significant values.
2.3 Pain perception and EEG beta band power
Using NPSs as a covariate, we obtained, for beta band power, a significant Coronal by Sagittal by NPS interaction, revealing a significantly higher beta power in Hi-Pain than Lo-Pain scorers at frontal and right-parietal leads (see Table 2 and Figure 2(a)). In addition, for beta power, the Coronal by Sagittal by NPS by Condition interaction was also significant. This interaction disclosed a significantly higher beta power in High-Pain scorers at Fz, FCz, Cz, F8, T5, and T6 leads during CP and at F3, F4, FC4, and P4 leads during Baseline (Table 2 and Figure 2(b)). No significant effects involving distress rating scores were observed for the EEG band power measures of interest.
2.4 Effects of BIS and FFFS on EEG delta and gamma powers
No significant effects involving any of RST-PQ traits were obtained for theta and alpha band power measures.
The ANCOVA performed on delta power scores, using BIS as a covariate, disclosed a significant effect for Coronal plane followed by a significant four-way interaction for Coronal by Sagittal by BIS by Condition. These effects revealed that during CP condition there was a higher delta power in Hi-BIS as compared to the Lo-BIS participants at P3, Pz, and P4 leads (Table 2 and Figure 3).
A similar analysis conducted on delta power scores, using FFFS trait as a covariate, yielded a main effect for this trait, indicating that Hi-FFFS participants had a higher delta power than Lo-FFFS ones (Table 2 and Figure 4(a)). Moreover, the two-way Coronal by FFFS was near the significance level (FDR corrected P = .059), and four-way Coronal by Sagittal by Condition by FFFS interactions were all significant (Table 2). These effects indicated that Hi-FFFS scorers, for the CP condition, had a relatively greater delta power than Lo-FFFS scorers at T3, T5, P3, T4, and T6 leads, while for the Baseline these differences disappeared (Figure 4(b)).
Finally, ANCOVA on EEG gamma power disclosed significant effects for both Sagittal and Coronal planes and a significant three-way Coronal by Condition by FFFS interaction (Table 2). This interaction indicated that, during the CP condition, Hi-FFFS participants had a lower gamma power than Lo-FFFS ones across all midline sites (Fz, Cz, Pz) and left and right posterior temporal (T5, T6) and parietal leads (P3, P4) (Figure 5).
3. Discussion
Findings from the present study did not disclose significant EEG band oscillation changes between CP and Baseline condition. This means that our results did not support findings from previous studies in which cold-pressor test led to an increase in delta (Chen, Dworkin, Haug, & Gehrig, Reference Chen, Dworkin, Haug and Gehrig1989; Ferracuti, Seri, Mattia, & Cruccu, Reference Ferracuti, Seri, Mattia and Cruccu1994; Huber et al., Reference Huber, Bartling, Pachur, Woikowsky-Biedau and Lautenbacher2006), theta (Chen, Rappelsberger, & Filz, Reference Chen, Rappelsberger and Filz1998; Russ, Campbell, Kakuma, Harrison, & Zanine, Reference Russ, Campbell, Kakuma, Harrison and Zanine1999), beta, and gamma activity (Chang et al., Reference Chang, Arendt-Nielsen and Chen2002b; Shao et al., Reference Shao, Shen, Yu, Wilder-Smith and Li2012). These differences could be due to the fact that we used an active Baseline task during which participants had to focus attention on task-relevant stimuli (i.e., they had to detect and count changes in the ongoing white noise) which may have produced a general activation response in this experimental condition (Peng et al., Reference Peng, Hu, Zhang and Hu2014). However, in the current study, ANCOVA analyses on the conventional EEG band oscillation changes, induced by CP from Baseline, disclosed that (a) beta power change was sensitive to individual differences in pain perception, (b) delta power to individual differences in BIS levels, and both delta and gamma powers to individual differences in FFFS traits.
In terms of pain perception (NPS), the ANCOVA performed on EEG beta band power scores disclosed a main effect for the covariate NPSs, indicating higher frontal beta activity in High-Pain scorers compared to Low-Pain scorers. This analysis also showed that these individual differences were more pronounced at Fz, Cz, F8, T5, and T6 leads during CP condition and at F3, F4, and P4 leads during Baseline condition (Table 2 and Figure 2). In the whole, these findings suggest a functional role of EEG beta activity in representing subjective experiences of tonic pain. Specifically, our finding of enhanced beta activity in pain-sensitive individuals (Table 2) is in line with those reported in previous studies using long-lasting tonic pain stimulations wherein tonic pain was found associated with decreased alpha and increased beta activities (Chang et al., Reference Chang, Arendt-Nielsen and Chen2002a; Chang, Arendt-Nielsen, Graven-Nielsen, Svensson, & Chen, Reference Chang, Arendt-Nielsen, Graven-Nielsen, Svensson and Chen2004; Chen & Rappelsberger, Reference Chen and Rappelsberger1994; Giehl, Meyer-Brandis, Kunz, & Lautenbacher, Reference Giehl, Meyer-Brandis, Kunz and Lautenbacher2014; Shao et al., Reference Shao, Shen, Yu, Wilder-Smith and Li2012) and with higher beta power density findings (Huber et al., Reference Huber, Bartling, Pachur, Woikowsky-Biedau and Lautenbacher2006; Ploner, Sorg, & Gross, Reference Ploner, Sorg and Gross2017). Research has also demonstrated that beta and gamma band activities enhance with heightened attention to pain stimulus (Hauck, Lorenz, & Engel, Reference Hauck, Lorenz and Engel2007; Tiemann, Schulz, Gross, & Ploner, Reference Tiemann, Schulz, Gross and Ploner2010), vary with conscious perception (Gross, Schnitzler, Timmermann, & Ploner, Reference Gross, Schnitzler, Timmermann and Ploner2007) and attention effects of pain (Tiemann et al., Reference Tiemann, Schulz, Gross and Ploner2010). Accordingly, our observations of enhanced relative fronto-temporal EEG beta activity to CP in Hi-Pain scorers may reflect the operation of an excitatory process employed for the encoding of subjective experiences of tonic pain. In contrast, the reduced beta activity in Lo-Pain scorers may reflect the disposition, in these individuals, toward an inhibitory attentional-shift from painful stimulus making a reduced pain perception. Moreover, the present data indicate that these EEG changes are specific to tonic CP, as compared to changes observed during a non-painful Baseline stimulation, this is since we did not find any significant association between NPSs and state anxiety or dispositional personality traits of interest.
In terms of individual differences in rRST traits, our statistical analyses disclosed that Hi-BIS, as compared to Lo-BIS participants, had a relatively higher EEG delta power increase during CP across frontal, temporal, and parietal leads (Table 2 and Figure 3). These relatively new findings appear in line with few reports suggesting delta responses as a modulator of signal detection and decision making (Başar, Başar-Eroglu, Karakaş, & Schürmann, Reference Başar, Başar-Eroglu, Karakaş and Schürmann2001; Schürmann, Başar-Eroglu, Kolev, & Başar, Reference Schürmann, Başar-Eroglu, Kolev and Başar2001), with studies indicating a role of delta oscillations in the synchronization of brain activity with autonomic functions in higher emotional involvement such as pain and in anxiety disorders (Knyazev, Reference Knyazev2012). Delta activity was also associated with the detection of motivationally salient stimuli of reward and ancestral defensive mechanisms (Knyazev, Reference Knyazev2007; Knyazev et al., Reference Knyazev, Slobodskoj-Plusnin and Bocharov2009) and to behavioral inhibition (Harmony, Reference Harmony2013; Kamarajan et al., Reference Kamarajan, Porjesz, Jones, Choi, Chorlian, Padmanabhapillai and Begleiter2004; Knyazev, Reference Knyazev2007; Putman, Reference Putman2011). More specifically, Kamarajan et al. (Reference Kamarajan, Porjesz, Jones, Choi, Chorlian, Padmanabhapillai and Begleiter2004) reported suppressions of frontal delta and theta responses in alcoholic individuals which are likely to show deficits in cognitive functions that are mediated by these oscillatory processes. Thus, the higher relative delta power, we obtained during CP, in Hi-BIS participants, may reflect the enhanced adaptive attempt devoted by these individuals to resolve the conflict associated with tonic pain perception (Amodio, Master, Yee, & Taylor, Reference Amodio, Master, Yee and Taylor2008; De Pascalis, Varriale, & D’Antuono, Reference De Pascalis, Varriale and D’Antuono2010), vice versa, the relative lower delta power we did find in Lo-BIS participants may reflect the reduced tendency to process tonic CP stimulation in these individuals. However, this interpretation remains a purely speculative attempt to explain current results considering that we have not found any significant association between NPS and BIS, FFFS, or BAS traits. It should be noted that a lack of association between pain perception and anxiety-related traits is not new. For example, in a previous personality-pain study (Coen et al., Reference Coen, Kano, Farmer, Kumari, Giampietro, Brammer and Aziz2011) no association between neuroticism and pain ratings has been reported during visceral pain perception, although a negative correlation between neuroticism and brain activity was obtained in regions associated with emotional and cognitive pain processing, including the parahippocampus, insula, thalamus, and ACC (Coen et al., Reference Coen, Kano, Farmer, Kumari, Giampietro, Brammer and Aziz2011). Additionally, in a previous fMRI-pain study (Kumari, Das, Wilson, Goswami, & Sharma, Reference Kumari, Das, Wilson, Goswami and Sharma2007) neuroticism correlated positively with the ratings of fear of a shock and negatively with brain activity in the anterior and posterior cingulate, superior/middle temporal gyrus extending to the hippocampus, precuneus, putamen, thalamus, and middle occipital gyrus. These observations support the view of reduced processing of pain in subjects with higher levels of neuroticism, especially the anxiety component of this trait (Kumari et al., Reference Kumari, Das, Wilson, Goswami and Sharma2007). Further, fMRI findings (Bishop, Duncan, Brett, & Lawrence, Reference Bishop, Duncan, Brett and Lawrence2004) found that higher state anxiety levels were associated with both less rostral ACC activity and reduced recruitment of lateral PFC, suggesting that higher state anxiety is associated with reduced top-down control over threat-related stimuli. Unfortunately, we failed to extend abovementioned findings to tonic CP stimulation since we did not find a significant association between state anxiety and EEG power changes within conventional frequency bands during CP. We also failed to find a significant association between midfrontal theta activity and BIS as reported in previous studies (Andersen et al., Reference Andersen, Moore, Venables and Corr2009; Cavanagh & Shackman, Reference Cavanagh and Shackman2015; Gray & McNaughton, Reference Gray and McNaughton2000; Moore et al., Reference Moore, Mills, Marshman and Corr2012; Neo & McNaughton, Reference Neo and McNaughton2011). This may depend from differences in the type of task used between the current and previous studies. For example, while in previous studies the BIS–theta relationship was found for designed tasks requiring rapid resolution of a cognitive goal conflict (e.g., Moore, Gale, Morris, & Forrester, Reference Moore, Gale, Morris and Forrester2006; Moore et al., Reference Moore, Mills, Marshman and Corr2012; Neo & McNaughton, Reference Neo and McNaughton2011), in the current study the conflict consisted in paying attention to a continuous white noise in order to rate it. Thus, further investigation is justified.
In terms of the FFFS trait, ANCOVA analysis disclosed that Hi-FFFS participants, compared to Lo-FFFS participants, had higher delta power changes to CP at right-temporal and left-parietal leads, whereas they had lower gamma power changes at midline fronto-central and right parietal leads (Table 2; Figures 4 and 5). It is important to note that in the present study painful stimulation cannot be avoided, and the main function of FFFS activation serves to actively avoid the treat (active avoidance; Corr & McNaughton, Reference Corr and McNaughton2012). Considering that in previous studies delta activity was found associated with functional cortical deafferentation of sensory inputs that interfere with internal concentration necessary to accomplish a given task (Buzsáki, Reference Buzsáki2006; Harmony, Reference Harmony2013), our findings can be explained assuming that in higher FFFS individuals the active avoidance behavior is manifested through the increased delta power necessary to inhibit painful sensory afferences that interfere with concentration necessary to rate pain sensation. This finding is interesting and needs of further replications.
According to Botvinick (Reference Botvinick2007), pain falls into a class of conflicting signals, as monetary loss and negative feedback, which are registered within the ACC and weighted as an aversive or costly event. This would have a direct impact on decision making, influencing subsequent adaptive behavioral adjustments in avoidance-learning mechanisms. As previously stated, we believe that in Hi-FFFS participants were mainly involved spontaneous defensive avoidance mechanisms to facilitate their cognitive control of painful experience (Deakin & Graeff, Reference Deakin and Graeff1991). In line with Botvinick (Reference Botvinick2007), Buzsáki (Reference Buzsáki2006), Knyazev (Reference Knyazev2012), and Harmony (Reference Harmony2013) suggestions, we think that in Hi-FFFS participants the relative increase in temporo-parietal delta activity taken together with the relative decrease in cortical gamma activity, across midfrontal, temporal, and parietal regions, can serve to activate active-avoidance control mechanisms in order to reduce focused attention on painful stimulation and to prevent negative outcome. These findings appear also in line with previous reports suggesting that a complex network, including sensory cortices, insula, hippocampus, amygdala, and periaqueductal gray, is activated to an incoming threat associated with painful stimulation (Corr & McNaughton, Reference Corr and McNaughton2012; Faull & Pattinson, Reference Faull and Pattinson2017; Mobbs et al., Reference Mobbs, Petrovic, Marchant, Hassabis, Weiskopf, Seymour and Frith2007).
It is important to underline that the present study has some limitations. First, the sample was restricted to right-handed women. Our findings thus may not be applicable to men or left-handed women. This is since it has been shown that right-handed females perceive a painful heat stimulus as more intense than do males (see, e.g., Paulson, Minoshima, Morrow, & Casey, Reference Paulson, Minoshima, Morrow and Casey1998) and this is associated with greater activation in the contralateral thalamus and anterior insula. In addition, pain threshold and tolerance in response to submerging a hand in very cold-water baths have been found higher on the right hand in dextral subjects (Pud, Golan, & Pesta, Reference Pud, Golan and Pesta2009; Sarlani, Farooq, & Greenspan, Reference Sarlani, Farooq and Greenspan2003; Schiff & Gagliese, Reference Schiff and Gagliese1994). Second, this study was exploratory in nature since personality traits, as measured by the RST-PQ, have not yet been studied in relation to tonic CP and EEG oscillations. Third, participants rated subjective pain and distress intensity just at the end of the cold stimulation condition, and not continuously monitored during tonic CP stimulation. Thus, the results are tentative and need verification through additional research.
Acknowledgement
We want to give special thanks to the technician Emiliano Pes and all students participants and allowing us to conduct this study. This research was supported in part by a grant from La Sapienza University of Rome, Italy (project: C26A15RC5R) to Cecilia Guariglia (2015).
Author contributions
V.D.P. designed the methods and experiment. P.S. and B.P. performed data acquisition and signal processing. V.D.P. performed statistical analyses, interpreted the results, and wrote the paper. P.J.C. co-worked on results interpretation and suggested data analyses to test hypotheses derived from the Reinforcement Sensitivity Theory (RST). P.J.C. supervised discussion of the findings within a RST framework. All authors have contributed to, seen, and approved the manuscript.
Conflicts of interest
The authors have nothing to disclose.









