Introduction
The prevalence of chronic conditions and multimorbidity is increasing, imposing an enormous burden on patients and the healthcare system. Living with multimorbidity can affect patients’ quality of life in significant ways (Reference Damarell, Morgan and Tieman1). It is argued that investing in self-management interventions has the potential to improve health outcomes and ease the pressure on healthcare systems (Reference Taylor, Pinnock and Epiphaniou2;Reference Barlow, Wright, Sheasby, Turner and Hainsworth3). Self-management requires patients to engage in a collaborative partnership with their families or carer and their health professionals, with a view to learn (and commit) to look after themselves while managing their condition (Reference Grey, Schulman-Green, Knafl and Reynolds4). Patient’s knowledge, confidence, and skills to self-manage their own health and care refereed as “patient activation” (Reference Hibbard, Stockard, Mahoney and Tusler5) play important role in managing their health and has better health outcomes.
The emerging use of (innovative) digital technologies in healthcare offers a potentially effective option for delivering self-management support strategies to some individuals. Examples include education, support, and self-management in diabetes, chronic obstructive pulmonary disease (Reference Morton, Dennison and May6;Reference Slevin, Kessie and Cullen7), and epilepsy (Reference Bruno, Simblett and Lang8). Despite their rapid diffusion, important scientific and practical challenges need to be overcome to ensure that digital health technologies (DHTs) are adopted by healthcare systems and, just as importantly, by patients (Reference Singh, Drouin and Newmark9).
Chronic kidney disease (CKD) is a slow, progressive, and irreversible decline in renal function, leading to end-stage renal disease and cardiovascular morbidity, with significant health and healthcare cost implications (Reference Go, Chertow, Fan, McCulloch and Hsu10;Reference Elshahat, Cockwell and Maxwell11). The management of CKD includes: slowing down the disease progression to kidney failure; and reducing cardiovascular disease risk by managing kidney functions and CKD progression risk factors, such as hypertension and diabetes. As CKD progresses toward end-stage, long-term CKD management requires a high-level patient involvement to reduce the overwhelming impact of CKD. Self-management of CKD incurs a high burden of implementation for patients, who are expected to manage aspects ranging from: attention to dietary and medical management to recognizing early warning signs, regulation of fluid intake, blood pressure, and electrolytes (Reference Peng, He and Huang12). The increasing use of self-management interventions for CKD in recent years demonstrates the growing importance of such interventions in managing this condition to improve health outcomes (Reference Donald, Kahlon and Beanlands13). Hypertension management is a key outcome of CKD self-management, and technology-enabled interventions can control blood pressure in CKD (Reference Jeddi, Nabovati and Amirazodi14).
Clinically and cost-effective DHTs that can transform health and social care delivery must also be patient-centered. Research and Development (R&D) effort to support the creation of DHTs, particularly those used by patients, should be informed by strong clinical evidence and patients’ values, priorities, and preferences (Reference Iglesias Urrutia, Erdem and Birks15;Reference Krahn and Naglie16). If an R&D team, the target patients, and their (informal) carers do not co-produce the design of an innovative DHT from the earliest stages of development, the potential benefits of these technologies may never come to realize (Reference Edelman and Barron17;Reference Shields, Brown, Wells, Capobianco and Vass18). Thus, it is important to invest in co-designing any new form of collaborative care to help individuals manage health in their daily lives, respond more quickly to changes in symptoms, and prevent relapse.
This study is a part of the UK Engineering and Physical Sciences Research Council-funded “Wearable Clinic” aimed to create a set of software tools for wearable technology to support patient self-managing their long-term conditions. In this exploratory study, we aimed to investigate and elicit the preferences of individuals with CKD toward wearable DHTs to support self-management of their condition. This is the first effort in the context of using early health technology assessment methods to support the development of new wearable DHT.
Methods
Discrete-choice experiments (DCEs) are survey-based methods commonly used to explore preferences for services or products (Reference Clark, Determann, Petrou, Moro and de Bekker-Grob19). They involve presenting individuals with hypothetical choices which differ in attributes and their magnitude or levels and ask participants to choose the alternative they prefer in each choice set. This enables researchers to understand the value that individuals place on various levels of the attributes that characterize the technology under development. The study was designed and reported in line with published recommendations (Reference Bridges, Hauber and Marshall20–Reference Hollin, Craig and Coast22). We describe below the process involved in identifying the important aspects of wearable DHT used in the DCE.
Phase 1: identification of the characteristics of a wearable DHT to support self-management of chronic conditions
A literature review was conducted to identify published studies that (i) investigated reasons why individuals with CKD may choose (or refuse) to use wearable DHTs to support their self-management activities, as well as any study that (ii) attempted to understand people’s preferences for, and perceptions of technology-based interventions (e.g., wearables, mHealth, telehealth, smartphone apps). The search terms used are provided in Supplementary Material S1. Two researchers independently screened the titles and abstracts of all the articles identified through the electronic search, and one reviewer extracted data for all included studies. An initial set of generalizable characteristics of wearable DHTs (i.e., attributes) was compiled for further use in Phase 2.
It included their appearance, choice of settings, the format in which feedback information is provided to users, degree, and extent of data entry, capabilities/functionalities, style of engagement with the user (frequency and type of information the device provides), and the time required by the user to interact with it. A brief description of these features is provided in Supplementary Material S2. Furthermore, we conducted qualitative interviews with technology developers to identify the potential purposes of the technology under development. This resulted in a list of nine purposes of DHT which was used in Phase 2.
Phase 2: qualitative interviews
The findings from Phase 1 were used to define a topic guide for a semi-structured focus group meeting (Supplementary Material S3). Focus groups involve organized discussions with individuals to gain information about their views and experiences of a topic. In contrast to individual interviews, focus groups capitalize on stimulating interaction between participants, often yielding additional insights (Reference Kitzinger23). In this study, the focus group was used for two purposes: (i) to identify DCE attributes and attribute levels; and (ii) to shed light on individuals’ views of the role and potential uses of wearable DHTs to support collaborative management of their condition.
We used multiple platforms to recruit people with CKD into our focus groups, such as patient/consumer groups, charities, social media, existing patient and public involvement networks, and online research recruitment platforms. As a result, 10 volunteers who initially expressed an interest in contributing to our research were invited to participate in the focus group, and four were subsequently accepted (based on scheduling and availability).
Data from the focus group were transcribed verbatim and qualitatively analyzed using the framework approach (Reference Gale, Heath, Cameron, Rashid and Redwood24). To provide context, participants were asked about what strategies they used to manage their health and prior experiences of using DHTs and/or wearable devices (e.g., wearable blood glucose monitoring devices). When asked about the features that would persuade them to use a wearable device to support their self-management efforts, respondents preferred a small, multifunctional device. Individuals indicated they were willing to compromise on aspects such as the portability, aesthetic, and appearance (how discreet it is) of the device as long as this was able to help them with more than just one task of their self-management routine. Participants valued features that allow them to (i) monitor symptoms, (ii) pick up any warning signs, (iii) track progress and alert them when extra support may be needed, (iv) provide flexibility in interacting with a care team, and (v) help manage the time and frequency of appointments with the care team (for detailed results, see Supplementary Material S4).
Phase 3: designing, piloting, and fielding the online DCE
The outcomes of Phases 1 and 2 provided us with a list of attributes and attribute levels to use in DCE, based on which we have designed the pilot survey. In DCE, participants were asked to choose between two hypothetical wearable DHTs presented to them and a “none of them” option to mimic the real-world scenario where individuals are free not to choose a device for whatever reason. Responses to these choice-based questions enabled us to analyze trade-offs people made. In turn, this allowed us to inform the design of technologies that have the greatest potential to maximize adoption, adherence, patient satisfaction, health outcome, and possibly reduce costs for the healthcare system.
DCE experimental set-up
We adopted a full profile balanced-overlap, nearly orthogonal experimental design (Reference Chrzan25) using Sawtooth Software Lighthouse Studio v9.6 to create alternative profiles of a hypothetical wearable DHT from different combinations of its attributes and associated levels (reported in Table 1). The final set of attributes and their levels were determined by the investigative team based on the feedback received from the patients during piloting. The experimental design included ten choice tasks, each having two alternatives described by five attributes with a number of levels (see Table 1 for the full list). As it was not feasible for a participant to respond to the full factorial design (i.e., sixty-four combinations of attribute levels), we blocked the full factorial design into ten blocks (versions). Each participant was then randomly assigned to each block when they clicked on the survey link.
Table 1. Final attributes and levels used in the experiment

GFR, glomerular filtration rate.
The experimental design ensured one-way and two-way frequency balance such that each attribute level appeared an almost equal number of times in the survey and paired with other levels of the attribute in choice tasks as an equal number of times as possible (two-way frequencies). The balanced overlap between choice tasks in the experimental design allowed us to measure marginal utilities put on attribute levels when controlling for any cases where certain level(s) might appear all the time. An example choice task can be found in Figure 1.

Figure 1. Example of choice sets used in the discrete-choice experiment.
Pretesting and piloting the online choice survey
The online survey was piloted via four in-depth face-to-face interviews using the think-aloud method (Reference Kløjgaard, Bech and Søgaard26) before deploying the final survey. The purpose of this exercise was to: investigate the face validity, test the understanding and relevance of the wording used to characterize attributes of the wearable DHT, as well as, assess the overall presentation and interpretation of the DCE survey. In this pilot, we recruited individuals with CKD purposively based on technology use experience, demographics, and disease stage. Participants were presented with choice tasks and asked to complete them using a think-aloud approach while responding to survey questions. In addition to any issues raised by participants while reading and completing the questionnaire, we looked at visual hints (e.g., body language, facial gestures, pauses) that may reveal the need to clarify and discuss specific aspects of the survey. Three researchers facilitated the process, clarified any issues that participants had, and documented issues related to the presentation and description of any of our online survey items, for example, wording, display of the survey.
Overall, pre-testing participants completed the choice tasks without difficulty. They suggested changing some of the wordings to add clarity and maintain consistency. For example, one of the suggestions was to rename option 3 “none of them” to “neither option 1 nor option 2.” In addition, there was confusion on the levels of two attributes, that is, information it provides (medical and non-medical) and the format of information it provides (number, text, image, audio). In the revised version, we provided examples of medical and non-medical information and images, and audio. Following the think-aloud interviews, the wording and display of the survey introduction and the choice tasks were revised and simplified. Altogether, the final design included ten choice tasks, each comprising two hypothetical technologies and one opt-out scenario, which were randomly allocated to participants.
Survey participants, measures, and procedures
We recruited patients with CKD using online advertisements circulated to patient/consumer organizations (e.g., HealthWatch), charities (e.g., Kidney Care UK), social media (e.g., Facebook groups, Twitter), patient and public involvement networks, and online recruitment platforms between July and October 2019. The eligibility criteria for the participants were being aged ≥18 years and diagnosed with stage 3 CKD and onwards, but not on dialysis. As a token of appreciation, participants were given a £20 Amazon gift voucher.
There is no formal basis for sample size calculation for choice surveys in healthcare, and estimation methods are currently developing (Reference de Bekker-Grob, Donkers, Jonker and Stolk27). Therefore, the sample required for this study was estimated based on pragmatic considerations, and these included taking into account likely participation rates based on prior experience recruiting individuals with long-term conditions. The required minimum sample size to estimate preferences was 100 respondents. However, a larger sample was targeted to allow for the analysis of preferences heterogeneity.
The online survey began with a patient information sheet, followed by screening questions to identify eligible respondents. Participants were provided with a consent form, information on the study, and instructions on how to complete the choice task. Respondents were asked to select the top five purposes from a list of nine non-mutually exclusive potential purposes of a wearable health technology that emerged from Phase 2. The survey concluded with questions about their health, prior experience of using any digital wearable devices to monitor and control their condition, socio-demographics, and views on the survey.
The study was approved by the University of Manchester’s Research Ethics Committee (reference no. 2019–3263-10408) and was conducted in accordance with the General Data Protection Regulation.
Statistical analyses
The analysis of data from the choice task in our online survey was based on the random utility maximization theory (Reference Thurstone28;Reference Manski29). As individuals might have different preferences, we used a latent class modeling approach (Reference Cunningham, Deal and Rimas30;Reference Erdem and Thompson31). The underlying theory for the latent class model (LCM) posits that participants’ preferences can be segmented into
![]() $ Q $
latent segments (classes). Preferences within each class are assumed to be the same (i.e., homogeneous) but differ across classes. The model allows us to explain individuals’ preferences from their choice data and simultaneously show how respondents’ characteristics, such as gender and age, influence class membership. The optimal number of classes (i.e., segments) is identified using model fit criteria such as Bayesian Information Criterion (BIC), Akaike Information Criterion (AIC), log-likelihood values, and the principle of model parsimony. Socio-demographics and other data collected as part of the online survey were analyzed using descriptive statistics. All statistical analyses were undertaken using Apollo package in R (Reference Hess and Palma32). We also fit mixed logit and multinomial logit regression models but they performed worse than latent class model (Supplementary Material S5).
$ Q $
latent segments (classes). Preferences within each class are assumed to be the same (i.e., homogeneous) but differ across classes. The model allows us to explain individuals’ preferences from their choice data and simultaneously show how respondents’ characteristics, such as gender and age, influence class membership. The optimal number of classes (i.e., segments) is identified using model fit criteria such as Bayesian Information Criterion (BIC), Akaike Information Criterion (AIC), log-likelihood values, and the principle of model parsimony. Socio-demographics and other data collected as part of the online survey were analyzed using descriptive statistics. All statistical analyses were undertaken using Apollo package in R (Reference Hess and Palma32). We also fit mixed logit and multinomial logit regression models but they performed worse than latent class model (Supplementary Material S5).
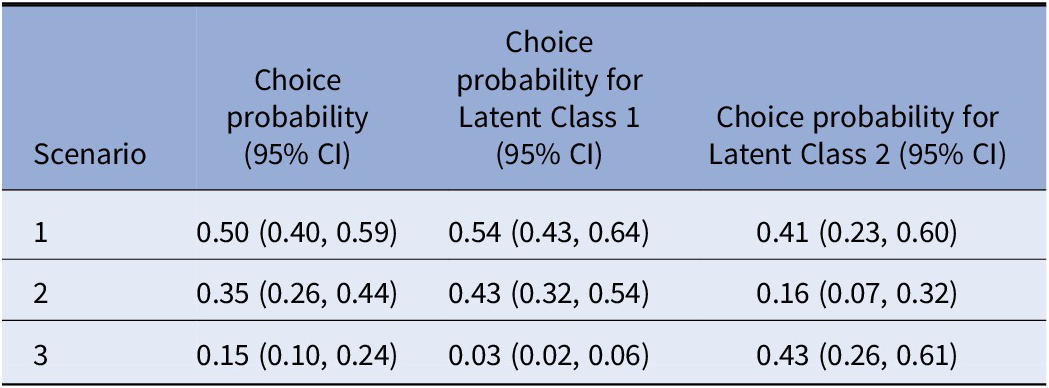
Furthermore, we performed scenario analyses for a number of policy-relevant scenarios to demonstrate the implications of our findings. We assume that there are three scenario options for wearable digital devices:
-
• Scenario 1: Device which has a discreet appearance, presents non-medical information with numbers and images, offers options to patients, and takes up a maximum of 30 min per day for an individual.
-
• Scenario 2: Device which has a noticeable appearance, presents medical information with numbers and texts, tells patients what to do, and takes up between 30 and 60 min per day for an individual.
-
• Scenario 3: No device
Using the LC model estimates, we calculate the predicted choice probabilities of these scenarios at an aggregate level (Table 2). We used Krinsky and Robb (Reference Krinsky and Robb33) technique to calculate confidence intervals (CIs) for the predicted update probabilities for given scenarios. This technique is based on taking a large number of draws (in our case r = 10,000) from a multivariate distribution with mean probabilities calculated using the estimated coefficients and covariances from their covariance matrix of the estimated coefficients. So, based on this r number of draws, we produced r simulated values of predicted probabilities. These values are then used to calculate the 95 percent CI. More detail on the calculator can be found in Supplementary Material S6.
Table 2. Scenario analyses
Results
The survey weblink was shared on multiple online platforms. In total, 233 unique hits on the survey website led to 142 (61 percent) completed surveys. We excluded twenty-nine responses that were completed in <5 min or were duplicates, and the final sample included 113 respondents.
Socio-demographic and health responses
Among the 113 respondents (Table 3), the sample included a slightly higher proportion of women (67 percent). Respondents were predominantly white, and over three-quarters of them were aged ≥45 years. The majority were not in full-time employment, and 51 percent of the respondents had a degree-level qualification or higher. Almost two-thirds of the participants (63 percent) had CKD stage III disease, and 61 percent of the sample received their CKD diagnosis more than 5 years ago. Only 6 percent of the respondents had previously used any digital wearable devices to self-manage their condition. Over four-fifth (81 percent) agreed that “they have the knowledge, skills, and confidence to take an active role in managing their own care.”
Table 3. Sample characteristics

CKD, chronic kidney disease; SD, standard deviation.
Latent class model results
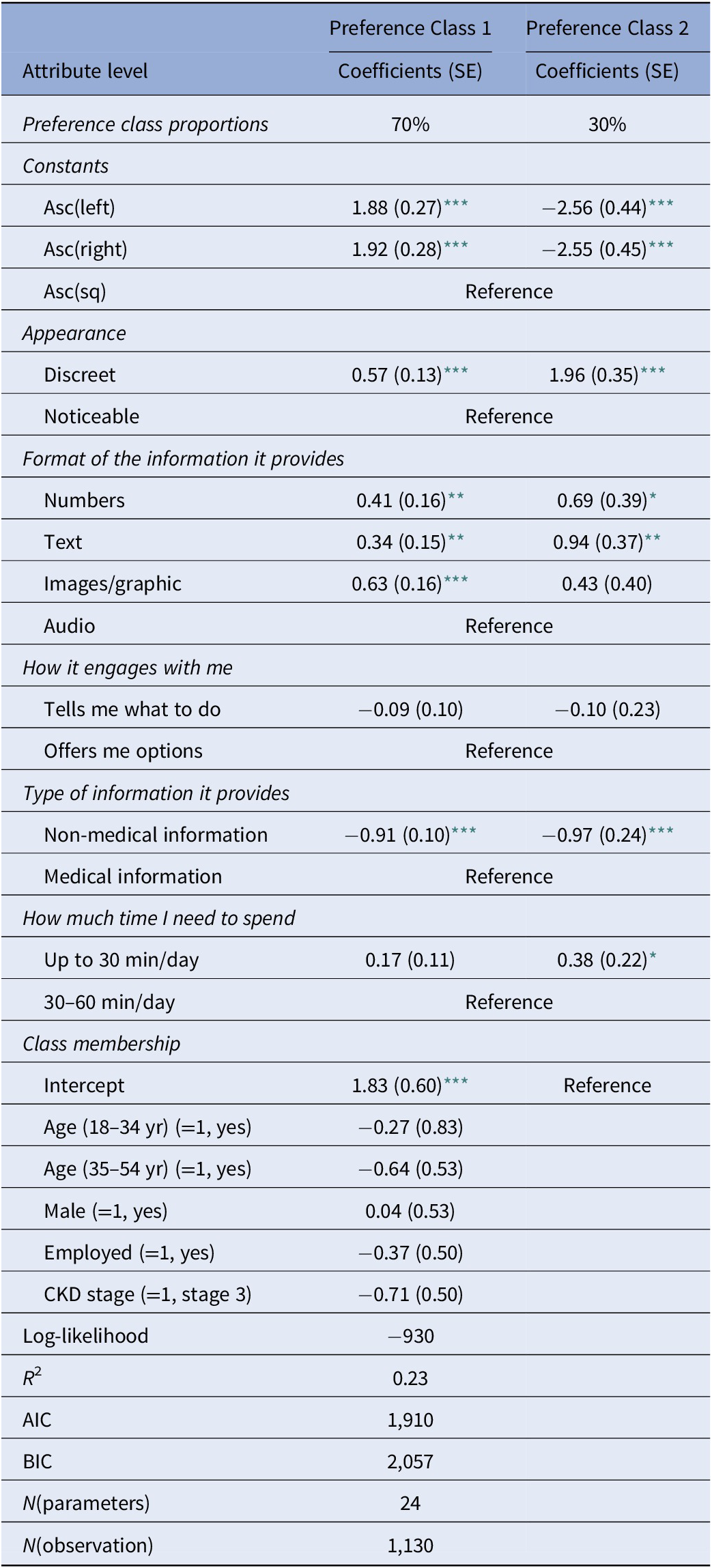
We analyzed the choice data using LCMs with up to four latent classes. Comparing the information criteria, log-likelihood measures, and model parsimony of these models, we decided on the model with two classes (Table 4). We also note that due to the small sample size, we also considered the size and composition of the latent classes when deciding on the final model. For example, a latent class as small as 5 percent of the sample (i.e., around eight people) would not give much reliable information; thus, it is useful to consider the sample size along with other measures when choosing the final model.
Table 4. Results of the latent class model
*** p < .01;
** p < .05;
* p < .10.
AIC, Akaike information criterion; ASC, alternative specific constant; BIC, Bayesian information Criterion; SE, standard error.
Class 1 accounted for 70 percent of the sample, and Class 2 accounted for 30 percent of the sample. The socio-demographic characteristics used in the class membership function were not statistically significant, indicating that none of the gender, age, employment status or CKD stage significantly determined the preference-class membership.
Focusing on the estimates for each attribute and their relative magnitude in both latent classes, we see that appearance and format are perceived to be the most important features of the digital wearable devices, as compared to engagement and information type a device can offer, as well as the time requirement from patients.
The majority (Class-1) preferred to have a wearable digital device, the minority (Class-2) preferred not to have it, as evident from significant and negative coefficients on alternative specific constants (asc). Despite this difference, both patient groups preferred a discreet wearable device, which uses numbers or texts and presents only medical information. While the use of images was favorable to the largest latent class, the smallest latent class did not prefer them. As for the engagement style, both groups did not indicate significant preferences, but looking at the coefficients, we can say that all patients were leaning toward wearable devices offering them options rather than telling them what to do. Another difference between the two patient classes was about the time they were prepared to spend. Whilst the large class was indifferent, the small class was more favorable toward a device requiring less than 30 min per day.
Overall, the majority of the patients preferred to have a wearable device, which has a discreet appearance, non-audio features when sharing information with users, and shares only medical information. The remaining small group did not prefer to have a wearable device. However, if they were using one, they preferred to have a device with discreet appearance, utilizing only numbers and texts, sharing only medical information, and requiring only up to 30 min per day.
What are the most relevant purposes of wearable health technology?
The survey asked participants to choose the top five potential purposes of wearable health technology from a list of nine. The most frequently selected relevant purposes were (i) help monitor or track symptoms of condition between follow-up appointments, (ii) help track their own progress and alert them at times when they may need extra support, (iii) educate them with new knowledge or skills relevant to their condition, (iv) prevent relapse by detecting warning signs that they may have missed, and (v) monitor (and remind when necessary) whether or not they have done specific tasks including following routines as part of care plan such as taking medications. Managing the time and frequency of appointments with people in the care team, sharing progress with others, and alerting when extra support is needed were the lowest-ranked relevant purpose of the wearable DHT.
Scenario analysis
We found that Scenario 1 had, on average, a 50 percent chance of being selected and Scenario 2 has a 35 percent chance of being chosen by patients. No-device option (Scenario 3) has a 15 percent chance of being chosen by patient groups. This demonstration highlights that the choice and adoption of a wearable digital device is highly dependent on the weights patients put on the attributes of these devices.
Considering the observed and unobserved heterogeneity, we can further explain the choice predictions using the LC estimates, including the class membership covariates. According to these choice probabilities, on average, we see that the largest latent class (class-1, 70 percent) chooses scenario 1 with 54 percent probability, scenario 2 with 43 percent probability, and a minimal likelihood of choosing no device option. However, the latent class-2 seems to favor having a no-device (43 percent), followed by scenarios 1 and 2.
Discussion
Over the past decade, there have been advances in technological innovations of wearable DHT and the rise of consumer health wearables (Reference Piwek, Ellis, Andrews and Joinson34;Reference Frist35). However, while these technologies can improve the quality of care, can be adapted on a large scale and at a low cost (Reference Kvedar, Coye and Everett36), their wider adoption remains hindered by acceptability, usability, and cost-effectiveness (Reference Loncar-Turukalo, Zdravevski, Machado da Silva, Chouvarda and Trajkovik37). This research investigated patients’ preferences for wearable DHTs that aim to help individuals manage CKD.
The results provide insights into what generalizable characteristics of wearable digital devices are more likely to be valued or accepted by target users (in this study, people with CKD) and how these preferences might differ for different patient groups. More specifically, we found that, on average, participants preferred a wearable DHT that is discreet, interacts with the user by providing options, provides medical information related to the monitoring of the condition and provides information in text, image or graphic formats.
However, our econometric analysis showed preference heterogeneity, indicating the need for considering different versions of wearable DHTs for different CKD patient groups. These initial results indicate users’ preferences toward wearable DHTs, how these differ between patient subgroups, and support the case for designing the device functionalities to meet the requirements of different subgroups of patients. The findings also provide insights to technology developers in the healthcare technology sector on how best to meet the needs of different CKD patients regarding their conditions. Incorporating user preferences early in the development pathway of DHT could help design targeted, person-centered technologies and improve adherence and disease management, facilitate shared decision-making, and lead health innovations. With the recent global growth in mobile/digital technology, involving patients earlier in adopting such technologies in healthcare can promote both clinical control and patient self-management.
There is an emerging consensus that users (e.g., patients and healthcare providers) should be involved at crucial decision points in developing the medical product life cycle (Reference Hoos, Anderson and Boutin38). Previous studies mainly looked at the use and effectiveness of technology-enabled interventions to support self-management of CKD (Reference Jeddi, Nabovati and Amirazodi14;Reference Campbell and Porter39), and indicated a positive effect of such interventions on clinical outcomes but did not consider patients’ perceptions and preferences which are crucial aspects for improving adherence and uptake of the resulting interventions. In their review, Jeddi et al. (Reference Jeddi, Nabovati and Amirazodi14) described the features of technology-based interventions to improve CKD self-management but did not indicate what features mattered the most to individuals with CKD. Moreover, patients’ preferences, perspectives, and values are often not included in formal health technology assessment processes (Reference Brooker, Carcone, Witteman and Krahn40).
Our findings showed that CKD patients’ preferences toward wearable DHTs differ between patient groups. In line with these findings, a recent review by Lin and Hwang (Reference Lin and Hwang41) showed that different levels of engagement and eagerness are required for a patient-centered approach to self-management support in CKD. In other chronic conditions, such as chronic obstructive pulmonary disease (COPD), a qualitative study on the use of wearables and self-management apps in patients with COPD found that people wanted to maintain control of the information and connect with the data (Reference Wu, Ginsburg, Son and Gershon42). Involving patients (and informal carers) in the co-design of wearable technology will be crucial to overcome potential barriers for use and ensure that technology design and development incorporates key components and/or features that may aid the self-management of chronic conditions.
In our study, patients with CKD preferred a wearable DHT that provides medical information as opposed to non-medical information, such as lifestyle advice. It is possible that they preferred the wearable DHT that provides disease-specific information in order to be alerted when a possible exacerbation occurs and that they can get non-medical information from other sources. In this regard, Vosbergen et al. (Reference Vosbergen, Mulder-Wiggers and Lacroix43) used mixed methods (qualitative interviews and online survey) using members of the general public to develop tailored health education messages and elicited chronic heart disease patients’ preferences for multiple message features. They found that patient-centered tailored messages using individual-based methods produced a manageable set of tailored messages, leading to increased patient engagement and improved processing of the message’s content.
Furthermore, the majority (80 percent) of the respondents agreed with the statement “I have the knowledge, skills, and confidence to take an active role in managing my own care”, indicating a certain degree of patient activation (Reference Hibbard and Gilburt44) and identifying the patient as a good target for self-management support. An important question that emerges, as the self-management tasks become more demanding, is the relationship between health literacy (including digital health literacy), health awareness, and self-care behaviors is complex (Reference Wong, Velasquez, Powe and Tuot45). It has been found that limited health literacy disproportionally affects people with CKD with low socio-economic status and of non-white ethnicity (Reference Taylor, Fraser and Dudley46); thus, healthcare providers must be aware of the potential equity implications associated with implementing a digital health-driven self-management support intervention in this patient population.
To the best of our knowledge, this is the first study that looked at CKD patients’ preferences to inform the development of a wearable DHT, which has the main function of supporting people with CKD to self-manage their condition. Inclusion of preference exploration (qualitative) and elicitation (quantitative) methods provide useful information for decision-making at different stages of the medical product development lifecycle. We used a patient-oriented approach to develop the DCE by involving patients and members of the public. This approach contributes to greater transparency, acceptability, and appropriateness of methods; and increases participation in research (Reference Aguiar, Harrison and Munro47).
Conducting choice surveys in the design phase of DHT raised a number of methodological considerations. Given the early phase of technology development, we used a pragmatic approach to estimate the sample size for our online choice survey. The fact that we were in an early stage of technology development meant that the attributes included in the choice task scenarios had to be kept broad. The list of attributes and levels can be refined further in the next phase of developing our wearable DHT.
The survey was administered online, and data were collected electronically. As it is typical with all online and offline surveys, it is possible that participants self-selected themselves into the study, which might cause the study results to be skewed toward this group. We see, for instance, that white female respondents were over-represented compared to the distribution of the general population of people with CKD in the UK. Increasing our sample’s size and diversity would help us further investigate preference heterogeneity; and the association between class membership, individual’ socio-economic characteristics (e.g., age, gender, and employment status), and disease severity.
Conclusion
The development of wearable DHTs to support people’s self-management of their condition must take into account their preferences to facilitate a move away from one-size-fits-all provisions. This will likely result in population health gains. Although this study focuses on designing and developing a specific technology in self-management for CKD, our research methodology is generalizable to inform and support the provision of nuanced person-centered products and services across a range of different health technologies and chronic conditions.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0266462322003233.
Acknowledgments
The authors would like to acknowledge and thank all participants who took part in the focus group and think-aloud interviews for providing insight into their experience; the individuals who responded to the questionnaire; public contributors Ms Suzy Bourke and Mr Rob Finnigan, for their comments on the earlier version of the online survey; and Dr Paul Laboi, York Teaching Hospital NHS Foundation Trust, for clinical input. The authors are grateful to Stella O’Brien for her comments on the earlier version of this manuscript from a PPI perspective; to John Mills (Vasculitis UK), Katherine Grady (Research for the Future), and Kidney Care UK, who supported and facilitated the recruitment of study participants. The authors appreciate the support from Kath Wright (University of York) and Paolo Fraccaro (University of Manchester) with the literature review.
Author contributions
C.P.I., S.E. and A.M. developed the study concept; V.S.G., C.P.I., S.E. and L.H. were involved in data curation; V.S.G. and S.E. performed formal statistical analysis; C.P.I., S.E., and A.M. designed the methodology; V.S.G. and L.H. recruited study participants. V.S.G. wrote the first draft of the manuscript and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding statement
This research was funded by the Engineering and Physical Sciences Research Council (grant EP/P010148/1; The Wearable Clinic: Connecting Health, Self and Care).
Conflicts of interest
The authors declare that they have nothing to disclose.
Consent to participate
Patients had to provide electronic consent before taking part in the study.
Ethics approval
The study was approved by the University of Manchester’s Research Ethics Committee and was conducted in accordance with the General Data Protection Regulation.
Availability of data and material
The datasets generated during and/or analyzed during the current study are not publicly available as no consent was sought from participants to allow sharing of data with third parties.







