Poor iron status, resulting from a long-term negative iron balance, is a major problem affecting people globally(1). Iron deficiency anaemia is highly prevalent in developing countries, especially among children and women. Dietary iron intake in these areas is low and does not meet requirements naturally. Apart from iron intake, iron status is also affected by other dietary factors, such as polyphenols from tea(Reference Tapiero, Gaté and Tew2). The assessment of the constituents in the diet that affect the bioavailability of iron will in theory help in correction of factors that could contribute to iron deficiency.
After water, tea is the most popular consumed beverage worldwide with an average intake per capita of 120 ml/d(Reference McKay and Blumberg3). Tea intake contributes significantly to the dietary intake of flavonoids. Apart from the potential protective role of flavanoids as antioxidants, these substances are assumed to affect negatively iron bioavailability by means of diminished solubility(Reference Fairweather-Tait and Teucher4).
Interventions with single meals have shown reduced uptake of iron from food when a test meal is accompanied by tea(Reference Hurrel, Reddy and Cook5, Reference Samman, Sandström and Toft6): iron absorption from test meals decreased from 12·1 (sd 4·5) % to 8·9 (sd 5·2) % (P < 0·01)(Reference Samman, Sandström and Toft6). A limitation of this approach is that it does not reflect normal dietary conditions. The association between tea and iron absorption was weaker when iron availability was assessed from the diet during a 2-week period than suggested by studies with single meals(Reference Cook, Dassenko and Lynch7). Furthermore, in a 16-week intervention trial investigating the possible differences between rooibos and black tea consumption on the iron status of black South African school children, no difference was shown between the effect of black tea in comparison with rooibos tea on markers of iron status(Reference Breet, Kruger, Jerling and Oosthuizen8). The flavonoid content of black tea is approximately 20–30 % higher when compared with that of herbal teas, and the addition of freshly boiled tap water to black tea leaves resulted in a concentration of 2 g/100 ml(Reference Marnewick, Joubert, Swart, Van der Westhuizen and Gelderblom9).
In an overview of sixteen observational studies, a negative association between tea consumption and iron status was found in populations with a marginal iron status, which were defined as children and as menstruating women. All but two studies were carried out in Westernized populations where iron status is usually in the normal range(Reference Temme and Van Hoydonck10).
So far, very little is known about the effect of tea consumption on iron status in black populations. Vorster et al. (Reference Vorster, Oosthuizen, Jerling, Veldman and Burger11) described rural black children and women aged 16–65 years as vulnerable groups for all levels of iron deficiency in South Africa, with usual iron intakes of less than 67 % of the recommended amount (US RDA). Moreover, black tea appears to be one of the drinks most frequently consumed by children(Reference Breet, Kruger, Jerling and Oosthuizen8, Reference Steyn and Labadarios12) and adults(Reference MacIntyre13) in South Africa and this might place the population at risk for iron deficiency.
Therefore, the aim of the present study was to further investigate the association between black tea consumption and iron status in a sample of black adults and in populations at risk for all levels of iron deficiency, living in the North West Province in South Africa.
Materials and methods
Sample
The THUSA (Transition and Health during Urbanization of South Africans) study was conducted from 1996 to 1998. For this cross-sectional study, ‘apparently healthy’ adults aged between 15 and 65 years were recruited from thirty-seven randomly selected sites in the North West Province of South Africa. Subjects with known diseases, pregnant and lactating women and subjects using chronic medication were excluded(Reference Vorster, Wissing and Venter14). In total, 1858 subjects were included in the THUSA study. For the present analysis we excluded subjects who were found to be infected with HIV (n 253), as this viral infection is assumed to be associated with iron accumulation and excessive iron storage. HIV disease progression might go along with increasing levels of serum ferritin and could therefore bias a possible correlation between tea consumption and iron status(Reference Savarino, Pescarmona and Boelaert15, Reference Gordeuk, Delanghe, Langlois and Boelaert16). The remaining 1605 subjects were stratified by sex because of physiological differences in iron metabolism between men and women. Subjects were recruited from five levels of urbanization: 28·7 % from deep rural areas; 15·6 % from farms; 16·9 % from informal housing areas; 27·8 % from established urban townships; and 11·0 % from ‘upper’ urban areas.
To ensure that volunteers were properly informed we provided all volunteers with written information explaining all aspects of the study. In addition and specifically for the benefit of illiterate volunteers, all information was also provided verbally in a language of their choice. All volunteers were given the opportunity to ask questions to clarify any aspects that were unclear. All volunteers signed an informed consent form and had the right to withdraw from the study at any time. The study was approved by the Medical Ethics Committee of the former Potchefstroom University for Christian Higher Education, now North-West University (Potchefstroom, South Africa).
Measurements
Data were collected by individual interviews conducted by researchers and trained fieldworkers. Anthropometric measurements and clinical and laboratory examinations were made. The THUSA study is described in detail elsewhere(Reference Vorster, Wissing and Venter14).
A demographic questionnaire was used to obtain descriptive information on the study population. A validated quantitative FFQ was used to collect information on dietary intake(Reference MacIntyre, Venter and Vorster17, Reference MacIntyre, Venter and Vorster18). Quantities consumed were assessed using validated photographs of different portion sizes(Reference Venter, MacIntyre and Vorster19), household measures and food models. Reproducibility of the FFQ was tested by a second administration of the FFQ to a randomly selected sub-sample of 144 volunteers 6–12 weeks after the initial interview(Reference MacIntyre, Venter and Vorster18). Relative validity of the FFQ was tested in a sub-sample of seventy-four volunteers by comparison to a 7 d weighed food record(Reference Venter, MacIntyre and Vorster19). The reported nutrient intakes were analysed using a program (Food Finder® Medical Research Council, Tygerberg, South Africa) based on the South African Food Composition Tables. Since tea often means any hot drink in this culture, brand names were asked as well. Tea consumption was defined as the intake of black tea only. Milk and/or sugar added to tea were recorded separately.
Anthropometric measurements included height and weight, and BMI (in kg/m2) was calculated for each participant. Haematocrit and Hb concentrations were measured in EDTA blood according to the centrifuge and cyanmethaemoglobin methods, respectively. Serum ferritin, transferrin and iron concentrations and total iron-binding capacity were determined using standard immunological, colorimetric and HPLC methods(Reference Vorster, Wissing and Venter14).
Statistical analysis
Statistical analyses for the reproducibility and relative validation studies were done using the Statistica for Windows (version 5) software (Statsoft, Tulsa, OK, USA). Correlations between energy and nutrient intakes derived from the first and second administrations of the FFQ, and the FFQ and the average of intakes from the 7 d weighed records were tested using Spearman rank correlation coefficients, differences in mean intakes between administrations and methods were tested using the t test for paired samples and the Bland–Altman method(Reference Bland and Altman20) was used to identify proportional bias and the limits of agreement. Detailed descriptions of the statistical analyses of the reproducibility and relative validity studies have been published elsewhere(Reference MacIntyre, Venter and Vorster17, Reference MacIntyre, Venter and Vorster18).
SPSS software package version 14.0 for Windows (SPSS Inc., Chicago, IL, USA) was used to perform statistical analyses, and P < 0·05 was considered statistically significant.
Mean values and standard deviations of descriptive variables and prevalence of iron deficiency and of iron deficiency anaemia were calculated. Variables that were not normally distributed were presented as geometric mean with 95 % CI after applying log-transformation. Iron deficiency was defined as a serum ferritin level < 15 μg/l, since iron stores are assumed to be depleted below these concentrations(1). Anaemia was defined as occurrence of subnormal Hb levels according to the WHO, i.e. Hb < 12 g/dl in non-pregnant adult women and Hb < 13 g/dl in men(1). Iron deficiency anaemia was defined as a combination of anaemia and iron deficiency.
Serum ferritin and Hb concentrations across categories of black tea consumption, defined as household measures of 180 ml, were determined in a multivariate ANOVA, simultaneously correcting for known confounding variables. Prevalences of iron deficiency and iron deficiency anaemia across black tea consumption categories and OR for iron deficiency anaemia at each level of black tea consumption, with no tea intake as a reference, were calculated. A test for trend of the OR across the tea categories was done using a logistic regression model including the numbers of cups of black tea per tea consumption category as a continuous variable. The association between black tea consumption and either iron deficiency or iron deficiency anaemia was evaluated in multiple linear regression models, adjusting for age, BMI, smoking status, and alcohol and iron intake.
Likewise, the analysis was repeated for sub-populations ‘at risk’, defined as (1) women ≤ 40 years; (2) subjects of both sexes with a low consumption of dietary iron, defined as the bottom quartile of iron intake ( ≤ 5·8 mg/d); and (3) subjects with a marginal iron status, defined as the bottom quartile of serum ferritin concentrations ( ≤ 26·6 μg/l).
Results
Complete demographic, dietary and physiological data were obtained from 1360 subjects. Demographic data of this population showed a mean household size of 6·0 and 41 % of all the subjects were employed at the moment of data collection. The total monthly household income was assessed to be R1000 (approximately 110 euro) or less for 74 % of the study population. Demographic factors, dietary intake and iron indicators of men and women are presented in Table 1. Significant differences between men and women were observed for mean values of Hb, serum iron and serum ferritin concentrations, total iron-binding capacity levels, transferrin saturation and haematocrit. All mean values of indicators of iron status fell within normal ranges.
Table 1 Characteristics of the subjects studied

Values were significantly different from those of the female group: *P < 0·05.
† Data were completed for 1360 subjects; for some variables the mean is based on data of a larger number of subjects.
‡ Packyears = (number of cigarettes × number of years smoking)/20.
§ Data presented as median and interquartile range.
The results of the reproducibility for reported iron intakes showed a significant correlation coefficient of 0·3 (95 % CI 0·12, 0·42), a small non-significant difference mean iron intake (1·2 mg; 95 % CI 0·39, 2·01) and weak, non-significant proportional bias (r 0·1; 95 % CI − 0·07, 0·26) between administrations of the FFQ(Reference MacIntyre, Venter and Vorster18). Regarding relative validity, there was a weak non-significant correlation coefficient of 0·2 (95 % CI − 0·03, 0·41) between iron intakes derived from the FFQ and the average of the intakes from the 7 d weighed record. There appeared to be slight, but not significant, underreporting of iron intakes on the FFQ compared to the average of the 7 d food record (difference (weighed record − FFQ) = 0·5 mg; 95 % CI − 0·6, 1·6), while the Bland–Altman method showed negligible proportional bias between the two methods (r 0·1; 95 % CI − 0·07, 0·26)(Reference Bland and Altman20).
In women, serum ferritin concentrations were positively correlated with Hb concentrations (Pearson correlation coefficient, r 0·26, P < 0·01). Dietary iron intake of women (7·6 mg; 95 % CI 7·3, 7·8) was low when compared to the estimated average requirement (8·1 mg/d for women 19–50 years; 5 mg/d for women>50 years according to US estimated average requirement(21)). Iron intake showed a weak negative correlation with black tea consumption in both men (r − 0·08, P = 0·041) and women (r − 0·07, P = 0·052). There was a strong positive correlation between iron and energy intake in both sexes (men r 0·78; women r 0·82; P < 0·05), but black tea consumption was not significantly correlated with energy intake. Age, BMI and alcohol intake showed significant related to serum ferritin and Hb concentrations and were, except for BMI, positively correlated with tea consumption as well. Serum ferritin concentrations in both sexes were higher in smokers than in non-smokers and in women Hb concentrations were higher among smokers than among non-smokers (P < 0·01).
In trend-anaysis, unadjusted serum ferritin levels across black tea consumption categories (Fig. 1) showed a decreasing concentration of serum ferritin in tea-consuming men, but this pattern was not consistent across the highest two categories of black tea intake. No clear trend in serum ferritin levels across categories of tea intake was seen in women. Adjustment for age, BMI, alcohol and iron intake, and smoking status did not alter these trends (Table 2): for both sexes, no trend was shown for the relationship between black tea consumption and Hb (men P = 0·33; women P = 0·49) or serum ferritin (men P = 0·059; women P = 0·49).

Fig. 1 Geometric means of unadjusted serum ferritin concentrations within tea consumption categories. ■, Men; □, women.
Table 2 Geometric means of iron indicators within categories of tea consumption†

† Adjusted for age, BMI, smoking status, and alcohol and iron intake.
OR were adjusted for age, BMI, alcohol and iron intake, and smoking status. Tea intake of 0 ml was used as the reference category (Table 3). Except for black tea intake of 181–360 ml in men, all OR for iron deficiency and iron deficiency anaemia showed broad confidence intervals without a trend across the tea consumption categories.
Table 3 Prevalence and OR† of iron deficiency and iron deficiency anaemia across categories of tea consumption

† Adjusted for age, BMI, smoking status, and alcohol and iron intake.
Multiple logistic regression with iron deficiency as a dependent variable and adjusted for confounders showed similar results: the relation between black tea consumption and status of iron deficiency was not significant in either men or women (men P = 0·056; women P = 0·74); similarly, iron deficiency anaemia was not significantly correlated to black tea consumption in either men or women (men P = 0·26; women P = 0·42).
Intake of iron did not show a significant correlation with status of iron deficiency or iron deficiency anaemia. Multiple logistic regression showed that only age and smoking status were predictive for iron deficiency in both sexes and for iron deficiency anaemia only in women.
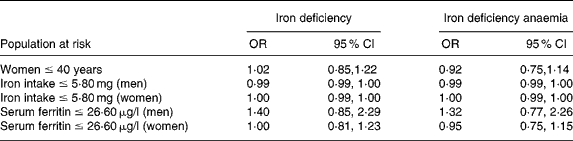
In order to test the hypothesis that the association between black tea and iron status is only present in high-risk groups, analyses were repeated in three populations that we defined as ‘at risk for iron deficiency’: (1) women ≤ 40 years (prevalence of iron deficiency (n/total): 29·8 % (151/507)); (2) subjects of both sexes with a dietary iron intake ≤ 5·8 mg/d (32·2 % (75/233) of the women and 12·4 % (18/145) of the men); and (3) men and women with serum ferritin levels ≤ 26·6 μg/l (53·2 % (167/314) in women and 43·9 % (29/66) in men). None of these at-risk populations showed significant differences in serum ferritin or Hb concentrations across tea consumption categories; linear regression with serum ferritin as dependent variable demonstrated no significant relation with black tea intake (Table 4). Using multiple logistic regression, adjusted for confounders, black tea consumption showed no increased risk for iron deficiency or iron deficiency anaemia in any of these sub-populations (Table 5).
Table 4 Multiple linear regression of tea consumption and serum ferritin on tea consumption in different populations at risk†

† Adjusted for age, BMI, smoking status, and alcohol and iron intake.
Table 5 Logistic regression of tea consumption and iron deficiency and iron deficiency anaemia in different populations at risk†
† Adjusted for age, BMI, smoking status, and alcohol and iron intake.
Discussion
In this large population of black African men and women we did not find consistent associations between black tea consumption and iron status. However, associations between black tea intake and serum ferritin concentration and between black tea intake and prevalence of iron deficiency in men were borderline significant and suggest a tendency for these associations. Age, sex and smoking status were the strongest predictor variables for iron status. Prevalence of both iron deficiency and iron deficiency anaemia was especially high in women. Iron stores were depleted in one of five women and this lack of iron was responsible for anaemia in 15 % of the women.
The question of the effect of the addition of milk to tea on a number of physiological outcomes has been of interest for a number of years. It is known that proteins can bind to flavonoids(Reference Kumar and Singh22, Reference Hagerman and Butler23) and it has been hypothesized that milk might have some functional effect on the ability of flavonoids to bind metals or act as antioxidants. A recent report suggests that milk protein could inhibit potential favourable effects of tea on flow-mediated dilation(Reference Lorenz, Jochmann and von24). It has, however, been shown that the addition of milk to black tea has no effect on antioxidant activity(Reference Leenen, Roodenburg, Tijburg and Wiseman25–Reference Richelle, Tavazzi and Offord27) and that the addition of milk to tea does not affect the bioavailability of flavonoids(Reference Hollman, Van Het Hof, Tijburg and Katan28, Reference Kyle, Morrice, McNeill and Duthie29). The most relevant work, however, was done by Hurrell et al. who studied the effects of different polyphenol-containing beverages on iron absorption from a bread meal in adult human subjects using the erythrocyte incorporation of radio-Fe as primary endpoint(Reference Hurrell, Reddy and Cook30) and showed that adding milk to tea has little or no influence on its iron inhibitory nature.
It is generally accepted that bioavailability of iron is influenced by a variety of dietary factors(Reference Fairweather-Tait and Teucher4), such as fibre, calcium, ascorbic acid and animal protein. Although experimental studies have shown that polyphenolic-containing liquids inhibit iron absorption(Reference Hurrel, Reddy and Cook5, Reference Samman, Sandström and Toft6, Reference Brune, Rossander and Hallberg31), we found that black tea consumption did not negatively affect either serum ferritin levels or iron status in this cross-sectional analysis. A similar conclusion was drawn from a study in the rural areas of China where even high levels (38 g dry weight/d) of black tea consumption were not correlated with iron status(Reference Root, Hu, Stephenson, Parker and Campbell32). Serum ferritin concentrations and prevalence of iron deficiency in the present black African study population were in the same range as those found in studies investigating the relationship between tea intake and iron status in European populations(Reference Gregory, Foster, Tyler and Wiseman33, Reference Galan, Yoon and Preziosi34) and ferritin concentrations were in line with those in a population of rural Chinese women as well(Reference Root, Hu, Stephenson, Parker and Campbell32).
From our regression models, adjusted for demographic and lifestyle variables, it can be concluded that black tea consumption as part of a complete diet is not a significant predictor of iron status, iron deficiency or iron deficiency anaemia, whereas sex, age and smoking status were the main determinants of iron status. Furthermore, iron intake was not significantly associated with iron status in the present study population.
The validity of reported nutrient intakes is always of concern in dietary intake studies. In the present study, while reported iron intakes were found to be reproducible, relative validity, on an individual level, as estimated from the Spearman rank correlation coefficient was found to be weak. Comparison of reported mean intakes and the Bland–Altman plot showed slight (but not significant) underreporting by the FFQ. Therefore, actual iron intakes may have been slightly higher than those reported in some subjects. Nevertheless, in light of the very weak association of iron status and iron intake it is unlikely that this difference would have led to different results.
Dietary iron in an absorbable form is essential for maintenance of an adequate iron status. However, considering the confounding variables, we did not find a significant relation between iron intake and iron status, which is in agreement with several other studies with a similar design(Reference Root, Hu, Stephenson, Parker and Campbell32, Reference Ball and Bartlett35, Reference Hunt and Roughead36). Demographic factors, smoking status and menstrual blood loss in females are shown to be important determinants of iron status as well(Reference Galan, Yoon and Preziosi34).
The present results from this apparently healthy African population agree with the final statements of several other observational studies. Results of the National Health and Nutrition Survey in the USA showed that both iron intake and tea consumption as part of a varied diet were not significant determinants of iron deficiency anaemia after controlling for iron and ascorbic acid intake. However, both age and sex contributed to prediction of iron status(Reference Mehta, Pritchard and Stegman37). Furthermore, the present findings are in line with an extended review of sixteen studies, almost all in Western populations(Reference Temme and Van Hoydonck10), and a review based on twelve observational studies in the UK(Reference Nelson and Poulter38) on the association between tea consumption and iron status. Both reviews concluded that tea intake did not affect iron status in people with adequate iron stores.
Previous findings suggest that consumption of black tea reduces iron availability when examined under laboratory conditions. However, our analysis implies that this effect diminishes when determined in free-living subjects who do not necessarily drink their tea with their meals under the same conditions as during an experiment. A possible explanation for the discrepancy between conclusions from experimental and observational studies might be that results from single test meals do not reflect complete dietary patterns(Reference Hunt39). More extreme dietary differences than would be considered practical in the habitual diet can be implemented in the study design and results from test meals might therefore overestimate the importance of the effects of black tea on iron status. In a 16-week clinical trial investigating the possible differences between rooibos and black tea on the iron status of black South African school children, no difference was found between the actions of black tea in comparison to rooibos tea on markers of iron status(Reference Breet, Kruger, Jerling and Oosthuizen8). These results suggest that black tea consumption with meals in the long term does not negatively affect parameters of iron status and supports the notion that results from single test meal studies may overstate the action of potential inhibitors of iron absorption on iron status.
In the present study polyphenol content of the black tea as well as tea intake in combination with other food items was not known. Cultural habits in South Africa show that tea is consumed both as a beverage between meals and as a drink accompanying meals(Reference MacIntyre13). Data from Kenya show that the majority of the Kenyan population consume tea with at least one meal(Reference Breet, Kruger, Jerling and Oosthuizen8, Reference Shell-Duncan and McDade40). At national level, tea is one of the five most commonly consumed foods in South Africa among children(Reference Steyn and Labadarios12). A report on South African food consumption studies undertaken amongst different population groups shows that the dietary pattern among adults was similar(Reference Nel and Steyn41).
Temme & Van Hoydonck(Reference Temme and Van Hoydonck10) mention that in populations with marginal iron status, defined as populations of children; of menstruating women (17–42 years); and of women of 19–43 years including a large percentage of iron-deficient women (16–40 %) there seems to be a negative association between tea consumption and iron status. Likewise, Nelson & Poulter(Reference Nelson and Poulter38) suggest that groups at risk of iron deficiency, described as children under 6 years; as adolescent girls; as women of age 18–49 years; and as women older than 75 years should not consume tea along with their meals. Although associations between tea consumption and iron status in children and elderly could not be determined with our data, we did not find any associations between black tea consumption and iron intake in several groups which are at risk for iron deficiency and iron deficiency anaemia.
Multiple regression analysis on women of menstruating age, which is one of the most cited examples of a population at risk, showed that black tea consumption did not contribute to an increased risk of iron deficiency or iron deficiency anaemia. Furthermore, also in sub-populations with a low iron intake or with depleted iron stores, no relation between black tea drinking and iron status was shown. Thus, the present results do not support the hypothesis that there is an association between black tea consumption and iron status in groups that are at high risk for iron deficiency.
Conclusion
Black tea consumption as part of a complete diet does not affect iron status in these black adult Africans, not even in sub-groups which are at a higher risk for iron deficiency.
Iron status of African adults was in general satisfactory in men, but iron deficiency was considerably prevalent in women. The likelihood of having iron deficiency or iron deficiency anaemia is not significantly explained by black tea consumption as part of a varied diet in this population. Therefore, we found no evidence that black tea consumption will have adverse effects on iron status populations at risk for iron deficiency or in populations with marginal iron status. Other strategies are urgently needed to address the high prevalence of iron deficiency in this group.
Acknowledgements
Biochemical analyses of serum were performed at the Department of Chemical Pathology, University of Pretoria. Financial support of the North-West (former Potchefstroom) University, the Foundation for Research Development, the Dry Bean Producers Organisation, Clover, the Medical Research Council and the South African Sugar Association is appreciated. We would like to acknowledge the efforts of Hester Vorster, who was the initiator and project manager of the THUSA study. We also thank Grieta Hanekom, Lizanne Muller and Machteld van Lieshout. P. S. H. performed statistical analysis and principally wrote the manuscript. J. C. J., T. H. and A. M.-B. contributed to the data analysis and writing of the paper. U. E. M. performed the field survey and prepared the dietary and nutritional information. None of the authors have any conflict of interest.








