To the Editor—Medical devices that are reprocessed in a sterile processing department must be cleaned, disinfected, and sterilized to prevent infection before apply to the patient. The preferred and most commonly used sterilization method is steam sterilization, in which saturated steam (moist heat in the form of saturated steam under pressure) is used as a sterilizing agent.Reference Nagpal and Shriniwas1 The steam sterilization process has several advantages: it is inexpensive, nontoxic, fast, accurate, and widely recognized. The basic principle of the steam sterilization process is to remove air and noncondensable gases (NCGs) from the sterilization chamber by gravitational system or by separate vacuum pump so that steam gets easy access for sterilization. However, when air removal is insufficient, the sterilization process is suboptimal, even if all other process parameters, such as steam pressure and temperature, are achieved. Therefore, the quality of the vacuum pump in relation to the chamber volume is very important for proper air removal and steam penetration purposes. In addition, door seals, gaskets, and other valves should be tested frequently to ensure that there is no leakage where air and NCGs (ie, carbon dioxide and hydrogen) can enter during the sterilization period.Reference Winter, Smith, Lappin, McDonagh and Kirk2
The presence of air and other NCGs inside the sterilization chamber is one of the biggest threats to the sterilization process. NCGs prevent steam from reaching the medical device, inhibiting thermal coagulation of spores during sterilization. Therefore, air pockets form (bubbles of NCGs) that isolate the goods to be sterilized and block further condensation of the steam before sterilization. In addition, formation of NCGs inside the sterilizer chamber (NCGs should be ≤0.15%) can slow down the heating process or the sterilization cycle may abort due to insufficient vacuum if the gases are not properly removed.
No reliable equipment monitoring device is available to monitor insufficient air removal inside the sterilizer chamber. However, operators can check the temperature and pressure against time via thermoelectrical measurement to interpret how much vacuum are achieved according to the sterilizer manufacturer. The measurement is based on simultaneous measurement of the temperature in the center of the pack and outside it. Some additional preventive actions can be taken to reduce the problem: (1) using a good quality vacuum pump because adequate air removal by good quality vacuum pump reduces the residual air in the chamber; (2) increasing the number of pulse-vacuum phase, which is determined by capacity of vacuum pump with chamber volume or density of the sterilizer; (3) performing timely maintenance of door gaskets, seals, and valves because door gaskets can be leaked by a high level of contaminants or uneven surface of sterilizer door and valves can be damaged by continuous use of sterilizer; and (4) using cold water (for deep vacuum) with a deionization system because hydrogen carbonate containing water can produce CO2 and carbonate if the ion exchanger fails during steam preparation.Reference van Wezel, van Gastel, de Ranitz and van Doornmalen Gomez Hoyos3
In 1963, the Bowie–Dick or air removal test for vacuum-assist sterilizers was developed to detect air and NCGs present in a sterilization cycle. To confirm their absence, an air removal cycle is run first in an empty chamber with exposure time of 3.5 minutes at 134°C.4 If the Bowie–Dick test is passed satisfactorily, then the following load cycle run with a biological indicator to monitor the lethality of a given sterilization process for biologically proven sterility assurance. Every test device should be placed inside the sterilizer chamber in the most challenging area, (eg, in front of the door or drain strainer) for a worst-case scenario. If the air is correctly removed, then steam can penetrate satisfactorily and the color of the chemical indicator changes significantly and uniformly.
Discussion
The Bowie–Dick test is an equipment-specific test that assesses how well the autoclave vacuum pump is working according to EN 285, the European large steam sterilizer standard. It is the only indicator in which air and NCGs are detected. The test pack is composed of 3 important elements: (1) a porous barrier layer with standardized porosity, (2) an indicator layer composed of a type-2 chemical indicator with thermochromic ink to detect steam quality, and (3) 2 transparent gas impervious layers composed of 2 polyester films with 0.01–0.03 mm thickness between which the indicator is placed.5 According to the weight of [4 kg, according to the American medical instrumentation standard] (AAMI/ANSI ST79), or 7 kg, according to the European (EN) and international (ISO) medical instrumentation standards, this test assesses the vacuum pump capacity, that how much air can be removed from the chamber and the individual package so that steam can penetrate well for proper sterilization.6,Reference Basu, Bhattacharya, Mahajan, Ramanan and Chandy7
Continuous quality monitoring of sterilization processes is of paramount importance in supplying sterile materials to patients. However a single Bowie-Dick test cannot give guarantee all over the day if the sterilizer is completely stopped for more than 3-4 hours at a time because NCGs (due to poor water quality) may accumulate inside the steam pipeline if water flow is stopped or stagnant. Moreover, even when the Bowie–Dick test has been successful, the process parameters can change with each subsequent cycle. Every delivery of steam to the sterilizer can have different characteristics that change in every cycle.
To ensure the air and NCG removal process and to control cost, we strongly recommend the use of a type-2 chemical indicator in every cycle (Table 1) by a reusable test device according to the European standard for nonbiological systems for use in sterilizers part 5, specification for indicator systems and process challenge devices for use in performance testing for small sterilizers type B and type S (EN 867-5). This process can confirm that the sterilizer chamber and packages are free from air and NCGs to assure optimum sterility according to the European standard requirements for medical devices to be designated sterile (EN 556).8
Table 1. Advantages of Type-2 Chemical Indicator Used in Every Autoclave Cycle
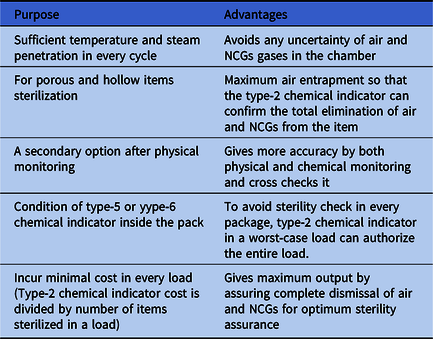
Note. NCG, noncondensable gas.
Acknowledgments
None.
Financial support
No financial support was provided relevant to this article.
Conflicts of interest
All authors report no conflicts of interest relevant to this article.



