Summations
-
∙ The human laminated cerebral neocortex is of recent evolutionary origin and is not essential for the regulation of reward-seeking and misery-fleeing emotional behaviour.
-
∙ Almost the entire telencephalon (endbrain) of human’s earliest vertebrate ancestors became incorporated into our amygdaloid and hippocampal complexes.
-
∙ The origin of many mental disorders can be found in the dysfunction of the amygdalo/hippocampal – habenular – upper brainstem circuitry.
-
∙ Studying the modulation of neuronal circuit functioning in primitive vertebrates may offer suitable models by which to study the mechanism of human mental disorders, and to develop fundamentally different psychotropic treatments.
Considerations
-
∙ The reciprocal functional connectivity between specific cortical areas, for example, anterior cingulate, subgenual cingulate, orbitofrontal and ventromedial prefrontal cortices, and the various components of the subcortical regulatory neuronal system, remains to be determined.
-
∙ It remains to be proven whether the connectivity of the corticoid amygdala through the extended amygdala and the hypothalamus regulates the lateral habenula, and that through the hippocampus and the septal nuclei the medial habenula is regulated.
-
∙ It remains to be proven that the connectivity between the subgenual cingulate gyrus (sCg) cortex, the nucleus accumbens shell, the ventral pallidum, the thalamus and the sCg constitutes (in part) a closed re-entry circuit.
Introduction
The human cerebrum largely consists of two massive hemispheres which are completely covered by an extensively in gyri and sulci folded, laminated, cortical layer (Reference Nieuwenhuys, Voogd and Van Huijzen1). The cortical surface is primarily so expanded by the C-shaped and outward-to-inward curving of the entire hemisphere during embryological development. As this cortical layer is less well developed in all other animals, which are believed to lack many human skills, it is obvious to most psychiatrists and psychologists that the cerebral cortex is the essential structure in the regulation of human behaviour. This observation is usually expanded to the belief that behavioural disturbances, as reflected by mental disorders such as depression or psychosis, are also primarily caused by cortical defects. Functional deficits of the frontal part of the cerebral cortex in particular are believed to play an essential role in, for example, both depression (Reference Rolls2) and schizophrenia (Reference Hulshoff Pol, Schnack and Bertens3,Reference Van Haren, Cahn, Hulshoff Pol and Kahn4). However, behaviour can, in essence, be considered to be a mechanism whereby the brain manages input in order to create a specific output which enables the organism to adapt to the ever changing circumstances within its biosphere. This capacity to compete with the ecological influences which endanger the internal processes is a characteristic of all organisms. Free living animals have far better possibilities than, for example, sea anemones (Anthozoa) to obtain food and procreative opportunities, and to escape from dangers by fleeing or hiding; this capacity results in more rapid growth and more extensive reproduction by the first types of free living animal species. To accomplish these goals, however, free-moving animals need several special skills: two of these essential behaviours are the showing of (1) appetitive-searching (reward-seeking) and (2) adversity-avoiding (misery-fleeing) activity. In order to maintain existence both as an individual and a species, the animal should obtain access to necessities including food, comfort, territory and possibilities to reproduce on the one hand, and on the other, should escape from threats, pain, discomfort and other factors which may decrease chances to stay alive and to have offspring. Being successful leads to feelings of pleasure (hedonia) or happiness (euphoria) in humans. As the first freely moving animals which were living about 560 million years ago must have had these capabilities too, their primitive brains must already have been capable of regulating these behaviours.
During the course of evolution, in the progression from being primitive animals to highly developed humans, the forebrain underwent tremendous changes, which finally resulted in the human cerebrum as it exists now. The question arises, however, whether or not the cerebral cortex also initiates and controls reward-seeking and misery-fleeing behaviour, which, if this were the case, would mean that during evolution these functions were taken over from more primitive parts of the brain. For this reason, it is of interest to study the evolutionary development of the brain in vertebrates: this cannot be accomplished by studying the fossil remains of these ancestors, but we can use the idea that during several stages of evolution the development of some species stopped. Current representatives of these animals are believed to have essentially the same appearance as the animals living several hundred million years ago, and therefore the brains of more primitive vertebrates may reflect earlier evolutionary stages of the current human brain. Hence, the brains of lampreys, sharks, lungfishes, frogs, turtles, opossums, rats and monkeys correspond to the brains of human ancestors from about 560 million years ago until recently (Reference Moreno and González5).
During the study of this evolutionary development we discovered that almost the entire endbrain of our earliest vertebrate ancestors became assimilated into the human amygdaloid and hippocampal complexes. Even the oldest part of the cerebral cortex, that is the insula of Reil, is essentially absent in these animals. Only after the branching out of mammals from the evolutionary line leading to higher reptiles and birds, does the laminated cerebral cortex begin to develop. However, a nuclear complex, which is too tiny to be studied in the human central nervous system with conventional neuroimaging techniques, the habenula, remains in control of regulating reward-seeking behaviour from the earliest vertebrates until humans as we are now. In this review article, we will summarise the evolutionary development of the forebrain (Reference Loonen and Ivanova6,Reference Loonen and Ivanova7). We will go on to describe how the amygdala, hippocampus, habenula and accumbens nucleus regulate reward-seeking and misery-fleeing behaviour, and finally, we will explain how this system might be involved in the pathogenesis of addiction (Reference Loonen, Schellekens and Ivanova8), depression (Reference Loonen and Ivanova9,Reference Loonen and Ivanova10), mania (Reference Loonen, Kupka and Ivanova11), delusions (Reference Loonen and Ivanova12) and specific stress disorders.
The brain of early vertebrates
The very first vertebrate is generally supposed to be an animal comparable with the modern lamprey. This animal has a head containing a brain and it has vertebrates, but not yet a lower jaw. The lamprey’s brain already has a primitive hemisphere, but this is small and insignificant (Fig. 1) (Reference Loonen and Ivanova6,Reference Nieuwenhuys and Nicholson13). Primitive cortical tissue is termed ‘pallium’ and the tissue aligning it, but which has a different cytostructure, is ‘subpallium’. Next to the hemisphere, the lamprey’s forebrain also includes a medial pallium, which lies more caudally and near the midline of the endbrain. In humans, motor activity is initiated and controlled by the cerebral cortex, but this does not appear to be the case in the lamprey. The subpallium of the lamprey was shown to contain an extrapyramidal system, which has a similar composition and organisation to its human subcortical equivalent, and which directs motor centres in the lower brain, thereby controlling motor behaviour (Reference Grillner, Robertson and Stephenson-Jones14–Reference Robertson, Kardamakis and Capantini16). This extrapyramidal system receives input from several other parts of the lamprey’s brain. Similar to the human system, the input part of this lamprey subpallial extrapyramidal equivalent is termed ‘striatum’ (Fig. 2), and the output area connected with the motor centres is called the pallidum. The activity of the striatum is regulated by dopaminergic neurons originating within the animal’s midbrain, which again is comparable with the human extrapyramidal system. The activity of these dopaminergic neurons, as well as that of the adrenergic and serotonergic centres within the upper brainstem, is in turn regulated by a specific nuclear complex situated caudally within the roof of the animal’s thalamus: the habenula (Figs 1 and 2). Sten Grillner’s group from the Karolinski Institute in Stockholm ascertained that the activity of this motor control system is influenced by a specific nucleus termed the ‘habenula-projecting globus pallidum’ (GPh; Fig. 2) (Reference Stephenson-Jones, Kardamakis, Robertson and Grillner17): it plays a critical role in enabling the basal ganglia to select actions and to evaluate their outcome. The GPh receives input from pallium and striatum, and controls the activity of the extrapyramidal system by affecting the activity of the midbrain dopaminergic neurons. It inhibits the animal’s behaviour when the motor activity is not rewarding, as, for example, when no food is obtained.
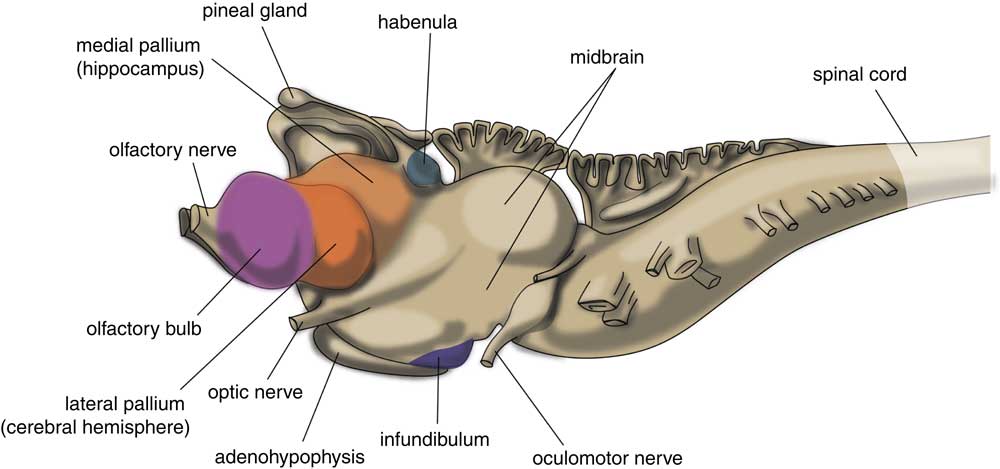
Fig. 1 Central nervous system of lamprey. Adapted from (Reference Nieuwenhuys and Nicholson13).
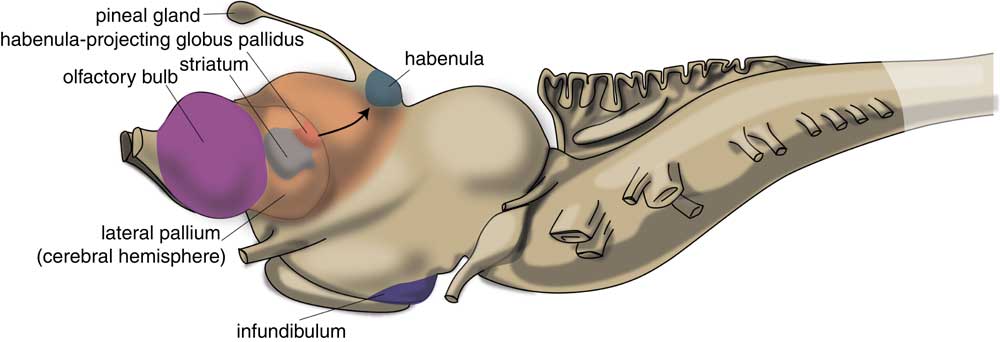
Fig. 2 Position of striatum and habenula-projecting globus pallidus of lamprey. Adapted from (Reference Stephenson-Jones, Kardamakis, Robertson and Grillner17).
Later in this article we will describe that this system, including the GPh, habenula and midbrain, is extremely well conserved during the evolutionary development from these earliest vertebrates up to modern humans. However, first, we should describe what happened with the early vertebrate’s telencephalon (endbrain).
The first land animals
The very first free-moving animals were living in water; it was after the evolution of lungfish (also known as salamanderfish) that the invasion of the continents started. Lungfish are freshwater fish which combine the ability to breathe air alongside the presence of lobed fins and a well-developed internal skeleton. A later evolutionary stage of development is represented by animals with a brain comparable with that of frogs and toads (Fig. 3) (Reference Ten Donkelaar18). An important change was shown to have taken place within the subpallium of these amphibians in comparison with lampreys, because it was divided into two parts (Reference Moreno and González19). The subpallium of the lamprey was maintained as part of the amygdala, and a new striatopallidum had developed, which gave rise to the human extrapyramidal system. However, the major part of the amphibian-like pallium does not at this point of evolution have the same function as the human cerebral cortex, so the corticostriatal equivalent of the human extrapyramidal system is still obscure (Reference Loonen and Ivanova7). The pallium can better considered as an ancestor to the corticoid parts of the amygdala and the hippocampus. The human amygdaloid complex is a heterogeneous group of 13 nuclei and cortical areas located in the medial side of the temporal lobe just rostral to the hippocampal formation (Reference Benarroch20,Reference Freese and Amaral21). The complex can be neuroanatomically divided into ‘deep nuclei’ and ‘superficial nuclei’, which have glutamate-using cortex-like cell types and ‘remaining’ or ‘extended amygdalar nuclei’, which contain γ-aminobutyric acid (GABA)-using neurons, like the striatum and pallidum. This is comparable with the lamprey’s pallium and subpallium. The amygdala has an important role in initiating the emotional response. The hippocampal complex is also a cortical structure localised more caudally within the medial edge of the temporal lobe. The hippocampus has an important role in episodic memory and contextual orientation.
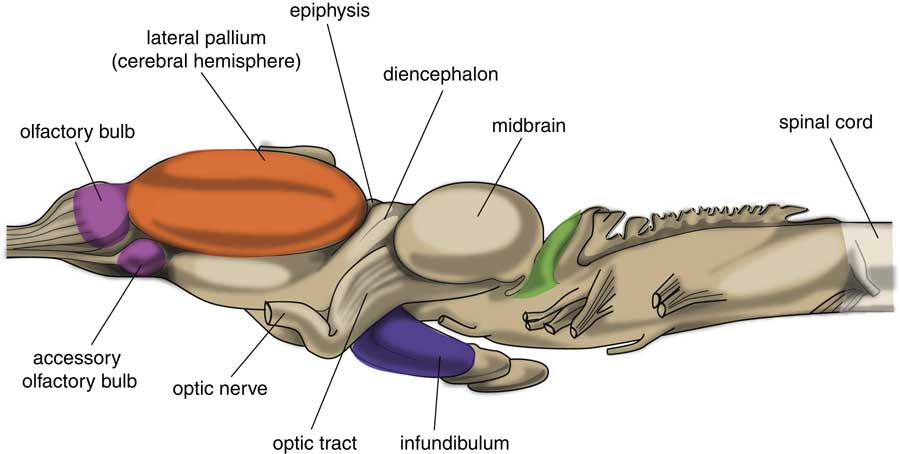
Fig. 3 Central nervous system of frog. Adapted from (Reference Ten Donkelaar18).
In the human brain the centromedial nuclei of the amygdala, the connecting extended amygdala, the bed nucleus of the stria terminalis (BST), the ventral striatum and the dorsal striatum are well within one line with each other as a series of nuclear structures (Fig. 4) (Reference Loonen22). However, this was apparently not already true in our amphibian-like ancestors. Within the anuran forebrain, the striatum (anterior) is continuous with the central and medial amygdala (posterior), and clearly separated from the dorsal/ventral pallidum and the BST and septum (Reference Moreno and González19). An important discovery during the study of the embryological development of anuran basal ganglia was the finding that the BST and part of the septum are also of pallidal instead of striatal origin (Reference González, Morona, Moreno, Bandín and López23,Reference Moreno, Morona and López24). This is interesting because the BST is a suitable structure to execute the limbic component of lamprey GPh. We will come back to this later.
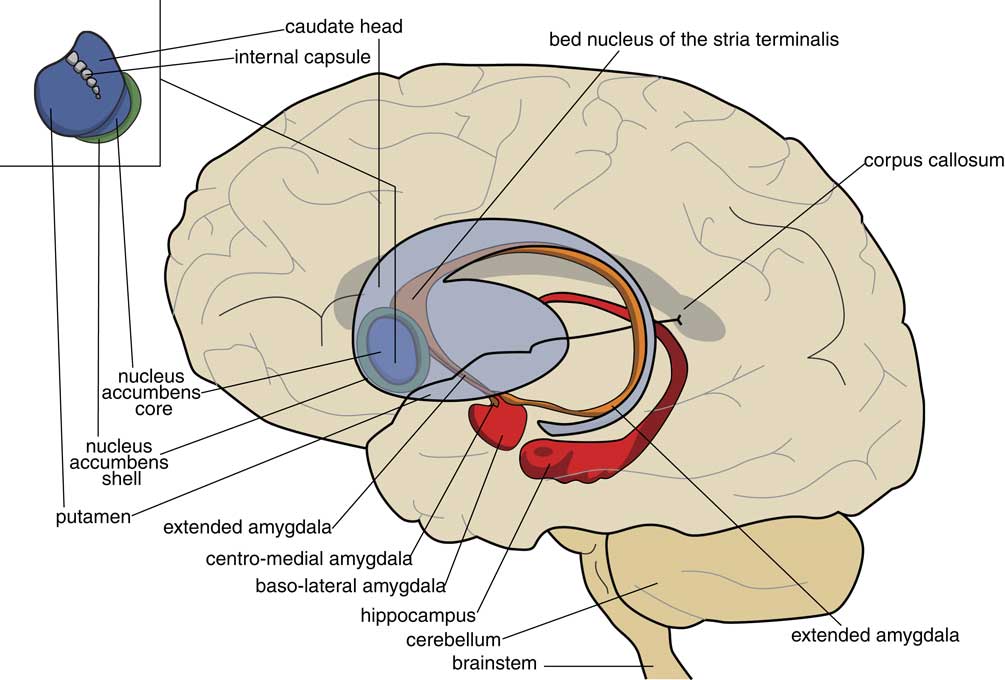
Fig. 4 Position of the limbic basal ganglia (extended amygdala and nucleus accumbens shell) relative to the extrapyramidal striatum (caudate nucleus, putamen, nucleus accumbens core) and hippocampus.
An important question arises whether the dorsal pallium of our amphibian-like ancestors may have given rise to the human neocortex, as is often assumed. According to the ‘Von Baerian’ theory, embryos of later-descendent species resemble the embryos of earlier-descendant species to the point of their divergence. In human embryos, the future insula is the first cortical structure to develop (Reference O’Rahilly and Müller25,Reference Kalani, Kalani, Gwinn, Keogh and Tse26). The primordial insula is initially located on the free dorsolateral surface of the primitive cerebral hemisphere and adjoining the cortical amygdaloid regions on one side, and the olfactory cortical regions at the other (Reference O’Rahilly and Müller25,Reference Nieuwenhuys27). This is comparable with the position of the dorsal pallium in lamprey or amphibian hemispheres. Moreover, specific neurons of lamprey dorsal pallium regulate the activity of certain brainstem motor centres (Reference Ocaña, Suryanarayana and Saitoh28). The described brainstem structures may correspond to brainstem structures for smooth pursuit eye movements and the pedunculopontine nucleus (Reference Loonen and Ivanova7). Both structures are connected with the cerebral neocortex in primates. The most likely explanation for the findings of Ocaña et al. (Reference Ocaña, Suryanarayana and Saitoh28) would be that the part of the lamprey dorsal pallium develops into the frontal motor cortex in primates (Reference Loonen and Ivanova7). Nevertheless, considering the connectivity of the dorsal thalamus and dorsal pallium of amphibians makes it evident that the roof of the amphibian hemisphere has not yet achieved its human input processing and output generating role (Reference Roth, Grunwald and Dicke29–Reference Laberge, Mühlenbrock-Lenter, Dicke and Roth31), but is still part of a more extensive ‘limbic’ behavioural control system including almost all pallial and subpallial regions. From the least developed vertebrates up until, and also including our ancestors with an amphibian-like brain, the dorsal pallium can be considered to be an extension of the medial pallium, which will give rise to the later hippocampus. However, a small part of this roof of the amphibian hemisphere probably gives rise to the insula and other parts of the human neocortex. This matches with the idea that the insular cortex in particular is involved in sensing emotional feelings such as anger, fear, lust, disgust and distress (Reference Loonen, Schellekens and Ivanova8,Reference Nieuwenhuys27,Reference Nagai, Kishi and Kato32,Reference Craig33), and which would therefore find its evolutionary basis in this origin of the insular cortex in the anterior edge of the dorsal pallium.
From amphibian-like animals to mammals
Primitive reptiles with a turtle-like brain precede more developed reptiles and birds on the one hand, and mammals on the other. Reptiles and birds do not have a cerebral neocortex, but instead have developed a unique structure called the dorsal ventricular ridge (Reference Medina and Abellán34). In primitive mammals, both a prominent dorsal thalamus and the cerebral neocortex have occurred. The complete (or extensive) thalamus consists of four parts, the hypothalamus, subthalamus, (dorsal) thalamus and the epithalamus. The subthalamus is part of the extrapyramidal system (Fig. 5) and will not be considered here in further detail. Up to turtle-like animals at least, the hypothalamus together with some brainstem motor centres completely controls the behavioural output of the animal. The epithalamus consists of the stria medullaris, the habenula and pineal gland in all vertebrates; it plays an important role in regulating the intensity of behaviour. The (dorsal) thalamus is a principle relay centre which conveys all sensory input (apart from smell) to the human cerebral cortex and also connects different parts of the latter structure with each other. In our mammalian ancestors, the cerebral neocortex developed into its current human form: this resulted in C-shaped and inward-to-outward curving of the two hemispheres with the displacement of the amygdala and hippocampus to the medial inside of the temporal lobe (Fig. 4). Parallel to the expansion of the cerebral cortex, a subcortical extrapyramidal circuit evolved in a modular fashion (Reference Grillner, Robertson and Stephenson-Jones14,Reference Robertson, Kardamakis and Capantini16). Every time a new function was acquired by a more developed ancestor of humans due to the expansion of the neocortex, a corresponding extrapyramidal circuit was created to support the execution of this function. This resulted in a modular expansion of the extrapyramidal system.
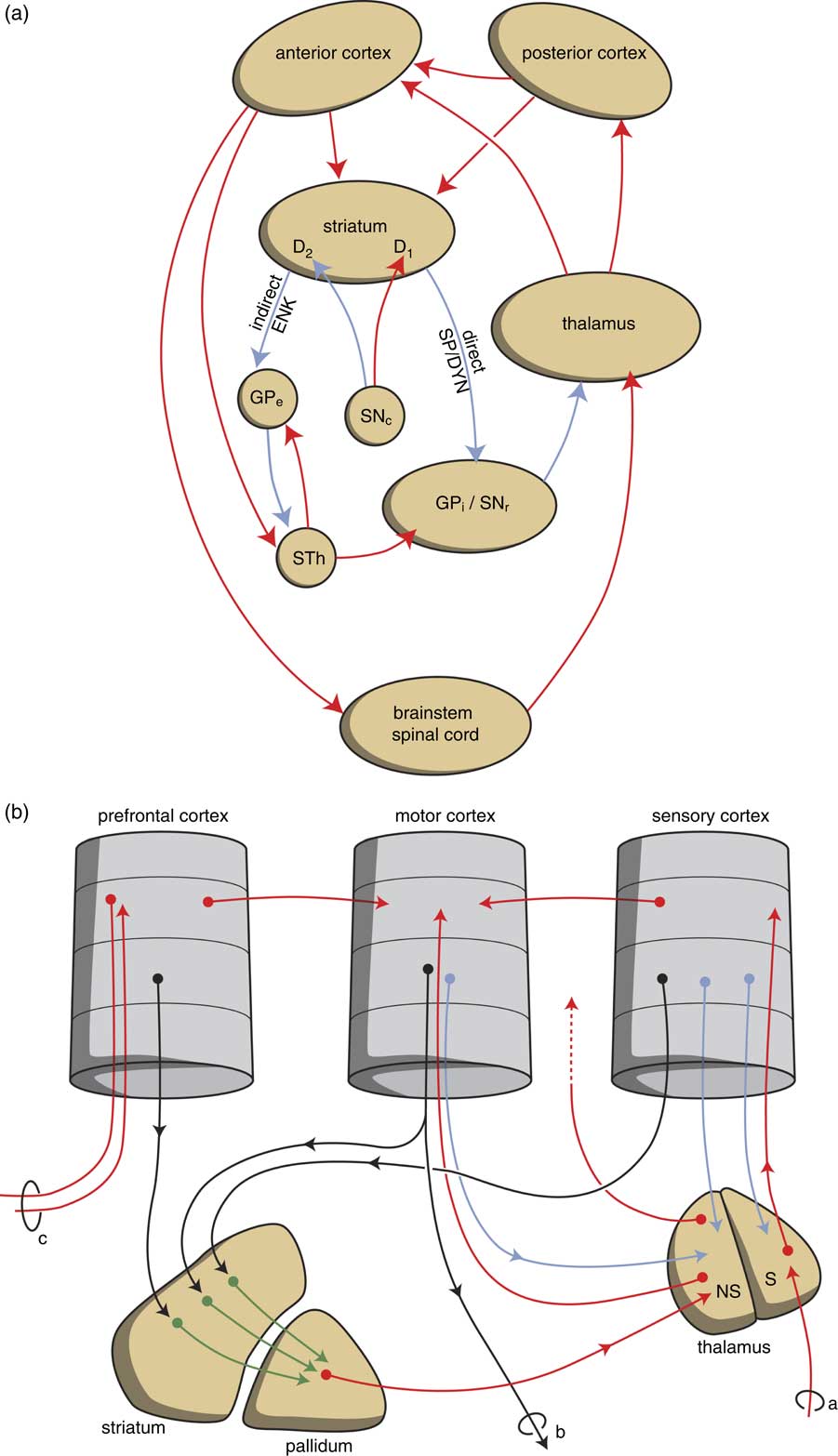
Fig. 5 Scheme representing the organisation of human extrapyramidal system. (a) Direct and indirect pathways lead to the activation (red) or inhibition (blue) of the anterior cortical endpoint. (b) Convergent pathways via the basal ganglia correct the serially connected intracortical connections. a, sensory input; b, motor output; c, to contralateral cortex; D1, medium spiny neurons carrying dopamine D1 receptors; D2, medium spiny neurons carrying dopamine D2 receptors; DYN, dynorphin; ENK, enkephalin; GPe, globus pallidus externa; GPi, globus pallidus interna; NS=non-specific part of the thalamus; red, black, green and blue arrows, neurochemically undetermined; S=specific part of the thalamus; SNc, substantia nigra pars compacta; SP, substance P; STh, subthalamic nucleus.
New cerebral capacities of mammals
In the jawed vertebrates succeeding our amphibian-like ancestors, the input to the dorsal thalamus was largely increased, and this in turn lead to a significant expansion of the dorsal pallium (Reference Butler35,Reference Butler36). However, this expansion occurred along different lines in non-synapsid (turtles, reptiles, birds) and synapsid (mammals) animals (Reference Butler36,Reference Montiel, Vasistha, Garcia-Moreno and Molnár37). The dorsal thalamus consists of two divisions called lemnothalamus and collothalamus. In non-synapsid animals, only the collothalamus with the corresponding lateral dorsal pallial field is largely expanded, whereas in the synapsid line leading to mammals both the collothalamus with lateral dorsal pallial fields and the lemnothalamus with the corresponding medial dorsal pallial fields developed (Reference Butler36). The medial part of the lemnothalamic division forms the subicular, cingulate, prefrontal, sensorimotor and related cortices in mammals. The lateral part forms the striate (visual) cortex. Specific fields within the collothalamic lateral division of the dorsal pallium form the extrastriate visual, auditory, secondary somatosensory and related cortices in mammals (Reference Butler36). The expansion just described probably resulted in a total displacement of ventral and medial pallial fields: the medial pallium became the hippocampus, and the ventral pallium became the most caudal edge of the frontal lobe (including olfactory tubercle) and cortical regions of the amygdaloid complex in the temporal lobe. The rest of the dorsolateral pallium (the primitive hemisphere) partly expanded and probably largely moved with the ventral and medial pallium, so it is safe to state that almost the entire forebrain of our amphibian-like ancestor probably evolved and continued in more modern animals as the amygdaloid and hippocampal complexes. Even the limbic areas such as the orbitofrontal cortex and the cingulate gyrus are of more recent, that is, mammalian, origin.
In amphibians, the start of an extrapyramidal system can be accepted, as reflected by the presence of a striatum and pallidum separate from the primitive extended amygdala (Reference Moreno and González19). However, the cerebral neocortex has not yet developed, the amphibian striatum is therefore probably comparable with the ventral, limbic part of the human extrapyramidal system to which the more dorsal circuits were added in later mammals. ‘Added’ is perhaps not an entirely correct term: it is probable that separate parts of the dorsal extrapyramidal circuits developed from the edges of the amphibian-like primitive striatum, whereas the remainder developed into the shell and core of the accumbens nucleus which receive input from the subgenual and anterior cingulate gyrus, respectively, next to the orbitofrontal cortex (Reference Dalley, Mar, Economidou and Robbins38,Reference Loonen and Stahl39).
The extrapyramidal circuitry serves to correct the intensity of the intracortical stimulation of the output generating frontal cortex. Within the posterior parts of the cerebral cortex incoming information is processed in a stepwise fashion and conveyed to anterior parts of the neocortex in order to activate it to produce output. In parallel, every part of the cerebral cortex also gives topographically arranged signals to the striatum as the start point of an extrapyramidal circuit (Reference Heimer40). These extrapyramidal circuits converge to the same cortical output units as are addressed by the corresponding intracortical connections, and are capable of correcting the intensity of their activation when necessary (Fig. 5) (Reference Loonen and Ivanova41). All connections have the capacity to memorise the intensity of the stimulation, and this complex reaction pattern results in a skilled response repertoire after proper training. This is most evident in complex motor movements, but the same is also true for other behavioural response patterns. The two sets of extrapyramidal circuits including the nucleus accumbens deserve special attention: the extrapyramidal circuits, including the core part of this nucleus, regulate motivation to show reward-seeking behaviour, and those including the shell part of the nucleus to express misery-fleeing behaviour (Reference Loonen and Ivanova7).
The role of the habenula
In the earliest vertebrates with a lamprey-like brain a prominent regulatory position is taken by the habenula. This nuclear complex and its output connectivity with the midbrain has been very well conserved during evolution. So, similar to the situation in lamprey, the habenula also modifies the activity of several regulatory centres in the upper part of the brainstem in primates (Reference Hikosaka42). The habenular complex consists of a larger lateral (LHb) and a smaller medial (MHb) division, each of which consist of a complex set of subdivisions. In humans, the habenular nuclei are very small (about 20–30 mm3 in each hemisphere), and cannot be studied with classical neuroimaging techniques (Reference Batalla, Homberg and Lipina43). The stria medullaris is the main habenular input and the fasciculus retroflexus the primary output structure of the habenula (Reference Benarroch44–Reference Sutherland47). The fasciculus retroflexus is divided into two regions: the outer region originates in the LHb and projects mainly to the rostromedial tegmental nucleus (RMTg) next to numerous monoaminergic nuclei in the midbrain and hindbrain (Reference Bianco and Wilson45). The RMTg, which is also named the ‘tail’ of the ventral tegmental area, is a small nucleus that contains mainly inhibitory GABAergic cells and thereby regulates activity of the ventral tegmental area/substantia nigra pars compacta and the dorsal raphe nucleus (Reference Benarroch44). LHb neurons also directly target the dopaminergic ventral tegmental area (Reference Lammel, Lim and Ran48) and substantia nigra pars compacta themselves, as well as the serotonergic median and dorsal raphe nucleus, cholinergic laterodorsal tegmentum and noradrenergic locus coeruleus (Reference Herkenham and Nauta49). The inner area of the fasciculus retroflexus originates in the MHb, and projects to the interpeduncular nucleus (Reference Benarroch44–Reference Sutherland47). The MHb contains both cholinergic neurons (in its ventral two-thirds) and dorsally located substance P-containing neurons, and is also the main source of the input of the interpeduncular nucleus (Reference Bianco and Wilson45,Reference Klemm46,Reference Morley50). The interpeduncular nucleus is a singular, unpaired structure located at the ventral midline of the midbrain (Reference Klemm46,Reference Morley50). The major efferent pathways originating in the interpeduncular nucleus project to the dorsal tegmental nucleus (Reference Morley50), the ventral tegmental area (Reference Klemm46) and the raphe nuclei (Reference Bianco and Wilson45,Reference Klemm46). The interpeduncular nucleus is well known for its widespread projections, both ascending and descending (Reference Klemm46,Reference Morley50). Hence, the habenular nuclei affect the monoaminergic centres (using dopamine, norepinephrine, serotonin), which regulate the intensity of reward-seeking and misery-fleeing behaviours. Animal experiments have shown that the lateral habenula is involved in regulating reward-seeking and the medial habenula in misery-fleeing behaviour by affecting the activity of brainstem regulatory centres (Reference Loonen and Ivanova7).
At the input side of the habenula, one specific structure deserves special attention: in lamprey, the activity of the LHb is regulated by the GPh. We have hypothesised that the same may be true in humans (Reference Loonen and Ivanova6,Reference Loonen and Ivanova7). Within the extrapyramidal circuits, the human homologue of GPh may be localised within the border region of the globus pallidus (GPb) (Reference Stephenson-Jones, Kardamakis, Robertson and Grillner17) and the ventral pallidum (Reference Hong and Hikosaka51). Within the limbic system, the GPh is probably represented by a group of neurons within the anteromedial division of the habenula-projecting bed nucleus of the stria terminalis (BSTh), which project heavily to the medial and caudal regions of the lateral habenula (Reference Dong and Swanson52,Reference Dong and Swanson53). These three human representatives of the GPh may take an essential position in mediating the critical role of the limbic and extrapyramidal basal ganglia for selecting actions and evaluating their outcome.
Amygdala and hippocampus
When considering the development and functioning of the endbrain of our earliest vertebrate ancestors, it becomes evident that in humans, next to an extrapyramidal system, a similar limbic cortical–subcortical–cortical circuit may also exist. Such a circuit starting and ending within the corticoid amygdala and including the output of the BST to the thalamus has actually been identified (Reference Dong, Petrovich and Swanson54,Reference Swanson55). Moreover, during a later stage of evolution a second circuit has also developed, including the output of the hypothalamus and including the mesial frontal neocortex, and which is also connected with the corticoid amygdala (Fig. 6). The BST is the pallidal part of the extended amygdala, which receives input from the centromedial (i.e. striatal) part and gives output to the thalamus. The centromedial amygdala receives input from the corticoid amygdala, which is essentially the same as the organisation within the extrapyramidal circuit with cortical–striatal–pallidal–thalamic–cortical connectivity (Fig. 5) (Reference Loonen and Ivanova41). The centromedial amygdala and BST also gives output to the hypothalamus and brainstem motor centres (Reference Loonen and Ivanova7). This is a direct result of how reward-seeking and misery-fleeing behaviour was regulated by the brain of our earliest vertebrate ancestors, and is reflected by the regulation of the emotional response in humans (Reference Loonen, Schellekens and Ivanova8,Reference Loonen and Ivanova9,Reference Loonen22,Reference Bruinsma and Loonen56). The corticoid part of the amygdala receives massive sensory input (Fig. 7) (Reference Freese and Amaral21,Reference Pitkänen57) and selects the information which is most relevant for current well-being: this selection process is termed ‘salience’. In interaction with contextual (memorised) details supplied by the hippocampus, it activates hypothalamic and brainstem centres to produce a relevant emotional response (like fear, anger, love, appetite, sexual desire or power dominance) (Reference Sewards and Sewards58). From the hypothalamus connectivity also exists between the thalamus and mesial frontal part of the neocortex. This is probably the mechanism which affects the motor output of higher vertebrates, including humans, by inducing the drive to seek food, warmth, comfort, etc., or to escape from pain, thirst, misery, etc. (Reference Sewards and Sewards58). This results finally in a limbic cortical–subcortical circuit that is more complex, but nevertheless essentially similar, to the well-known extrapyramidal system, provided that one realises that the cerebral neocortex was included within the circuit on a later evolutionary moment (Fig. 6).
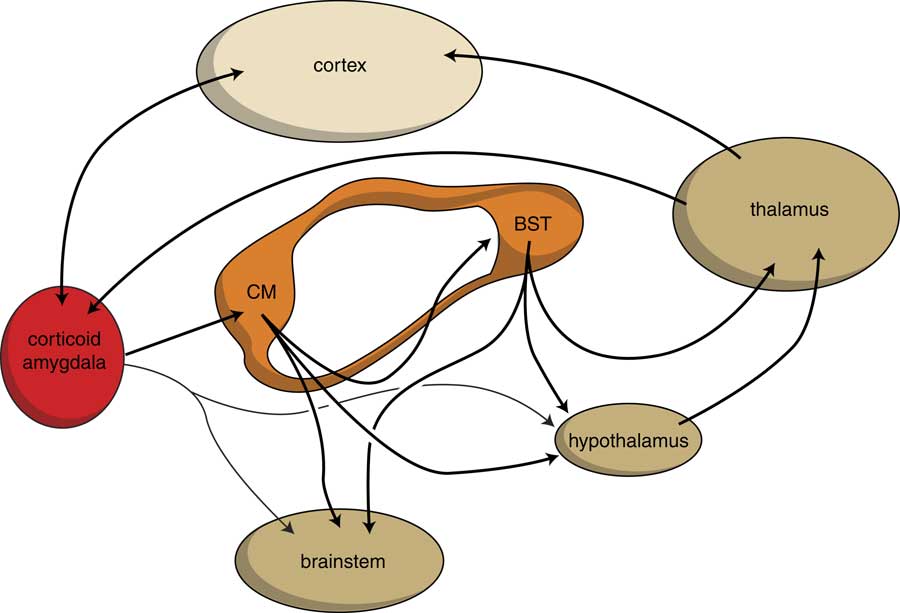
Fig. 6 Limbic cortical–subcortical regulatory circuit. BST, bed nucleus of the stria terminalis; CM, centromedial amygdala; dark yellow, diencephalon and brainstem; light yellow, neocortex; orange, extended amygdala; red, corticoid amygdala.
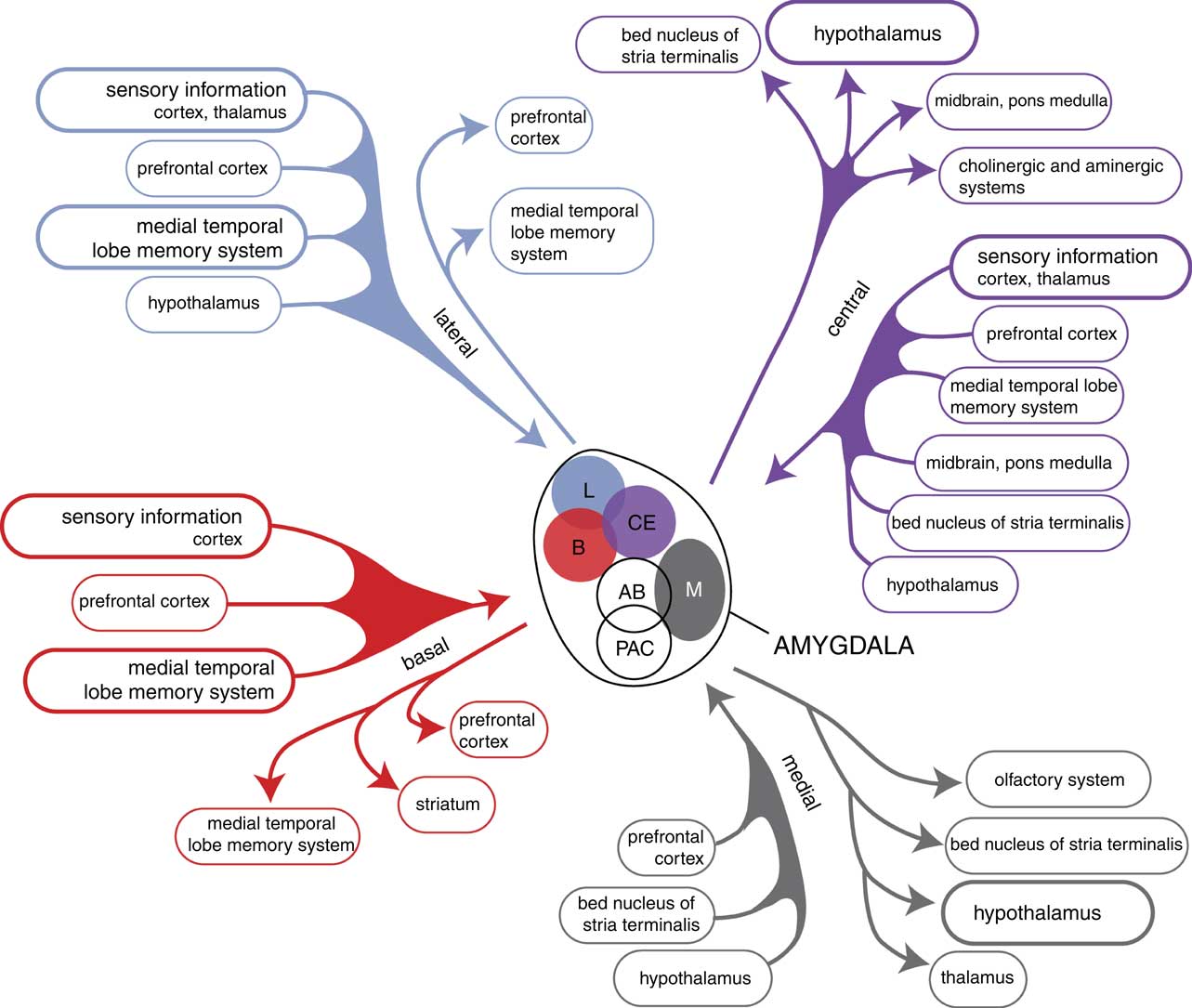
Fig. 7 Overview of the connectivity of the rat amygdaloid complex. Adapted from (Reference Pitkänen57) with permission of the author.
The amygdaloid complex is also heavily connected with the habenula. As we have already described, the corticoid amygdala gives output to the centromedial nucleus, which is in turn connected with the BST (Fig. 8). We have suggested that the habenula-projecting nerve cells within the anteromedial division of the BSTh have the same character as the lamprey GPh (glutamatergic) neurons (Reference Loonen and Ivanova7). Moreover, information from the amygdaloid complex may reach the lateral habenula via its connectivity with the hypothalamus (Reference Benarroch44). The deep corticoid amygdalar areas are reciprocally connected with the hippocampal complex, but this last structure can also be considered to represent an output channel of the amygdala (Reference Loonen and Ivanova7). Via the fornix, the hippocampus sends a GABAergic connection to the medial septum and a glutamatergic connection to the lateral septum (Reference Nieuwenhuys, Voogd and Van Huijzen1,Reference Khakpai, Nasehi, Haeri-Rohani, Eidi and Zarrindast59). The medial septum is a primary source of input to the medial habenula (Reference Benarroch44,Reference Klemm46). Moreover, the medial habenula has direct output to the lateral habenula and may regulate the latter’s activity (Reference Loonen and Ivanova6). It should be realised that the lateral habenula can be considered as an anti-reward nucleus (Reference Batalla, Homberg and Lipina43). Via the GABAergic RMTg this nucleus tonically inhibits the ventral tegmental area, which in turn affects the activity of accumbens nucleus within the forebrain. The core and shell parts of the nucleus accumbens are included in two extrapyramidal re-entry circuits starting and ending within the anterior cingulate cortex (BA24) and subgenual cingulate cortex (BA25), respectively (Fig. 9) (Reference Dalley, Mar, Economidou and Robbins38,Reference Loonen and Stahl39). They increase the intensity of (motivation to) reward-seeking and misery-fleeing behaviour and are both stimulated by dopaminergic terminals. However, the shell part is also stimulated by β-adrenergic receptors of adrenergic terminals from the locus coeruleus complex (Reference Loonen and Ivanova10,Reference Loonen and Stahl39).
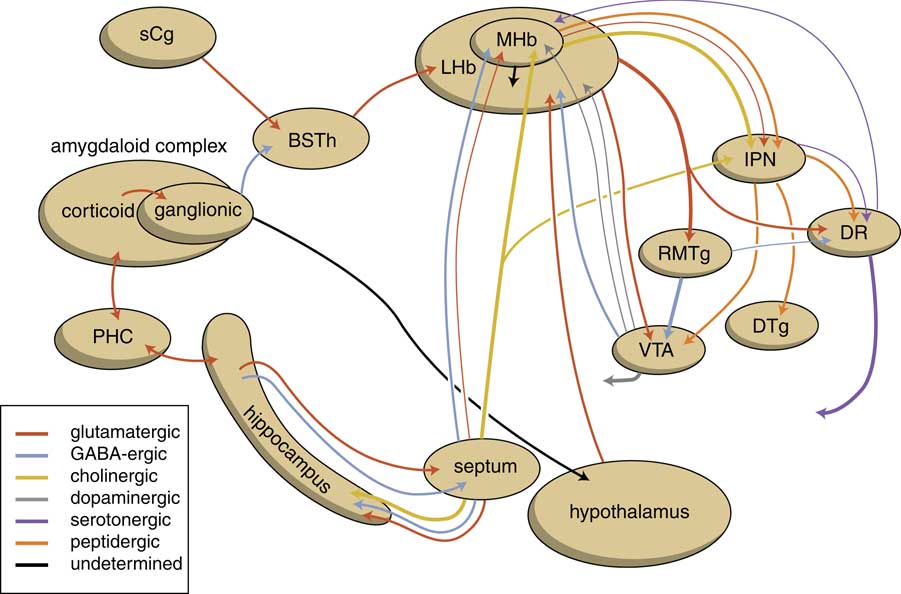
Fig. 8 Scheme showing the connectivity of the amygdalo–hippocampal system to the midbrain through the habenular complex. BSTh, habenula-projecting part of the bed nucleus of the stria terminalis; DR, dorsal raphe nucleus; DTg, dorsal tegmental nucleus; IPN, interpeduncular nucleus; LHb, lateral habenula; MHb, medial habenula; PHC, parahippocampal cortex; RMTg, rostromedial tegmental nucleus; sCg, subgenual cingulate gyrus; VTA, ventral tegmental area.
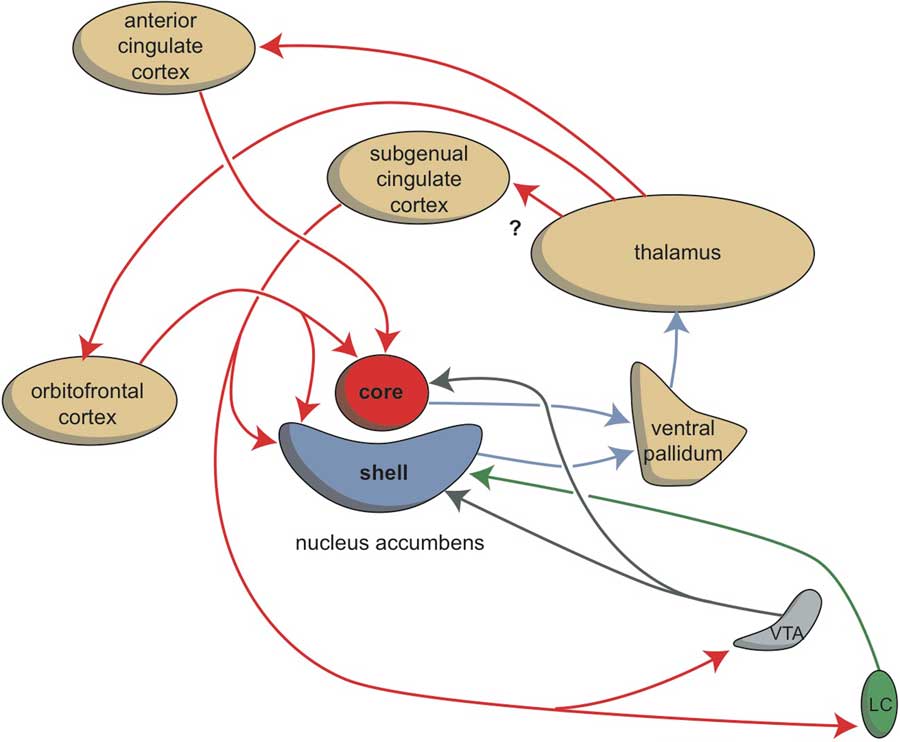
Fig. 9 Stimulation of the core and shell of the nucleus accumbens. Adapted from (Reference Dalley, Mar, Economidou and Robbins38) with permission of the author. VTA, ventral tegmental area; LC, locus coeruleus; red arrows, glutamatergic; blue arrows, GABAergic; grey arrows, dopaminergic; green arrow, adrenergic.
Secondary role for the cerebral cortex
The cerebral cortex inevitably also plays an important role in regulating these emotional responses. However, in our opinion, it seems unlikely that the cerebral cortex has entirely taken over the primary regulatory role of the subcortex. Nevertheless, the voluminous cerebral cortex with its highly developed recurrent collateral connections and extensive interactions with the amygdalar–hippocampal system is highly suited in its ability to compute what challenges the complex input actually represent, what rules appear to be involved, what reward or distress may be connected to it, what type of response would be most beneficial and how this response could best be executed. The learning capability of the intracortical and extrapyramidal connections – these connections are capable of increasing (by long-term potentiation) or decreasing (by long-term depression) their sensitivity to stimulation – ensure that the individual can acquire the necessary skills by playing, trying, training and studying. The first step of the initiation of an intuitive emotional response and its dominance over other response types may be determined by subcortical structures: this subcortical signal may activate a complex behavioural response executed by specific cerebral cortical mechanisms. In addition, the frontal cortex can strongly modify the response type initiated by the amygdala, by affecting the corticoid part of this complex but also of more distant parts of the amygdala–habenula–brainstem connectivity. In modulating the output of the amygdala, a specific role is attributed to the ventromedial areas of the prefrontal cortex (Reference Kim, Loucks and Palmer60,Reference Roy, Shohamy and Wager61). The subgenual cingulate cortex (BA25) is inter alia connected to the medial preoptic area, the BST, the diagonal band of Broca and the lateral septum (Reference Hamani, Mayberg, Stone, Laxton, Haber and Lozano62). Thereafter, projections from BA25 run caudally through the substantia innominata to innervate the amygdala and parts of the hypothalamus (Reference Hamani, Mayberg, Stone, Laxton, Haber and Lozano62). In addition, the lateral habenula in particular receives input from the neocortex. Primary sources of cortical inputs are the anterior insula, the midcingulate (dorsal anterior cingulate) cortex, the pregenual anterior cingulate cortex, and the ventral frontopolar cortex (Reference Benarroch44). Hence, mutual interactions appear to exist between the cerebral cortex and the subcortical regulatory system. The majority of these interactions and their putative significance have yet to be elucidated.
The opposite is undoubtedly also true; subcortical structures are connected with probably all areas of the cerebral cortex and certainly affect its functioning. Hence, subcortical structures regulating the emotional response may also determine – for example – the efficacy of processing sensory information, the capacity to identify and memorise this information, the capacity to select relevant cues (vigilance) and the adequacy of the working memory. This may be related to the many cognitive symptoms of mood and anxiety disorders.
An essential quality of the human cerebral cortex also deserves attention: within the cerebral cortex every input and output can be replaced by specific verbal or written abstract language symbols. This results in consciousness, imagination and the capacity of reasoning. These capacities allow the human individual to reflect on their current and future situation, which might lead to depressive or anxious symptoms and to construct specific beliefs explaining their bizarre feelings forming the background of delusional thinking. Consciousness also allows us to become aware of emotions, which in our opinion may be a function of one of the most ancient parts of the cerebral cortex, that is the insula of Reil (Reference Loonen, Schellekens and Ivanova8). Whether our primitive ancestors (up to mammals) could also sense emotions is unclear; we can also not be certain that they could sense vital emotions like pain, hunger and thirst. However, if these animals could be aware of anything, sensing vital emotions is probably the only thing they could actually have awareness of, because they still lacked a cerebral cortex for a sufficiently detailed processing of the input. Therefore, feeling pleasure and happiness as a positive reinforcer for behaviour can be as ancient as the existence of freely moving animals. This does also not exclude the existence of forerunners of mental disorders involving a disturbance of these emotions.
Consequences of this theory for the pathogenesis of mental disorders
In a series of lectures and articles, we have applied the above framework for describing the regulation of emotional behaviour in order to explain how dysregulation within the amygdalo–hippocampal–habenular connectivity can result in the symptoms of specific mental disorders. As a starting point, we have to state that we do not believe in the categorical disease model which is applied in the common disease classification systems such as the Diagnostic and Statistical Manual of Mental Disorders. In our opinion, combinations of neurobiological dysfunctions result in common mental disorders, and these should better not be considered to represent disease entities. Irrespective of such a viewpoint, specific (epi)genetic, developmental and environmental factors can contribute to certain mental disorders, and not to others, but looking for a specific and unique causal mechanism will probably remain fruitless. A second point is that modern man is living in circumstances which are essentially unsuitable and unhealthy. Our life expectancy at birth has more than doubled in two centuries and has more than tripled since the period up until and including the Middle Ages (Reference Loonen and Ivanova10). We are physically equipped to contemplate daily life challenges which are never encountered anymore: we hardly move, we feed ourselves unhealthy, use too much alcohol, tobacco, coffee and other illicit drugs, and our socio-sexual relationships are at best unnatural. Mental disorders are mainly maladaptation behavioural disorders, probably more often due to overshooting than to insufficient adaptation. This leads to the suggestion that neuroplastic changes (due to neuronal, endocrinal or immunological processes) lead to behavioural dysfunctions which are essential factors in common mental disorders. This is more or less obvious when the maladaptation results in an exaggerated and/or long-lasting stress response. Continuous overactivity of the ventral extrapyramidal re-entry circuit starting from and ending within the subgenual cingulate cortex (BA25) and including the shell part of the nucleus accumbens may lead to a depressive state. Continuous reactivation of a circuit, including the lateral habenula-projecting neurons of the GPb, may lead to obsessions and compulsions. Inappropriate activation of the maternal separation reaction leads to experiencing panic attacks. Adverse interactivity between the corticoid amygdala and the hippocampal complex may lead to the symptoms of post-traumatic stress reactions. Altered interactivity between the separate component of the neuronal framework due to maladaptive neuroplastic changes may result from neuronal (midbrain monoaminergic centres), endocrine (hypothalamus–pituitary gland–adrenal gland axis) and immunological (cerebral cytokines) influences that are primarily aimed to increase the chances of survival.
As has been stated above, the amygdala plays an essential role in selecting that sensory information that is vital to safeguard well-being and even life within the individual’s biotope. This process of attentive salience may be aberrant in psychotic disorders, leading to exaggeration of the importance of certain sensory input and form the biological basis of delusions (Reference Kapur63,Reference Howes and Kapur64). The delusional belief itself is then believed to result from the cognitive (i.e. cortical) process trying to explain these weird experiences. It has been suggested that due to a dysregulated, hyperdopaminergic state, environmental events and internal representations become associated with important elements of one’s experiences and induce the creation of this cognitive construct (the delusion). In our opinion it is also possible, however, that the hyperdopaminergic state results from a dysfunction somewhere else within the neocortical–amygdalar–habenular–mesencephalic chain (Reference Loonen and Ivanova12). The aberrant salience would then correspond to exaggeration of a normal function of this regulatory system; this would match the idea that psychotic disorders also result from maladaptation due to overstressing the system. That the hyperdopaminergic state results in psychomotor agitation (ventral striatum) and aberrant sensations/observations (parahippocampal gyrus) would then also become within the line of the whole behavioural pattern.
Two mental conditions can in particular be attributed to a dysfunction within the habenular complex: mania and addiction. Activation of the hankering for a drug induced by relapse after a period of abstinence closely corresponds to the role of the GPh in food-obtaining behaviour in the lamprey (Reference Loonen, Schellekens and Ivanova8). Moreover, the habenula may mediate the transition of pleasure to misery as an essential component of the development of addiction (Reference Batalla, Homberg and Lipina43). In bipolar disorder, affective episodes may be induced by similar neuroplastic changes causing unipolar depression. An extra biological factor, however, may cause the lateral habenula to not invariably decrease reward-seeking behaviour as occurs in depressive disorder. In bipolar disorder the activity of the reward-seeking system varies, which can result in anhedonic depression, agitated depression, mixed depressive state, mixed manic state, dysphoric mania and euphoric mania (Reference Loonen, Kupka and Ivanova11).
Conclusion
Studying the evolutionary development of the forebrain in vertebrates induces new ideas about the mechanism of the human behavioural response and the putative background of mental disorders. We want to share these insights with the readers of this journal. The starting point, and a great source of inspiration for us, was gaining knowledge of the work of Sten Grillner’s group. In our opinion, research in biological psychiatry has been dominated by neuroimaging techniques that are not very suitable for investigating the interactions between some of the most relevant neuronal circuitries. We hope to have contributed to the induction of an important change in the most significant targets for research in this area. Application of this knowledge may lead to the development of new models for the mechanism of human mental disorders, and new strategies for their treatment by studying the functioning of neuronal systems within the brains of primitive vertebrates.
Acknowledgements
The authors are grateful to the educational committee (chair: Gregers Wegener) of the Collegium Internationale NeuroPsychopharmacologicum which enabled them to work out these ideas in a series of educational courses chaired by Brian Leonard in Siberia, Russian Federation, between 2013 and 2017.












