INTRODUCTION
In 2014, the World Health Organization (WHO) reported nearly 9·6 million new cases of active tuberculosis (TB) and 1·5 million TB-related deaths [1]. Numerous national TB control programmes (NTPs) have scaled up the WHO recommended Directly Observed Therapy Short Course (DOTS) strategy, although current declines in TB incidence rates are considered too gradual to meet global WHO TB elimination targets by 2050 [Reference Lonnroth and Raviglione2–Reference Dye, Floyd and Jamison5]. Recent evidence suggests that active case-finding (ACF) of TB cases in the community may substantially improve TB case detection and contribute to overall reductions in TB prevalence and incidence in high-burden, low-resource settings more rapidly. In some instances, ACF has been attributed to TB prevalence reductions of 30–50% annually [Reference Dye, Floyd and Jamison5–Reference Corbett7].
In 2012, the WHO outlined new household-contact tracing recommendations for low- and middle-income countries (LMICs), including the systematic evaluation of all household contacts exposed to smear-positive TB cases, the diagnosis of active TB among them and the provision of appropriate treatment to these secondary cases. Despite the WHO-led recommendations for evaluating household contacts, in most high TB burden countries systematic active case detection of household contacts is rarely undertaken routinely, and is reserved primarily for children aged <5 years, HIV co-infected contacts, and contacts of known drug-resistant TB cases [8]. The systematic and routine screening of household contact tracing undertaken by routine TB programmes in LMICs is limited, even where national guidelines exist [8]. This is due, in part, to the perceived need to focus on passive case detection and treatment of drug-sensitive and drug-resistant cases alone. Additionally, there are resource implications of evaluating all household contacts including the human resources for identifying, locating and evaluating these exposed persons [Reference Fox9–Reference Morrison, Pai and Hopewell11].
Despite an established NTP with a DOTS strategy, Peru has among the highest TB and multidrug-resistant (MDR)-TB rates in the Western Hemisphere. Previous research has consistently shown that household contacts have higher rates of active TB and latent TB than the general community even in settings with low rates of human immunodeficiency virus (HIV) [Reference Fox9, Reference Morrison, Pai and Hopewell11–Reference Becerra14]. However, few studies have considered the cost-effectiveness of implementing a provider-initiated ACF programme within a routine operational TB programme. We estimated the cost-effectiveness of ACF of household contacts of TB cases in high-burden areas in comparison to passive case-finding (PCF) by public healthcare centre staff within a well-functioning routine DOTS-based NTP in a high-TB, low-HIV incidence area [Reference Fox, Dobler and Marks15].
METHODS
This economic evaluation was conducted from a health system perspective. The target population in the analytical model was households of index smear-positive pulmonary TB patients entering treatment in Peru.
Decision analytical model
A decision analysis model was developed to determine if ACF for TB in household contacts of index smear-positive TB patients was cost-effective compared to a passive case-finding strategy (TreeAge Software Inc., USA). The baseline pathway was the current PCF programme (algorithm 1), where symptomatic persons in the community, including household contacts, self-report to healthcare for TB diagnosis by sputum-smear microscopy and clinical evaluation. The PCF programme in Peru can periodically include other ad hoc-enhanced activities, such as periodic case-finding in health centres or in the community at large, or counselling of cases to inform their household contacts to be screened, where the initiative relies on symptomatic persons presenting for care. These strategies are not routine, and rarely undertaken. Xpert MTB/RIF (Cepheid, USA) is an automated polymerase chain reaction (PCR) test which can detect the Mycobacterium tuberculosis complex and rifampicin resistance. This novel diagnostic test is not currently in use for routine diagnostic TB in Lima; however, it is recommended by the WHO and therefore was considered in analyses to account for diagnostic tools that have greater sensitivity than routinely used sputum-smear microscopy.
Decision analysis estimated the probability and costs for each of the following three strategies compared to the baseline PCF programme: (i) the addition of an ACF programme with household visits to screen household contacts for TB using sputum-smear microscopy and symptom evaluation (algorithm 2), (ii) the addition of Xpert MTB/RIF in a single sample (algorithm 3) from persons presenting to a clinic for screening, and (iii) the addition of an ACF programme with household visits to screen household contacts of index TB cases for TB using symptom evaluation and an Xpert MTB/RIF in a single sample (algorithm 4) (Fig. 1).

Fig. 1. Decision analysis model. Not all branches are shown. Note that in algorithms 3 and 4, routine case-finding tests such as sputum smears are done as part of the Xpert MTB/RIF diagnostic test pathway. DS-TB, Drug-sensitive tuberculosis; MDR, multidrug resistant.
In all pathways of the decision analysis, all household contacts of newly diagnosed smear-positive TB cases enrolled in a Ministry of Health TB programme were included. The baseline TB prevalence of 0·2% was based on estimates from routine reported NTP data for San Juan de Lurigancho (SJL), a district with 34 NTP health centres and a population of ~ 900 000 persons. All household contacts detected with active TB were assumed to enter appropriate TB treatment. Following treatment initiation, the household contacts with TB were assumed to have similar probabilities for treatment outcomes and costs regardless of how they were detected.
Input parameters
Probabilities of transition through decision tree
Index TB case patients with active pulmonary TB are followed through a single cycle of household contact screening at the time of patient diagnosis. We defined the probabilities of the likelihood that each path was followed under each screening strategy. The input probabilities for each branch were obtained from locally available NTP data where available and from the published literature (Table 1). Each input probability consisted of a base case value included in the main analyses and a range of estimates (low value and high value) that were included in sensitivity analyses (Table 1).
Table 1. Model input probabilities and source of the parameter
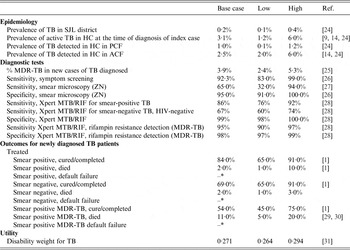
HC, Household contact; PCF, passive case-finding; ACF, active case-finding; ZN, Ziehl-Neelsen; MDR, multidrug resistant.
* = 1 – (probabilities of the cured/completed and died).
Programme direct costs
Government expenditures and health system-related costs were included. Estimated costs were derived from primary data collection and NTP estimates for sputum-smear diagnostics, DOTS treatment, personnel time and programme management of TB cases including training and supervision of staff, materials (e.g. TB programme forms) and supplies (e.g. N95 respirators) and supplemented with published data. The ingredient-costing approach was used whereby all input quantities of cost to perform tests and to deliver treatment were used to arrive at an average cost per test per index TB case (Table 2). Average programme costs for ACF home visits and time for evaluating household contacts were compiled using reports from local NTP healthcare staff and validated on a convenience sample of health centres by the study research team. These included transportation to home visits, nursing time to undertake the home visit and cost estimates of the sputum smear and clinical evaluation for each household contact evaluated within SJL health centres by NTP TB staff. The average salary of nurses and physicians in the programme were estimated for individual household contacts and also per household for household visits. The costs assumed a single home visit per index TB case for an average number of four contacts. These data were recorded on case report forms, including time of departure and time of return to health centre. This information was collected for a period of 4 weeks from a convenience sample of centres and used to calculate an average reported time per activity. Directly measured programme costs originate from an ongoing pragmatic stepped-wedge cluster randomized trial of ACF in SJL district [Reference Shah16]. In addition estimated costs of materials, training and start-up of the ACF programme, supervisory and central programme management costs were included from study logs of the ACF study underway in SJL district. The cost of Xpert MTB/RIF of US$16.38 per cartridge was assumed, and cost of first-line and second-line treatments were estimated at US$350 and US$2423, respectively. Treatment regimen costs included cost of drugs plus DOTS programme supervision for the duration of regimens, cultures and smears, and costs associated with treatment of adverse events in Peru [Reference Resch17, Reference Suárez18]. Patients’ out-of-pocket expenses and costs related to their illness, including loss of work or livelihood during TB disease and treatment, were not included.
Table 2. Programme costs for active case finding of household contacts in San Juan de Lurigancho district in Lima, Peru

* 1 Peruvian Nuevos Soles (S/) = 0·29 US$.
HC, Household contact; ACF, active case-finding.
Where applicable, costs of drug-sensitive TB treatment and MDR-TB treatment were included as were costs for the Xpert cartridge as follows: cost of drug-sensitive treatment = first-line DOTS (US$350·00); cost of MDR-TB treatment DOTS (US$2423·00); cost of Xpert MTB/RIF per cartridge (US$16·86).
Model outcomes
The primary outcomes were the TB cases detected in household contacts, estimated disability-adjusted life years (DALYs) and expected costs for each strategy. Incremental cost-effectiveness ratios (ICERs) are expressed as 2014 US dollars per DALY averted.
Uncertainty and sensitivity analysis
We undertook one-way sensitivity on all model parameters by varying their values across a range of plausible values. We varied the range of plausible values for the sensitivities of the diagnostic tests used to screen household contacts, the cost of the ACF programme (including staffing costs), the proportion of secondary active TB cases detected in contacts through PCF or ACF, and treatment outcomes. Each range of parameters was varied to observe its effect on the overall ICER, which are presented in tornado diagrams, a graph to compare the relative importance of the uncertainty of the variables. In our sensitivity analyses we accounted for the unknown threshold willingness-to-pay (WTP) (lambda) and the potential of small differences in the case detection rates between the ACF and PCF strategies. According to the World Bank, the 2014 gross national income (GNI) per capita (Atlas method) for Peru was US$6360 [19]. The GNI was used as the WTP threshold; an intervention was not considered cost-effective if the cost per DALY averted exceeded this value.
Ethics approval
This analysis used publically available data for which ethical approval was not required. Ethical approvals were granted by the Human Ethics Research Boards of McGill University Health Centre (Montreal, Quebec, Canada), Universidad Peruana Cayetano Heredia (Lima, Peru) and Direccion de Salud Lima IV Este (Lima, Peru) for data estimates used from a separate trial of ACF of household contacts in SJL district.
RESULTS
Incremental costs
The base-case scenario PCF costs were estimated per index case household screened. The addition of an ACF programme including a home visit to evaluate household contacts of index TB cases increased the costs by US$48·80. The implementation of an Xpert MTB/RIF test to evaluate household contacts detected through the routine PCF programme or in addition to the ACF programme resulted in an incremental cost of US$137·87 and US$245·33 per index case household, respectively (Table 3).
Table 3. Incremental cost, DALYs and incremental cost-effectiveness of screening 2500 index TB case households
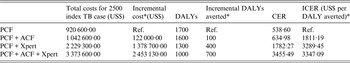
DALY, Disability-adjusted life year; PCF, routine passive case-finding programme; ACF, active case-finding programme; Xpert, Xpert MTB/RIF diagnostic test
CER, cost effectiveness ratio; ICER, incremental cost-effectiveness ratio.
* All referencing baseline PCF-only strategy.
Incremental effects
The PCF strategy was associated with the highest DALYs per index TB case household screened, while the strategy using ACF and the addition of the Xpert MTB/RIF resulted in the fewest DALYs per index TB case household screened. Compared to the baseline PCF strategy, the addition of the ACF strategy resulted in an additional 100 DALYs averted for 2500 index TB case households screened, 400 DALYs averted for the addition of Xpert MTB/RIF to the detection of household contacts in the PCF programme and 700 DALYS averted for implementing ACF with Xpert MTB/RIF as a diagnostic tool in addition to sputum-smear microscopy.
Cost-effectiveness
Compared with the baseline PCF programme alone, implementing ACF for index TB cases was associated with an ICER of US$1811 per DALY averted. Incorporating an Xpert MTB/RIF to evaluate one sputum sample in the evaluation of all index TB cases’ household contacts detected in the PCF strategy alone and with the addition of an ACF strategy was associated with an ICER of US$3289 and US$3347 per DALY averted, respectively. All of the alternatives to PCF alone were considered highly cost-effective compared to the WTP for Peru (GNI, US$6360), which is the threshold value within which an intervention would be considered cost-effective.
Sensitivity analysis
Each alternative strategy was compared to the baseline PCF strategy in one-way sensitivity analyses (Figs 2–4). The ACF strategy was considered highly cost-effective compared to the PCF routine strategy. This included increasing the cost of ACF for index TB case households by up to 30%, resulting in an ICER of US$6300 per DALY averted. This increase in costs represents potential increases in operational costs, for example the costs to undertake multiple household visits to complete screening of contacts, increases to programme staff time to actively seek out contacts pending evaluations, or overtime salary costs related to ACF activities completed on evening or weekends. In sensitivity analyses, we considered Xpert MTB/RIF in ACF and if included for all index cases, which remained cost-effective compared to PCF algorithms. However, in all algorithms where a more costly and sensitive test, in this case Xpert MTB/RIF, was integrated, the overall ICER per DALY averted was no longer cost-effective if the proportion of treated household contacts with TB had increasing rates of treatment failure or default.

Fig. 2. One-way sensitivity analysis of incremental cost-effectiveness ratio (ICER) comparing active case-finding (ACF) of index TB case households to passive case-finding (PCF). Note that for Tornado diagrams (Figs 2–4) the x axis refers to the ICER, a negative ICER refers to US$ per DALYs averted. A baseline of −1811 refers to the ICER US$ per DALY averted of ACF compared to PCF (corresponding with Table 3). Range of values is included in the key (cost range in US$), probabilities range from 0 to 1.
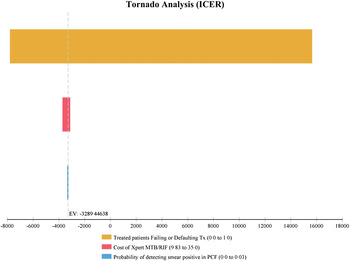
Fig. 3. One-way sensitivity analysis of incremental cost-effectiveness ratio (ICER) comparing passive case-finding of index TB case household contacts with an integrated Xpert MTB/RIF (PCF + XPERT) to passive case-finding strategy using sputum-smear microscopy only.

Fig. 4. One-way sensitivity analysis of incremental cost-effectiveness ratio (ICER) comparing active case-finding of index TB case household contacts with an integrated Xpert MTB/RIF test (ACF + XPERT) to passive case-finding strategy using sputum-smear microscopy only.
DISCUSSION
We found that an ACF programme for screening index TB cases’ household contacts using sputum-smear microscopy was highly cost-effective compared to a standard PCF programme for a TB-endemic urban area of Peru. Compared to the baseline PCF programme, integrating a more sensitive and costly test, such as Xpert MTB/RIF, to a PCF or ACF programme to screen household contacts also was cost-effective. Specifically, the additional cost per DALY averted for ACF alone or combined with an Xpert MTB/RIF diagnostic programme remained well below the threshold of US$6390 for Peru (Table 3).
The current analysis reflects evaluating household contacts at the time the index TB patient is enrolled in DOTS treatment. We assumed a single home visit for an average number of four contacts; however, actual household composition can be smaller or larger. We incorporated direct measures of costs of undertaking ACF including the time spent by front-line staff to undertake these activities in addition to responsibilities of a basic PCF TB programme. In order to account for potential variability in ACF costs, the cost of the ACF programme was increased across a range up to US$40 (or 30% increase in the estimated cost per index case home screened). This upper limit could account for either multiple home visits for the same contact, repeated attempts to capture pending contacts not captured in the initial home visit, increases in the time required to undertake any of the activities related to contact tracing, increases to salaries or to account for overtime costs if contact tracing was required on evenings or weekends. In sensitivity analysis, the ACF strategy remained cost-effective compared to PCF considering an increase in the cost of the ACF programme of up to 30%. However, above this increase, ACF for index case households was no longer cost-effective when considering the WTP threshold.
Few studies on the cost-effectiveness of household contact tracing and ACF within routine programmes from TB-endemic areas and LMICs have been published [8, Reference Yadav20, Reference Azman, Golub and Dowdy21]. Our findings are consistent with those reported from modelling estimates of cost-effectiveness or from primary data studies where available. In Cambodia, a randomized trial of an ACF programme was found to be highly cost-effective compared to the PCF (US$330 per DALY averted), although the ACF approach used included notifying all household and symptomatic neighbourhood contacts of registered TB patients of the past 2 years to attend screening at mobile centres [Reference Yadav20]. Recent theoretical modelling studies of the cost-effectiveness of ACF using data from India, China and South Africa also found ACF to be highly cost-effective [Reference Azman, Golub and Dowdy21]. These authors reported a wide range of ICER estimates, from $1200 per case detected in India up to $9400 per case detected in South Africa; however, all were cost-effective when considering the respective national WTP threshold.
In both the PCF and ACF strategies, the addition of Xpert MTB/RIF diagnostic testing was cost-effective under baseline conditions. The Xpert MTB/RIF is more sensitive for diagnosing TB than sputum microscopy, and can also detect rifampin resistance, which is a marker for MDR-TB [Reference Boehme22, Reference Boehme23]. During one-way sensitivity analyses, integrating Xpert MTB/RIF for screening household contacts only remained cost-effective if high treatment cure rates were observed; the overall cost-effectiveness of integrating Xpert MTB/RIF decreased with increasing rates of default or failure from TB treatment. These findings suggest that if household contacts detected with TB or MDR-TB through ACF have inadequate treatment or high treatment default rates, a corresponding fall in cost-effectiveness would be observed. This situation could be of particular concern in Peru, where primary MDR-TB rates are increasing (5·3% of new TB cases) [1]. MDR-TB cases require longer, more costly and complex treatment regimens with lower treatment success rates than drug-sensitive therapy [1].
These results are subject to limitations. First, the current analytical model does not consider the benefits of future TB cases prevented over time. However, if ACF results in earlier detection of household contacts with active TB, this programme will be even more cost-effective over time by reducing the spread of TB in the community. The analysis does not consider recurrent cases of TB or household contacts who develop TB beyond the initial screening. However, these analyses assumed that the costs and effectiveness of the PCF programme continue with the introduction of any new strategies, so the alternative strategies have the same chance of detecting late cases as the baseline strategy. Finally, this analysis was undertaken from the programme perspective and does not consider out-of-pocket expenses for patients or the overall societal perspective. Costs such as transportation to health centres for household contacts, loss of work or livelihood, patient and caregiver time, are all among the potential impacts to patients and were not considered in the current analysis. Although hardship for TB patients is an important consideration, accurate measurement of these costs is challenging and unlikely to vary much across the different strategies considered for case-finding. Future studies should integrate data on the overall economic burden that can be averted to households with an ACF programme.
Our analysis has included estimates of the time and cost of undertaking ACF of household contacts by routine programme staff, which contributes to the growing literature on ACF and household contact screening in LMICs within routine Ministry of Health programmes. The determination of whether ACF of index TB cases’ household contacts performed within a systematic routine public health TB programme is cost-effective in practice will depend on how well it is implemented and monitored. Our findings assume that the quality of the ACF remains at consistently high levels and completion rates, which would involve the deployment of adequate human resources with sufficient supplies to undertake household contact tracing activities. This analysis is based on estimates and ranges of ACF and strategically screening household contacts of TB cases, including those figures available from published systematic reviews and meta-analyses collected in a range of contexts. Additionally, the programme costs of ACF within the TB DOTS programme considered effectiveness within a densely populated urban area. Validating these results with ACF data from LMICs within routine public health systems is an urgent need for programmes considering implementation.
CONCLUSIONS
Improving case detection and case-finding in the community are potential avenues to enrol TB patients more rapidly into appropriate treatment regimens. We found that a provider-initiated ACF programme for household contacts of index TB cases, including provider-initiated home visits to locate contacts, can be highly cost-effective compared to the current routine passive programme. This cost-effectiveness analysis is among the very few to include the local programme perspective and actual ACF programme costs from an existing routine NTP programme. These findings are generalizable to similar regions with an established NTP, where TB rates are high and HIV rates are relatively low, and with comparable middle-income resource levels to urban Lima, Peru. In analyses, the integration of an Xpert MTB/RIF diagnostic test resulted in higher incremental costs then the PCF or ACF programmes with sputum-smear microscopy alone, although remaining cost-effective. Practical experiences from implementations studies of ACF within strong NTP programmes in LMICs will contribute to understanding the real-world cost-effectiveness of case-finding interventions within operational settings.
ACKNOWLEDGEMENTS
The authors acknowledge the participation of the TB programme physicians and nurses in SJL district. Funding was provided by the Canadian Institutes of Health Research (CIHR). Additional support for the primary author was provided by a Steinberg Global Health Postdoctoral Fellowship, CIHR Doctoral Research Award and Michael Smith Travel Supplement and the International Development Research Centre (IDRC) Doctoral Research Award.
DECLARATION OF INTEREST
None.










