Implications
Marbling is a very important characteristic of meat quality in ruminants due to its effect on flavor and juiciness. In addition, fatty acid composition of lipids in meat is also important for human health and, therefore, this subject has been extensively studied in recent decades. In this sense, nutrigenomic has been used to better understand the cellular mechanisms influencing marbling and fatty acid profile in meat. This knowledge may affect livestock sector, creating possibilities for the industry to produce substances or chemical compounds that can modulate gene expression and, therefore, improving meat quality. In addition, this knowledge will permit nutritionists to use feedstuffs and additives in order to modulate the expression of target genes and increase meat quality.
Introduction
Since the 1980s, consumers have become more concerned about the quality of food and how it could directly influence health. This concern became more pronounced during the 2000s, influencing consumption (Van Wezemael et al., Reference Van Wezemael, Verbeke, de Barcellos, Scholderer and Perez-Cueto2010) and, consequently, directing research for healthier food production. In this sense, research around the world has been conducted with the goal to improve fatty acid profile in ruminant meat, aiming to increase concentrations of beneficial fatty acids to improve human health and reduce fatty acids that could have some detrimental effect (Scollan et al., Reference Scollan, Dannenberger, Nuernberg, Richardson, MacKintosh, Hocquette and Moloney2014).
However, when analyzing research published in the last 10 years on this subject (Supplementary Table S1) in the main journals of Animal Science and Meat Science, it has been found that results showing that manipulating beef fatty acid profile in a way that is beneficial for human health are limited. It was considered as beneficial for human health concentrations of CLA, oleic and hypercholesterolemic fatty acids and n-6/n-3 ratio. Of the 28 articles selected, almost half (13) reported no improvement on fatty acid profile through dietary changes (Figure 1). In addition, specifically for CLA, a fatty acid linked with cancer prevention, reduction of atherosclerosis, improvement of the immune response, as well as changes in protein and energy metabolism (Whigham; Cook & Atkinson, 2000), only eight studies reported increased concentrations in muscle. Therefore, despite efforts to alter fatty acid profile in ruminant meat through dietary changes (i.e. use of high polyunsaturated fatty acids (PUFA) ingredients, grass-fed or feedlot in finishing phase), it has been found that this objective is hard to achieve. For this reason, the following questions arise: is it not possible to reduce/alter significantly ruminal biohydrogenation? Or, although fatty acid profile in the rumen is altered, does the muscle tend to maintain a pattern of their concentrations? To help answer these questions, research on how different fatty acids modulate expression of genes involved in muscle lipid metabolism have become important (Ladeira et al., Reference Ladeira, Schoonmaker, Gionbelli, Dias, Gionbelli, Carvalho and Teixeira2016), of which the main results are shown in this review.

Figure 1 Effect of diets on ruminant muscle fatty acid profile. Improving fatty acid profile considered increase concentrations of conjugated linoleic acid (CLA) c9,t11-C18:2 and oleic acid; and decrease hypercholesterolemic fatty acids and n-6/n-3 ratio. Data were obtained in researches published in the last 10 years on this subject in the main journals of Animal Science and Meat Science (Supplementary Table S1). FA=fatty acid.
Another important issue is marbling, a key factor for the production of high quality meat in some markets, such as the United States, Japan, Korea, Australia, Canada and Brazil. In this case, the knowledge about the metabolic mechanisms influencing lipogenesis and intramuscular fat deposition is fundamental to produce high marbling meat. According to Smith and Crouse (Reference Smith and Crouse1984), glucose incorporation into fatty acids was significantly greater in intramuscular adipose than in subcutaneous adipose, and glucose was quantitatively the primary lipid precursor (51% to 76%) in intramuscular adipose tissue compared with acetate (10% to 26%). Therefore, it is necessary to understand the regulation of genes involved in starch digestion and glucose absorption in the small intestine, liver gluconeogenesis and glucose uptake by the muscle when the goal is to produce high marbling meat.
The marbling content in adult animals is dependent on the number and size of adipose intramuscular cells, and the potential amount of these cells is greatly affected during prenatal and early postnatal life (Zhu et al., Reference Zhu, Han, Tong, Ma, Kimzey, Underwood, Ziao, Hess, Ford, Nathanielsz and Du2008). Environmental factors such as nutrition can affect gene expression in the animal through epigenetic effects varying the differentiation and proliferation of adipose cells. Therefore, the regulation of marbling and fatty acid profile starts with the maternal influences on fetal gene expression to modulate the differentiation and proliferation.
Therefore, this review has the objective to present a critical analysis of published results and new concepts on how nutrition affects expression of genes involved in adipogenesis, lipogenesis and fatty acid profile of ruminant meat.
Fetal programming of adipogenesis and marbling
Adipogenesis begins during the prenatal phase, which may have long-term influence on fat deposition and meat quality. Therefore, before discussing the direct effect of nutrients on genes during the finishing phase, it is necessary to examine the effect of nutrition of the dam on adipogenesis of the offspring through nutrients affecting the expression of genes in the fetus. This effect is part of the concept known as ‘fetal programming’. How nutrition of the dam affects offspring is complex. The dam can influence progeny phenotype by providing half of the fetal genes and, besides that, epigenetic markings, through somatic epigenetic reprogramming, via the ooplasmic contribution to the fetus and via the provision of the intrauterine environment (Aiken and Ozanne, Reference Aiken and Ozanne2014).
Fetal programming may have a direct effect on progeny development and have transgenerational effects, transmitting a genetic inheritance to generations that were not exposed to the initial signal (Heard and Martienssen Robert, Reference Heard and Martienssen2014). These epigenetic changes may affect adiposity and meat quality because several organoleptic characteristics are dependent on metabolic processes in the live animal and postmortem.
Epigenetics effects
Epigenetics is a concept of changes in gene functions related to parental inheritance without altering the base sequences of the DNA and can act as a key mechanism that allows phenotypic plasticity regarding a fixed genotype (Heard and Martienssen Robert, Reference Heard and Martienssen2014). Alterations in chromatin structure by DNA methylation, histone modification and noncoding microRNAs are the most common mechanisms in epigenetics and regulate timing and intensity of gene expression allowing those changes to pass through generations (Link et al., Reference Link, Balaguer and Goel2010). The combination of these three epigenetic mechanisms are responsible for controlling genetic expression, maintaining a robust combination that allows this regulation to be passed from one generation to another. The way that epigenetics is influenced by a nutritional stimuli was simplified and described by Mathers (Reference Mathers2008), and is called the 4Rs of nutritional epigenomes. First the animal RECEIVED a nutritional stimuli and it is RECORDED by the genome. Then this exposure is REMEMBERED by following cell generations, and finally is REVEALED in changed gene expression, cell function and overall health.
Maternal nutrition and offspring adipogenesis and marbling
In utero development of muscle and adipose tissue are important events that impact the ultimate quantity and quality of meat produced. Nutrient restriction or excess during fetal and neonatal development can have long-term consequences on offspring adiposity (Figure 2), particularly if they occur during critical periods of adipose development. The development of adipocytes, which will generate brown adipose tissue, starts in early gestation, and ~80% of fetal adipogenesis occurs in the final few weeks of gestation (Symonds et al., Reference Symonds, Stephenson, Gardner and Budge2007). Adipocyte hyperplasia occurs primarily during late fetal development and early postnatal life in cattle (Zhu et al., Reference Zhu, Han, Tong, Ma, Kimzey, Underwood, Ziao, Hess, Ford, Nathanielsz and Du2008). Although preadipocytes can proliferate and differentiate in adults, their capacity appears to be limited to the developmental stage in early life (Martin et al., Reference Martin, Hausman and Hausman1998).

Figure 2 Long-term effects of maternal nutrition according to gestation period in ruminant offspring development and performance. 1Greenwood et al. (Reference Greenwood, Cafe, Hearnshaw and Hennessy2005); 2Long et al. (Reference Long, Tousley, Underwood, Paisley, Means, Hess, Du and Ford2012); 3Blair et al. (Reference Blair, Mohrhauser, Taylor, Underwood, Pritchard and Wertz-Lutz2013); 4Mohrhauser et al. (Reference Mohrhauser, Taylor, Underwood, Pritchard, Wertz-Lutz and Blair2015); 5Underwood et al. (Reference Underwood, Tong, Price, Roberts, Grings, Hess, Means and Du2010); 6Summers et al. (Reference Summers, Blair and Funston2015); 7Wilson et al. (Reference Wilson, Long, Faulkner and Shike2016); and 8Larson et al. (Reference Larson, Martin, Adams and Funston2009).
Fetal programming may occur during cell division in response to a recent stimulus and transferred to other cells (Bonasio and Reinberg, Reference Bonasio, Tu and Reinberg2010). For example, maternal under-nutrition may cause adaptation in the offspring, leading to metabolic changes to ‘save’ energy, resulting in greater fat deposition and lesser muscle mass in the progeny (Blair et al., Reference Blair, Mohrhauser, Taylor, Underwood, Pritchard and Wertz-Lutz2013). The reason why nutrition of the dam can change cell tissues proliferation is that mesenchymal stem cells (MSC) originate muscle and adipose tissues by the action of transcription factors, regulating the involvement and differentiation of these cells and muscle composition (Du et al., Reference Du, Tong, Zhao, Underwood, Zhu, Ford and Nathanielsz2010). Transcription factors can act in many ways, as influenced by concentrations, cell-to-cell interactions and the extra-cellular matrix (Ladeira et al., Reference Ladeira, Schoonmaker, Gionbelli, Dias, Gionbelli, Carvalho and Teixeira2016).
The main transcription factor for MSC differentiation is Wingless and Int (Wnt) signaling. According to Du et al. (Reference Du, Tong, Zhao, Underwood, Zhu, Ford and Nathanielsz2010), the Wnt signaling pathway increases myogenesis and reduces adipogenesis in skeletal muscle, regulating body fat and reducing obesity susceptibility. Therefore, it is possible that adequate maternal nutrition during gestation will increase Wnt signaling, promoting more myogenesis in early- and mid-gestation. Then, during late-gestation, after a satisfactory myogenesis, Wnt signaling could be inhibited to increase adipogenesis.
Maternal nutrition may affect Wnt expression in an epigenetic manner. Maternal over-nutrition during pregnancy impairs myogenesis and elevates adipogenesis, which is partially explained by down-regulation of the Wnt signaling pathway (Yan et al., Reference Yan, Zhu, Dodson and Du2012). Dietary supplementation with methyl donor groups, such as folic acid, vitamin B12, choline and betaine, increases Wnt expression and reduces adipogenic differentiation (Funston and Summers, Reference Funston and Summers2013). Likewise, maternal nutrient restriction may increase visceral adipogenesis (Wang et al., Reference Wang, Yang, Harris, Nelson, Busboom, Zhu and Du2016). In addition, over-nourishment of beef cows during gestation enhanced the messenger RNA (mRNA) expression of adipogenic markers and collagen deposition without affecting myogenesis in skeletal muscle of beef cattle fetuses (Duarte et al., Reference Duarte, Paulino, Das, Wei, Serão, Fu, Harris, Dodson and Du2013).
The Zinc finger protein 423 (ZFP423) is another transcriptional factor involved in the regulation of adipogenesis. The ZFP423 stimulates expression of another transcription factor, known as peroxisome proliferator-activated receptor-gamma (PPARG), increasing adipogenic differentiation (Gupta et al., Reference Gupta, Arany, Seale, Mepani, Ye, Conroe, Roby, Kulaga, Reed and Spiegelman2010). Peroxisome proliferator-activated receptor isoforms function as heterodimers with a retinoid X receptor (RXR), and both bind to a specific DNA sequence, inducing or repressing its expression (Ladeira et al., Reference Ladeira, Schoonmaker, Gionbelli, Dias, Gionbelli, Carvalho and Teixeira2016). Because PPARα and PPARγ use fatty acids as endogenous ligands, it is suggested that they can be regulated by diet (Bispham et al., Reference Bispham, Gopalakrishnan, Dandrea, Wilson, Budge, Keisler, Broughton, Stephenson and Symonds2003). However, the bovine fetus has low concentrations of free fatty acids and this mechanism may be limited. Bispham et al. (Reference Bispham, Gopalakrishnan, Dandrea, Wilson, Budge, Keisler, Broughton, Stephenson and Symonds2003) observed that maternal dietary restriction increased PPARG expression in fetus inducing greater amounts of adipose tissue. Similar results were observed by Paradis et al. (Reference Paradis, Wood, Swanson, Miller, McBride and Fitzsimmons2017), who explain this effect as a consequence of an epigenetic effect, where mobilization of fatty acids from maternal adipose tissue increases expression of PPARA in the dam and fetus. Yang et al. (Reference Yang, Liang, Rogers, Zhao, Zhu and Du2013) observed that maternal obesity reduces DNA methylation in the promoter region of ZFP423 and adipogenic progenitors in mice fetuses. In addition, Gionbelli et al. (Reference Gionbelli, Veloso, Rotta, Valadares Filho, Carvalho, Marcondes, Cunha, MAS, Prezotto, Duarte and Gionbelli2018) has shown that ZFP423 and PPARG were more expressed at 139 days of gestation in fetuses of cattle whose dams were over-nourished during gestation.
Non-myogenic progenitors as fibro-adipogenic precursors (FAP) are also involved in adipocyte formation. Fibro-adipogenic precursors has an adipogenic and fibrogenic capacity, act in the recovery of muscle damage (Uezumi et al., Reference Uezumi, Ikemoto-Uezumi and Tsuchida2014) and are responsible for the development of intramuscular fat. Late gestation is the period of greater production of intramuscular adipocytes, consequently, manipulation of maternal diet for greater expression of FAP could lead to greater marbling and meat quality, but more research is necessary to further examine these responses.
Although there is evidence that an increase in intramuscular adipocyte number may occur in the late stages of development (Cianzio et al., Reference Cianzio, Topel, Whitehurst, Beitz and Self1985), late gestation and neonate phases are considered the best time for manipulation of the diet in order to increase marbling of progeny because of the large abundance of multipotent cells (Du et al., Reference Du, Tong, Zhao, Underwood, Zhu, Ford and Nathanielsz2010). In this case, after birth and until 250 days of life, there is still formation of adipose cells, but after this period the effects of dietary manipulation are conditioned to the hypertrophy of the existing adipocytes.
Deoxyribonucleic acid methylation also changes during the lifetime of the animal and can be manipulated by nutrition (Gueant et al., Reference Gueant, Elakoum, Ziegler, Coelho, Feigerlova, Daval and Gueant-Rodriguez2014). Vitamin A supplementation to the dam promotes adipogenic commitment through the increase of cellular retinoic acid binding protein 2 (CRABP-II) delivering retinoic acid for binding to retinoic acid receptor (RAR). Therefore, vitamin A supplementation during early development is expected to increase adipogenesis, which is supported by studies in mice (Wang et al., Reference Wang, Fu, Liang, Wang, Yang, Zou, Nie, Zhao, Gao, Zhu, de Avila, Maricelli, Rodgers and Du2017). In Wang et al. (Reference Wang, Fu, Liang, Wang, Yang, Zou, Nie, Zhao, Gao, Zhu, de Avila, Maricelli, Rodgers and Du2017) study, authors identified that vitamin A affects fetal and offspring adipogenesis through promoting angiogenesis. In this case, retinoic acid up-regulated Vegfa and Vegfr2 expression, which consequently increased the population of platelet derived growth factor receptor α+ adipose progenitor cells in adipose tissue. Therefore, this finding shows that the increase of adipocytes in early life may increase intramuscular fat deposition in the finishing phase of ruminant animals. On the other hand, vitamin A supplementation in finishing cattle increases fatty acid binding protein (FABP)5 and stimulates PPAR activation leading to an increase of lipid oxidation and reducing adipocyte hypertrophy and marbling (Wang et al., Reference Wang, Yang, Harris, Nelson, Busboom, Zhu and Du2016).
Although the way maternal nutrition affects gene expression and fetal development is not totally clear, it is well known that maternal nutrition and metabolism affect nutrient supply in the fetus and it may alter metabolism by reducing availability of methyl donors and specific amino acids involved in DNA methylation and histone modification (Paradis et al., Reference Paradis, Wood, Swanson, Miller, McBride and Fitzsimmons2017).
Nutrigenomic and glucose metabolism
Starch digestion
Ruminants evolved with cellulose supplying the majority of metabolizable energy for the rumen microbial fermentation. Cereal grains, which are primarily composed of starch, are a major feedstuff for ruminant production systems, especially in feedlots and dairies. Ruminants do not produce salivary α-amylase, so the first site of starch digestion is the rumen in which starch is fermented to volatile fatty acids (Kotarski et al., Reference Kotarski, Waniski and Thurn1992). Harmon et al. (Reference Harmon, Yamka and Elam2004) also reported a linear relationship between starch intake and starch digested in the rumen, suggesting that there are no limits to ruminal starch digestion. However, rapid and excessive fermentation of readily fermentable carbohydrate can result in ruminal and systemic acidosis (Owens et al., Reference Owens, Secrist, Hill and Gill1998). Therefore, factors regulating the rate of fermentation, such as grain source and processing method, must be considered in relation to forage source and inclusion level.
From 4% to 60% of dietary starch intake passes to the small intestine in cattle fed high-concentrate diets, depending on grain source and processing (Theurer, Reference Theurer1986). The starch that passes to the small intestine is first hydrolyzed by pancreatic α-amylase. The mucosal disaccharidases of the small intestine then hydrolyze the starch breakdown products. Once free glucose is formed, it is absorbed by mucosa primarily via sodium-dependent glucose transporter 1 (SGLT1; Bauer et al., Reference Bauer, Harmon, Bohnert, Branco and Huntington2001). Harmon et al. (Reference Harmon, Yamka and Elam2004) summarized several studies and reported that only 55% and 53%, respectively, of starch entering the small intestine disappears in the small intestine of cattle fed high-concentrate diets. Kreikemeier et al. (Reference Kreikemeier, Harmon, Brandt, Avery and Johnson1991) simultaneously evaluated small intestinal carbohydrate disappearance and portal appearance of glucose in steers infused abomasally with increasing amounts of glucose, corn dextrin or cornstarch. Only the glucose infusion resulted in a linear proportional increase in net portal glucose absorption, suggesting a possible limit in carbohydrase activity. This is further supported by the fact that 15 times as much starch as glucose flows past the ileum when starch is infused post-ruminally at a rate of 60 g/h. This suggests that inadequate α-amylase activity may be responsible for the limited capacity for starch digestion in the small intestine of ruminants.
The regulation of pancreatic digestive enzyme production and secretion in ruminants is complex and differs from that of non-ruminants (Swanson and Harmon, Reference Swanson and Harmon2002). Generally, the complexity of pre-gastric fermentation in ruminants makes the relationship between diet composition and nutrient regulation of enzymes difficult to discern. Dietary energy and post-ruminal flow of starch and protein and their breakdown products are thought to be the major factors influencing pancreatic exocrine function. Dietary energy typically has resulted in increased pancreatic content or secretion of α-amylase (Swanson and Harmon, Reference Swanson and Harmon2002). Generally, increasing duodenal flow of starch, partially hydrolyzed starch, or glucose decreases pancreatic content and secretion of α-amylase in ruminants (Swanson et al., Reference Swanson, Matthews, Woods and Harmon2002). Interestingly, increase in post-ruminal flow of protein has resulted in increased starch digestion and secretion of α-amylase (Richards et al., Reference Richards, Swanson, Paton, Harmon and Huntington2003).
Less research has been conducted quantifying α-amylase mRNA and protein abundance than content or secretion of enzyme activity. Pancreatic α-amylase mRNA tended to be lower, and protein abundance and activity (U/g pancreas and U/g protein) were lower in calves receiving abomasal partially hydrolyzed starch. α-amylase protein and activity seems to have a similar magnitude of response to diet or abomasal infusion treatment in calves (Swanson et al., Reference Swanson, Matthews, Woods and Harmon2002), suggesting that regulation by post-translational modification of α-amylase is not responsible for dietary or small intestinal adaptation of α-amylase expression in ruminants. This, along with the observation that α-amylase mRNA and protein do not respond to post-ruminal nutrients in a directly proportional manner, suggests that dietary/small intestinal adaptation of α-amylase expression is regulated at least in part by translational events in ruminants. This differs from non-ruminants, in that changes in mRNA mediate the observed alterations in protein synthesis of pancreatic α-amylase and proteases in response to dietary changes in carbohydrate and protein (Scheele, Reference Scheele1994). Cao et al. (Reference Cao, Yang, Guo, Zheng, Wang, Cai and Yao2018), investigating the effect of a duodenal infusion of leucine and phenylalanine on pancreatic development and enzyme gene expression in dairy goats, found that diet with 9 g/day of leucine and 2 g/day of phenylalanine increased amylase mRNA levels, and 2 g/day of phenylalanine increased lipase mRNA levels.
Intestinal regulation of solute carrier family 5 member 1 (SLC5A1) expression, which encodes the SGLT1, also seems to be complex in ruminants with inconsistent responses in mRNA and protein expression in response to luminal carbohydrates (Rodriguez et al., Reference Rodriguez, Guimaraes, Matthews, McLeod, Baldwin and Harmon2004). In a study, using Bos taurus and Bos indicus bulls, Carvalho (Reference Carvalho2015) did not find the effects of diet or breed on the abundance of SLC5A mRNA in the duodenum and jejunum. In this research, diets had different starch concentrations and degrees of corn processing. According to Liao et al. (Reference Liao, Harmon, Vanzant, McLeod, Boling and Matthews2010), the SGLT1 transporter has high affinity for monosaccharides, and they observed that SLC5A expression was greater in the duodenum when there was infusion of hydrolyzed starch in the rumen, and only the ileal epithelium responded to the infusion of hydrolyzed starch in the abomasum.
Up to now, what is signaling the changes in pancreatic α-amylase and intestinal SLC5A1 expression are not well understood in ruminants and likely includes substrate, endocrine and neuroendocrine factors. Because of the differences in digestive physiology resulting in differences in the flow and composition of digesta flowing through the digestive tract between ruminants and non-ruminants, differences likely exist in the regulation of digestive enzyme and nutrient transporter gene expression.
Liver gluconeogenesis
Glucose supply is one of the main factors affecting lipogenesis, marbling and beef quality in ruminants. Ruminants differ from non-ruminants in that they often absorb very little glucose from the diet as discussed above. Therefore, gluconeogenesis is critical to provide glucose, which is a universal fuel for cellular, tissue and whole-animal functions. Ruminants also differ in that propionate, a byproduct of ruminal fermentation, is the major precursor for gluconeogenesis. In addition, Harmon et al. (Reference Harmon, Britton, Prior and Stock1985) demonstrated that high-grain diets increase l-lactate absorption, which also contribute significantly to gluconeogenesis. Therefore, the importance of pathways involved in precursor entry into gluconeogenesis differ between ruminants and non-ruminants. For example, Zhang et al. (Reference Zhang, Koser and Donkin2016) has suggested that the induction of cytosolic phosphoenolpyruvate carboxykinase transcription in response to propionate is much greater than in response to cyclic adenosine monophosphate and dexamethasone and this effect is not repressed by insulin as it is in non-ruminants. It is less well understood how glucose sensing influences metabolism in the liver in ruminants. Glucose sensing in the liver of non-ruminants is thought to have impacts on energy metabolism and maintenance of blood glucose concentrations (Oosterveer and Schoonjans, Reference Oosterveer and Schoonjans2014).
According to Koser et al. (Reference Koser, Thomas and Donkin2008), bovine phosphoenolpyruvate carboxykinase 1 (PCK1) expression, the gene responsible to encode PEPCK, is positively regulated by propionate, constituting a feed-forward mechanism of substrate control for hepatic gluconeogenesis that is linked to the final products of rumen fermentation. However, despite the positive effect of propionate in PCK1 expression, Ladeira et al. (Reference Ladeira, Schoonmaker, Gionbelli, Dias, Gionbelli, Carvalho and Teixeira2016) reported that glycerol seemed to exert a negative feedback in glycerol kinase-1 expression. Still, according to these authors, researches evaluating transcription factors and mechanisms regulating the expression of genes involved in liver gluconeogenesis in ruminants are necessary. Some important transcription factors in which it is necessary to study are PPARG coactivator 1α and PPARG, due to their effects on PCK1 regulation in mice.
Muscle uptake and insulin sensitive
The transport of monosaccharides, including glucose, across cellular membranes is mediated by members of the glucose transporter (GLUT) family that are encoded by the solute carrier family 2 (SLC2) genes (Mueckler and Thorens, Reference Mueckler and Thorens2013). Skeletal muscle makes up a large proportion of the overall mass of mammals and thus is a large contributor to overall nutrient and energy needs. In addition, it is thought that because of the differences in carbohydrate digestion between ruminants and non-ruminants (described above) that ruminants are more insulin-resistant than non-ruminants. This may be supported by data suggesting that insulin has a lesser effect on GLUT4 translocation in bovine than in porcine skeletal muscle (Duhlmeier et al., Reference Duhlmeier, Hacker, Widdel, von Engelhardt and Sallmann2005). However, other research (Duehlmeier et al., Reference Duehlmeier, Sammet, Widdel, von Engelhardt, Wernery, Kinne and Sallmann2007) has suggested that GLUT1 may be of greater importance than GLUT4 for glucose uptake in skeletal muscle in ruminants. This is interesting as GLUT1 is thought to account for the basal glucose uptake and GLUT4 is thought to account for insulin-stimulated glucose uptake (De Koster and Opsomer, Reference De Koster and Opsomer2013), reinforcing the hypothesis that ruminants have greater insulin resistance. In addition, Hocquette et al. (Reference Hocquette, Bornes, Balage, Ferre, Grizard and Vermorel1995) found that, in ruminants, glucose is the main energy-yielding substrate for glycolytic but not for oxidative muscles, and that insulin responsiveness may be lower in oxidative than in other skeletal muscles. Therefore, more insulin resistance or lesser glucose uptake by GLUT4 action in adipose cells would lead to a lower glucose available to fatty acid synthesis, based on the hypothesis proposed by Smith and Crouse (Reference Smith and Crouse1984) in which glucose is the main substrate for lipogenesis in intramuscular fat tissue. According to Hocquette et al. (Reference Hocquette, Gondret, Baéza, Médale, Jurie and Pethick2010), a variety of genes may be used as markers of adipocyte development (such as GLUT4, lipoprotein lipase (LPL) and lipogenic enzymes), and adipogenesis (PPARG and sterol regulatory element binding transcription factor 1 (SREBF1)) and they would be of great potential for predicting subsequent intramuscular fat (IMF) development. Higher level of GLUT4 expression and higher activities of metabolic enzymes involved in the conversion of glucose into long-chain fatty acids were detected in intramuscular adipose tissue compared with subcutaneous in cattle (Hocquette et al., Reference Hocquette, Jurie, Bonnet and Pethick2005).
It is less clear how diet or management influences insulin sensitivity. Dietary chromium supplementation has been shown to increase insulin sensitivity (Spears et al., Reference Spears, Whisnant, Huntington, Lloyd, Fry, Krafka, Lamptey and Hyda2012), whereas increasing dietary energy intake (Sternbauer and Luthman, Reference Sternbauer and Luthman2002) did not influence insulin sensitivity. Interestingly, early weaning has been shown to enhance insulin sensitivity (Zezeski et al., Reference Zezeski, McCracken, Poole, Al Naib, Smith, McCann and Rhoads2017) and temperamental cattle have been shown to respond to glucose and insulin differently than calm cattle (Burdick Sanchez et al., Reference Burdick Sanchez, Carroll, Broadway, Hughes, Roberts, Richeson, Schmidt and Vann2016). The mechanisms mediating differences in insulin sensitivity in ruminants have not been extensively studied but differences are likely because of changes in expression of the insulin receptor, which mediates the trafficking of the glucose transporter, GLUT4, to the cell membrane (Smith, Reference Smith2017).
Nutrigenomic and lipogenesis
Marbling
Beef marbling is dependent of the energy content in the diet (Smith and Crouse, Reference Smith and Crouse1984) and therefore, for more intramuscular fat deposition, it is necessary for the diet to have high dietary energy. In addition, the hypertrophy or filling of adipocytes with lipids is an important component of intramuscular fat development. Robelin (Reference Robelin1986) reported that fat deposition from birth to maturity in Friesian or Charolais bulls was primarily (70%) due to increases in cell volume or adipocyte hypertrophy. As mentioned before, early studies demonstrated that acetate and glucose are the major precursors used for biosynthesis of fatty acids in ruminants, where intramuscular adipocytes prefer glucose, and subcutaneous adipocytes prefer acetate as lipogenic substrates (Smith and Crouse, Reference Smith and Crouse1984, May et al., Reference May, Burney, Wilson, Savell, Herring, Lunt, Baker, Sanders and Smith1995). On the other hand, Nayananjalie et al. (Reference Nayananjalie, Wiles, Gerrard, McCann and Hanigan2015) detected that acetate is the main precursor for lipid synthesis across fat depots. In addition, according to Choi et al. (Reference Choi, Silvey, Johnson, Doumit, Chung, Sawyer, Go and Smith2014), as cattle become heavier, the contribution of glucose to fat synthesis decreases whereas the use of acetate for fat synthesis increases in intramuscular adipose tissue (Figure 3). In this study, acetate was the main substrate for intramuscular fat and not glucose. Regardless of which substrate is used, the carbon sources or fatty acids must get transported through the circulation and into the cell for hypertrophy to proceed.

Figure 3 Fatty acid biosynthesis from acetate and glucose in intramuscular (i.m.) and subcutaneous (s.c) adipose tissues of Angus steers at 12, 14 and 16 months of age. Adapted from Choi et al. (Reference Choi, Silvey, Johnson, Doumit, Chung, Sawyer, Go and Smith2014).
Fatty acid transport and lipolysis in muscle tissue also influences intramuscular fat deposition. Historically, uptake of fatty acids into the cell was believed to be by passive diffusion; however, current research shows that various membrane-associated proteins or fatty acid transporters facilitate the entry of fatty acids into the cell (Glatz et al., Reference Glatz, Luiken and Bonen2010). These transporters may also play a role in coordinating lipid metabolism and have been implicated in the development of metabolic diseases (Glatz et al., Reference Glatz, Luiken and Bonen2010; Kitessa and Abeywardena, Reference Kitessa and Abeywardena2016). In addition, as well as enzymes involved in fatty acid uptake (i.e. lipoprotein lipase), membrane transporters are regulated by transcriptional and translational mechanisms, which will affect fatty acid uptake and adipocyte filling.
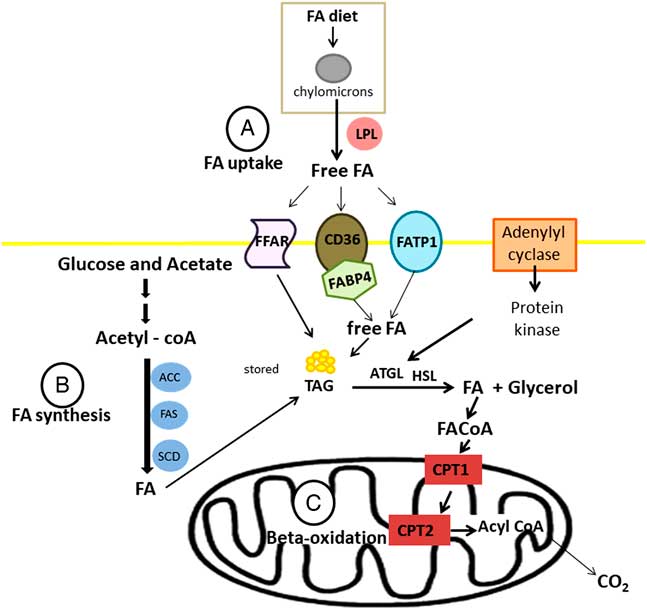
Fatty acids are transported into the cell by three groups of fatty acid transporters: fatty acid translocase (CD36), fatty acid transport protein (FATP) or FABP in association with acyl-CoA synthase (Figure 4a). There are six subgroups of FATP and FATP1 is located in white adipose tissues and skeletal muscle (Kitessa and Abeywardena, Reference Kitessa and Abeywardena2016). There are 12 different FABPs with FABP3 and FABP4 (also known as AP2) expressed in skeletal muscle and adipose tissues, respectively. Moore et al. (Reference Moore, Ekeren, Lunt and Smith1991) was the first study that reported FABP in bovine skeletal muscle. In addition, current research shows that there are also G-protein coupled receptors (GPR), also known as free fatty acid receptors (FFARs), that are on the cell membrane of bovine adipose tissues and transport fatty acids into the cell (Smith et al., Reference Smith, Go, Johnson, Chung, Choi, Sawyer, Silvey, Gilmore and Kim2012). There are four known FFARs and they have specificity for certain types of fatty acids. FFAR2 (GPR43) and FFAR3 (GPR41) have high affinity for short-chain saturated fatty acids like acetate and propionate; whereas FFAR1 (GPR40) has high affinity for medium-chain fatty acids and long-chain fatty acids, and FFAR4 (GPR120) has high affinity for long-chain fatty acids and is activated by various PUFA (Miyamoto et al., Reference Miyamoto, Hasegawa, Kasubuchi, Ichimura, Nakajima and Kimura2016).

Figure 4 Synthesis (a), uptake (b) and oxidation (c) of fatty acid (FA) on ruminant adipose tissue. LPL=lipoprotein lipase; ACC=acetyl-CoA carboxylase; FAS=fatty acid synthase, SCD=stearoyl-CoA desaturase; FFAR=free fatty acid receptors; CD36=fatty acid translocase; FATP=fatty acid transport protein; FABP4=fatty acid-binding protein 4; TAG=triacylglyceride; ATGL=adipose triglyceride lipase; HSL=hormone sensitive lipase; CPT=carnitine palmitoyltransferase.
Considering lipogenesis, the de novo fatty acid synthesis occurs by the action of the acetyl-CoA carboxylase (which is encode by the gene ACACA) and fatty acid synthase (FASN) (Ladeira et al., Reference Ladeira, Schoonmaker, Gionbelli, Dias, Gionbelli, Carvalho and Teixeira2016). Following their synthesis or uptake by adipocytes, fatty acids might be exposed to the action of the enzyme stearoyl-CoA desaturase (SCD1) (Figure 4b), inserting double bounds in the chain. During lipolysis, fatty acids needs to be oxidized into the mitochondrial via carnitine palmitoyl transferase enzyme (CPT1) (Bionaz et al., Reference Bionaz, Thering and Loor2012). Inside mitochondria, CPT2 converts the long-chain acylcarnitine back to long-chain acyl-CoA, and then long-chain acyl-CoA enters β-oxidation pathway (Figure 4c).
Therefore, increasing lipogenesis, fatty acid uptake and decreasing lipolysis are associated with greater IMF deposition. In other words, changes in the balance between synthesis and degradation can cause an increase or decrease in IMF. Corroborating this assertion, Teixeira et al. (Reference Teixeira, Oliveira, Chizzotti, Chalfun-Junior, Coelho, Gionbelli, Paiva, Carvalho and Ladeira2017) reported that Nellore bulls fed ground corn had greater expression of genes related to synthesis (ACACA and SCD1), absorption (FABP4 and LPL) and degradation (CPT2) (Table 1), characterizing greater lipid turnover in these animals, which could be responsible for less IMF. According to Knutson et al. (Reference Knutson, Sun, Fontoura, Gaspers, Liu, Carlin, Bauer, Swanson and Ward2017), intramuscular fat deposition is more complex than subcutaneous fat in beef cattle; it is affected by the genetic propensity to marble, nutritional plane throughout life, animal weight, age and environmental factors.
Table 1 Average pH, t10,c12-C18:2 content and relative gene expression of lipogenic and transcription factors in longissimus muscle of Angus or Nellore young bulls fed ground corn (GC) diet or whole shelled corn (WSC) diet

ACACA=acetyl-CoA carboxylase α; CPT2=carnitine palmitoyltransferase 2; FABP4=fatty acid binding protein 4; SCD1=stearoyl-CoA desaturase 1; SREBF1=sterol regulatory element binding transcription factor 1.
Source: Teixeira et al. (Reference Teixeira, Oliveira, Chizzotti, Chalfun-Junior, Coelho, Gionbelli, Paiva, Carvalho and Ladeira2017).
1 Diet contained 58% GC, 30% corn silage, 10% soybean and 2% mineral supplement.
2 Diet contained 85% WSC with 15% of a pelleted protein, mineral and vitamin supplement.
3 Breed and diet interaction.
Duarte et al. (Reference Duarte, Paulino, Das, Wei, Serão, Fu, Harris, Dodson and Du2013), studying Wagyu and Angus cattle, found that Wagyu had more IMF, and more expression of ZFP423, which induced adipogenesis and the upregulation of PPARG. Bong et al. (Reference Bong, Jeong, Rajasekar, Cho, Kwon, Kim, Paek and Baik2012), comparing gene expression in bulls and steers, found that steers have greater expression of lipogenic genes (ACACA and FASN), lipid uptake (LPL, CD36 and FATP1) and less lipolytic (adipose triglyceride lipase or official name: patatin like phospholipase domain containing 2 – PNPLA2). Therefore, steers had less lipid turnover, resulting in high marbling muscles (11.0% and 3.0%). These results are supported by positive correlations between IMF and ACACA, FASN, LPL, CD36, FATP1 and negative correlation with PNPLA2 (Jeong et al., Reference Jeong, Kwon, Im, Seo and Baik2012).
In research carried out by Duckett et al. (Supplementary Material S1), changes in the gene expression of fatty acid transporters in the longissimus muscle of lambs supplemented with linolenic acid or palmitoleic acid compared with a control that received no supplemental oil were examined. Authors found that supplementation with C18:3 increased mRNA expression of CD36, FFAR2, FFAR4 and FFAR1 over the control (Figure 5). On the other hand, supplementation with C16:1 increased mRNA expression of FFAR1 and reduced mRNA expression of FFAR4 compared with control. Glucose transporter 4 expression was also down regulated in both C18:3 and C16:1 supplemented lambs. Chorner et al. (Reference Chorner, Barbeau, Castellani, Wright, Chabowski and Holloway2016) also reported that α-linolenic acid supplementation may result in increased intramuscular lipid content and whole body fat due to the greater rate of lipid transport (FATP and FAT/CD36). In a study of Oliveira et al. (Reference Oliveira, Chalfun-Junior, Chizzotti, Barreto, Coelho, Paiva, Coelho, Teixeira, Schoonmaker and Ladeira2014), the greater C18 fatty acid content in a soybean diet was responsible for greater expression of LPL and FABP4. Therefore, fatty acid profile of the diet may change the expression of membrane transporters, increasing fatty acid uptake. According to Jurie et al. (Reference Jurie, Cassar-Malek, Bonnet, Leroux, Bauchart, Boulesteix, Pethick and Hocquette2007), FABP4 may be used as a marker of intramuscular adipocytes.

Figure 5 Gene expression of fatty acid and glucose membrane transporters in longissimus muscle of lambs supplemented with linolenic acid (C18:3; 56%) or palmitoleic acid (C16:1; 56%); * P<0.05; ** P<0.01. CD36=fatty acid translocase; FATP=fatty acid transport protein; FFAR=free fatty acid receptors; FABP=fatty acid-binding protein; GLUT4=glucose transporter type 4.
These results indicate that the expression of CD36, GLUT4 and FFARs in skeletal muscle is altered with dietary fatty acid supplementation. CD36 and GLUT4 are both located within the cytosol of cells and move to the plasma membrane when activated to allow the entry of fatty acids or glucose, respectively, into the cell. During insulin resistance states, CD36 is believed to permanently move to the plasma membrane for greater uptake of fatty acids into the cell; whereas, GLUT4 becomes internalized resulting in lower glucose uptake by the cell (Kitessa and Abeywardena, Reference Kitessa and Abeywardena2016). These changes in CD36 and GLUT4 with insulin resistance can result in the intramyocellular uptake of fatty acids and deposition of lipid within the muscle. In Figure 5, Duckett et al.’s (Supplementary Material S1) results indicate that C18:3 supplementation up-regulated CD36 and down-regulated GLUT4 expression in skeletal muscle. Kitessa and Abeywardena (Reference Kitessa and Abeywardena2016) suggest that ceramide and/or diacylglycerol species may regulate these changes in the skeletal muscle and further research is underway to characterize these species in our samples.
In Duckett et al.’s study, expression of FASN and SCD1 also tended to be down regulated with C18:3 supplementation (Table 2), which would suggest that de novo lipogenesis was not stimulated. In this regard, Choi et al. (Reference Choi, Park, Johnson, Chung, Choi, Kim, Kim and Smith2015) reported that oleic acid and linoleic acid down-regulated SCD1 expression in bovine subcutaneous and intramuscular preadipocytes. Also, feeding palm oil (high in oleic acid) or soybean oil (high in PUFA), to growing cattle, down-regulated SCD1 expression in subcutaneous adipose tissue (Choi et al., Reference Choi, Park, Choi, Li, Kim, Kim, Jeong, Johnson, Zan and Smith2016). Therefore, the down-regulation of SCD1 expression by fatty acids appears to be related to monounsaturated fatty acids (MUFA) and PUFA in general. In addition, SREBF1 and SCAP expression, the genes responsible to encode the sterol regulatory element binding protein-1c (SREBP-1c) and SREBP cleavage-activating protein which are regulators of lipid synthesis in animal cells (Matsuda et al., Reference Matsuda, Korn, Hammer, Moon, Komuro, Horton, Goldstein, Brown and Shimomura2001), were not altered with oil supplementation (Table 2).
Table 2 Fold-changes in relative gene expression of lipogenic and transcription factors or activators in longissimus muscle of lambs supplemented with α-linolenic acid or palmitoleic acid

ACACA=acetyl-CoA carboxylase α; FASN=fatty acid synthase; SCD1=stearoyl-CoA desaturase 1; SCAP=SREBF Chaperone; SREBF1=sterol regulatory element binding transcription factor 1; PGC1A=PPARG coactivator 1α.
*P<0.10.
Overall, these results indicate that fatty acid transporters may play an important role in the uptake of fatty acids into the cell for intramuscular fat deposition. When we supplement oils rich in certain types of fatty acids, these fatty acids are increased in the skeletal muscle and may alter metabolism depending on fatty acid type. In Duckett study, C18:3 supplementation increased linolenic acid accumulation and intramuscular fat deposition; whereas, C16:1 supplementation increased palmitoleic acid accumulation in the muscle but did not alter intramuscular fat content. These results are consistent with previous research examining exogenous palmitoleic acid and intramuscular fat reduction in obese sheep (Duckett et al., Reference Duckett, Volpi-Lagreca, Alende and Long2014). Further exploration into other lipid intermediates through lipidomics will be useful in investigating the potential mechanism of action of specific fatty acids that are supplied in the diet and how they alter overall lipid accumulation and metabolism.
It is well established that diets with high energy are also responsible for the production of meat with greater marbling. However, Teixeira et al. (Reference Teixeira, Oliveira, Chizzotti, Chalfun-Junior, Coelho, Gionbelli, Paiva, Carvalho and Ladeira2017) reported that bulls fed a diet with whole shelled corn and no forage did not increase intramuscular fat because this diet reduced rumen pH and increased t10,c12-C18:2, which reduced SREBF1 expression (Figure 6). In this sense, SREBP-1c is an important transcription factor regulating lipogenesis, having positive correlation with expression of ACACA (Oliveira et al., Reference Oliveira, Chalfun-Junior, Chizzotti, Barreto, Coelho, Paiva, Coelho, Teixeira, Schoonmaker and Ladeira2014) and SCD1 (Waters et al., Reference Waters, Kelly, O’Boyle, Moloney and Kenny2009).

Figure 6 Effect of rumen pH on t10,c12-C18:2, sterol regulatory element binding transcription factor 1 (SREBF1) expression and lipogenesis in bovine muscle. PUFA=polyunsaturated fatty acids; FA=fatty acids; ACACA=acetyl-CoA carboxylase A; FASN=fatty acid synthase; SCD1=stearoyl-CoA desaturase 1; TAG=triacyl-glyceride.
In other studies, Cooke et al. (Reference Cooke, Bohnert, Moriel, Hess and Mills2011) and Mangrum et al. (Reference Mangrum, Tuttle, Duckett, Sell, Krehbiel and Long2016) reported that animals receiving rumen undegradable unsaturated fatty acid, that contained a high percentage of oleic and linoleic acid, had greater intramuscular fat and marbling scores. On the other hand, Schoonmaker et al. (Reference Schoonmaker, Trenkle and Beitz2010) showed that animals fed wet distillers grains (rich in PUFA) had decreased marbling score. Therefore, more studies are necessary to understand the effects of specific fatty acids and their isomers on lipogenesis, fatty acid uptake and lipolysis which will affect meat intramuscular fat.
Gene expression and fatty acid profile
As mentioned before, there is interest in the manipulation of meat fatty acid profile, due to their possible beneficial and or detrimental action on human health. Conjugated linoleic acid c9,t11-C18:2 has been suggested to be an anticarcinogenic and hypolipidemic and reduces the risk of diabetes (Vahmani et al., Reference Vahmani, Mapiye, Prieto, Rolland, McAllister, Aalhus and Dugan2015) and some saturated fatty acids (SFA) also increase high-density lipoprotein (HDL)-cholesterol (Kris-Etherton et al.,Reference Kris-Etherton, Allison, Denke, Dietschy, Emken and Nicolosi1995). Furthermore, PUFA participates in several biological processes relevant to human health (Berton et al., Reference Berton, Fonseca, Gimenez, Utembergue, Cesar, Coutinho, de Lemos, Aboujaoude, Pereira, Silva, Stafuzza, Feitosa, HLJ, Olivieri, Peripolli, Tonussi, Gordo, Espigolan, Ferrinho, Mueller, de Albuquerque, de Oliveira, Duckett and Baldi2016). On the contrary, lauric, myristic and palmitic fatty acids are hypercholesterolemic because of the observed rise in low-density lipoprotein content in the blood (Wood et al., Reference Wood, Richardson, Nute, Fisher, Campo, Kasapidou, Sheard and Enser2003).
Meat fatty acid profile also plays an important role in the oxidative stability during the cooking process, which affects beef tenderness, flavor and juiciness. Age of animal, breed type and diet are the major factors influencing fatty acid composition of meat (Smith et al., Reference Smith, Gill, Lunt and Brooks2009a). In ruminant species, meat has a greater variety of fatty acids compared with meat from non-ruminant species due to microbial biohydrogenation in the rumen (Vahmani et al., Reference Vahmani, Mapiye, Prieto, Rolland, McAllister, Aalhus and Dugan2015).
Associated with the factors above, muscle fatty acid composition may control or be controlled by transcription factors which will affect expression of genes involved in the lipid metabolism (Table 3). According to Jump (Reference Jump2008), fatty acids act on the nucleus by binding to and regulating the activity of specific nuclear receptors or transcription factors, thus playing a central role regulating expression of genes involved in fatty acid uptake by muscle cells.
Table 3 Fatty acids effects on expression of genes in ruminants associated with lipid metabolism

PUFA=polyunsaturated fatty acids; PPARA=peroxisome proliferator-activated receptor α; PPARG=peroxisome proliferator-activated receptor gamma; SREBF1=sterol regulatory element-binding protein-1c; SCD1=stearoyl-CoA desaturase; ACACA=acetyl-CoA carboxylase α; FASN=fatty acid synthase; LPL=lipoprotein lipase; FABP4=fatty acid-binding protein 4; MUFA=monounsaturated fatty acids.
Interactions between nutrients from the diet and expression of genes involved in lipid metabolism have many possibilities regarding the deposition of fatty acids in the tissue. Diets rich in PUFA are important for the regulation of SCD1 expression in the muscle of beef cattle, altering fatty acid profile in the beef (Waters et al., Reference Waters, Kelly, O’Boyle, Moloney and Kenny2009). Furthermore, Herdmann et al. (Reference Herdmann, Nuernberg, Martin, Nuernberg and Doran2010) reported that animals fed greater n-3 PUFA content on the diet have less SCD1 expression and thus, less CLA c9,t11-C18:2 and oleic acid on muscle. Ladeira et al. (Reference Ladeira, Santarosa, Chizzotti, Ramos, Machado Neto, Oliveira, Carvalho, Lopes and Ribeiro2014) also demonstrated an increase of CLA concentration in the muscle of animals fed soybean compared with those fed rumen-protected fat, and this result may be due to the greater gene expression of SCD1 in the muscle (Oliveira et al., Reference Oliveira, Chalfun-Junior, Chizzotti, Barreto, Coelho, Paiva, Coelho, Teixeira, Schoonmaker and Ladeira2014).
Other researchers reported that feeding of diets high in saturated fatty acids to pigs (Smith et al., Reference Smith, Mersmann, Smith and Britain1999), high stearic acids diets in mice (Sampath et al., Reference Sampath, Miyazaki, Dobrzyn and Ntambi2007) or high energy grain diets to finishing cattle (Duckett et al., Reference Duckett, Pratt and Pavan2009) significantly up-regulates SCD1 expression in adipose tissues and increases adipose deposition.
Diets that increase t10,c12-C18:2 may be responsible for reducing SREBF1 expression, which consequently reduces fat biosynthesis by reducing the gene expression and activity of key enzymes (Obsen et al., Reference Obsen, Faergeman, Chung, Martinez, Gobern, Loreau, Wabitsch, Mandrup and McIntosh2012). Furthermore, t10,c12-C18:2 either reduces directly SCD1 expression, which consequently reduces MUFA synthesis (Smith et al., Reference Smith, Kawachi, Choi, Choi, Wu and Sawyer2009b). Usually, high-concentrate or high ether extract diets result in changes in rumen biohydrogenation increasing production of t10,c12-C18:2 and decreasing c9,t11-C18:2. Therefore, it is necessary to control the rumen environment in order to avoid the excessive production of t10,c12-C18:2, which will reduce intramuscular adipocyte differentiation.
Previous reports showed that high levels of PUFA suppress SREBF1 content by inhibiting proteolytic activation and decreasing mRNA stability (Nakamura et al., Reference Nakamura, Yudell and Loor2014). For example, long-chain n-3 PUFA such as docosahexaenoic and EPAs are nuclear suppressors of SREBF1 via inhibition of transcription and by increasing mRNA turnover (Rodríguez-Cruz and Serna, Reference Rodríguez-Cruz and Serna2017). This result is responsible for decreased lipogenesis, because of decreasing expression of ACACA and FASN in muscle, thus reducing concentration of products from de novo fatty acid synthesis (Hiller et al., Reference Hiller, Herdmann and Nuernberg2011). Other nutrients may be responsible to affect the expression of transcription factors. For example, González-Calvo et al. (Reference González-Calvo, Joy, Alberti, Ripoll, Molino, Serrano and Calvo2014) reported that vitamin E supplementation upregulated SREBF1 expression in the longissimus thoracis of lambs.
Beyond SREBP-1c, another transcription factor stands out in lipid metabolism, the PPARs (Ladeira et al., Reference Ladeira, Schoonmaker, Gionbelli, Dias, Gionbelli, Carvalho and Teixeira2016). The PPARs are a family of nuclear receptors that bind to fatty acids and perform significant functions in the regulation of nutrient metabolism and energy homeostasis (Lemay and Hwang, Reference Lemay and Hwang2006). Peroxisome proliferator-activated receptor isoforms act as heterodimers with RXR, and both bind to a specific DNA sequence in the promoter region of the gene, inducing or repressing its expression (Poulsen et al., Reference Poulsen, Siersbæk and Mandrup2012). Peroxisome proliferator-activated receptors are activated by a large variety of fatty acids that are binding the ligand dependent activation function present in PPAR structure, and they thereby serve as major transcriptional sensors of fatty acids (Poulsen et al., Reference Poulsen, Siersbæk and Mandrup2012).
In general, depending on the fatty acid, PUFA can activate in different intensity PPAR isotypes (Bionaz et al., Reference Bionaz, Chen, Khan and Loor2013). Activation of PPARG by PUFA (mainly DHA and EPA) results in a positive functional response in tumor cells (Grygiel-Górniak, Reference Grygiel-Górniak2014). Likewise, PUFA can bind to PPARα, at physiologic concentrations, and expression of several genes involved in fatty acid metabolism including their transport, synthesis and β oxidation (Rodríguez-Cruz and Serna, Reference Rodríguez-Cruz and Serna2017). According to Wolfrum et al. (Reference Wolfrum, Borrmann, Börchers and Spener2001), PUFA are more potent agonists than SFA to PPARA and, in general, in non-ruminant species that have been studied, PPARA has greater affinity for unsaturated fatty acids than for saturated fatty acids (Bionaz et al., Reference Bionaz, Chen, Khan and Loor2013).
Final considerations
To date, the knowledge shows that gene expression is a metabolic factor affecting marbling and fatty acid profile in ruminant meat. However, it is necessary to understand better this complex mechanism discovering how specific fatty acids act and who are the transcription factors of the transcription factors. The possible effects of fatty acids on DNA and histones or their chemical modifications are other important mechanism to be studied.
Acknowledgements
The authors thank the Federal University of Lavras, Brazilian agencies Capes, CNPq and Fapemig, National Institute of Science and Technology in Animal Science (INCT-Ciência Animal), North Dakota State University, Purdue University and Clemson University.
Declaration of interest
There is no conflict of interest.
Ethics statement
Every cited study was carried out according to the ethical guidelines adopted by the international ethics committees on animal use.
Software and data repository resources
None of the data were deposited in an official repository.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S1751731118001933