It is well established that a diet rich in fruit and vegetables can play a role in preventing cancer as well as other chronic degenerative disorders, such as CVDReference Kris-Etherton, Hecker, Bonanome, Coval, Binkoski, Hilpert, Griel and Etherton1–Reference Pomerleau, Lock and McKee5.
It is widely accepted and supported by many epidemiological studies that phytochemicals contained in vegetables and fruits, including flavonoids and other types of polyphenolic compounds, show complementary and overlapping mechanisms of action, such as antioxidant effects, stimulation of the immune system, antibacterial and antiviral effects, modulation of hormone and detoxification enzymes metabolism, regulation of gene expression in cell proliferation and apoptosisReference Dragsted, Strube and Larsen6–Reference Karou, Dicko, Simpore and Traore11. Nevertheless, the few studies on the DNA repair genes OGG1 and ERCC1 reported no changes in gene expression levels in vivo after a fruit and vegetable or antioxidant-rich dietReference Collins, Harrington, Drew and Melvin12, Reference Moller, Vogel, Pedersen, Dragsted, Sandstrom and Loft13.
Epidemiological studies have suggested a protective role of dietary flavonoids against CHD and possibly cancerReference Janssen, Mensik, Cox, Harryvan, Hovenier, Hollman and Katan14–Reference Ren, Qiao, Wang, Zhu and Zhang17. In two prospective large cohort studies, a significant inverse association of flavonoid/flavone intake with lung cancer risk was foundReference Knekt, Jarvinen, Seppanen, Hellovaara, Teppo, Pukkala and Aromaa18, Reference Hirvonen, Virtamo, Korhonen, Albanes and Pietinen19, while no clear effect of flavonoid intake and risk of bladder cancer was reported.
Although the effect of flavonoids on gene expression has been little studied in human subjects, some studies suggested a flavonoid-mediated modulation of the expression of the CYP family genes, whose products are involved in carcinogen activation and detoxificationReference Ciolino and Yeh20–Reference Murray22. Exposure to polycyclic aromatic hydrocarbons contained in cigarette smoke causes the induction of the CYP1A gene family, regulated by the cytosolic protein aryl hydrocarbon receptor (AHR). Flavonoids can either inhibit or activate human CYP depending upon their structures, concentration and experimental conditionsReference Murray22, Reference Moon, Wang and Morris23. An inhibition of CYP1A1 expression in HepG2 cells by resveratrol was previously reportedReference Ciolino and Yeh20, while two dietary flavonoids showed different effects on the modulation of CYP1A1 expression in MCF-7 human breast cancer cellsReference Ciolino, Daschner and Yeh24.
Tobacco smoking is an important risk factor for several types of human cancer including bladder cancer25–Reference Schollnberger, Manuguerra, Bijwaard, Boshuizen, Altenburg, Rispens, Brugmans and Vineis27. Several compounds contained in cigarette smoke can damage DNA directly (i.e. polycyclic aromatic hydrocarbons and nitrosamines) or through the generation of reactive oxygen speciesReference Nair, Ohshima, Nair and Bartsch28, Reference Phillips29. Oxidants, either present in cigarette smoke and/or formed as a metabolic by-product in the lung and bladder cells of smokers, may trigger oxidative damage to DNA and cellular components, contributing to carcinogenesis. Smoke carcinogens and free radicals generate several different types of DNA damageReference Godschalk, Nair, van Schooten, Risch, Drings, Kayser, Dienemann and Bartsch30.
Within the framework of a larger randomized controlled trial to test the ability of flavonoids to increase urinary anti-mutagenicity and inhibit the formation of DNA damage in the bladder exfoliated cells in a group of healthy male heavy smokersReference Malaveille, Hautefeuille, Pignatelli, Talaska, Vineis and Bartsch31, Reference Malaveille, Fiorini, Bianchini, Davico, Bertinetti, Allegro, Hautefeuille, Sacerdote and Vineis32, we hypothesize that smokers ingesting dietary phenolics might be partially protected against the harmful effects of tobacco compounds. In a subgroup, we examined whether administration of foods rich in flavonoids modulated some DNA repair and metabolic gene expression.
All the DNA repair genes investigated could be potentially involved in repairing DNA damage due to tobacco smoke compounds, which induce bulky DNA adduct formation, base oxidation and give other base alterations and single and double strand breaks.
DNA repair genes were selected because of: (i) the possible role in repairing oxidative DNA damage (base excision repair genes: APE1; OGG1; XRCC1); (ii) the involvement in repairing other DNA adducts of carcinogens whose reactivity is possibly modulated by the antioxidant activity exerted by the flavonoids (nucleotide excision repair genes: ERCC1; ERCC2; ERCC4; XPA; XPC; MGMT); (iii) the repair of double strand breaks (e.g. XRCC3 gene), which was also observed after flavonoid treatment in in vitro studiesReference Strick, Strissel, Borgers, Smith and Rowley33.
Materials and methods
Subjects
In the overall dietary supplementation studyReference Malaveille, Hautefeuille, Pignatelli, Talaska, Vineis and Bartsch31, Reference Malaveille, Fiorini, Bianchini, Davico, Bertinetti, Allegro, Hautefeuille, Sacerdote and Vineis32, ninety healthy heavy smokers of air-cured tobacco were recruited among Italian blood donors. Trial participants were all males, aged 35–70 years, resident in the Torino metropolitan area (northern Italy), healthy on the basis of a medical questionnaire and had normal values for urine and blood parameters (cholesterol, TAG, glycaemia, blood pressure, etc.), as tested in the context of selection for blood donation. All the volunteers gave written informed consent according to ethical standard requirements.
In the context of the randomized controlled trial described earlier, we nested a sub-study on DNA repair and metabolic gene expression among nine subjects (GE subgroup) recruited within the ‘flavonoid supplement’ group, one of the three arms of the trial (see below).
Study design
Volunteers were randomly assigned to three groups, corresponding to three different diets: (i) normal isoenergetic diet (with an adequate administration of fruit and vegetables; (ii) rich in flavonoids, but not based on supplementation; (iii) based on supplementation of flavonoids in the form of green tea, bilberry juice and soya products. A 2-week diet period proved to be suitable to reveal changes in biomarkers of fruit and vegetable intakeReference Brevik, Rasmussen, Drevon and Andersen34. In the present study, the volunteers followed the prescribed diet for 1 month in order to guarantee a complete turnover of the bladder cells used for the DNA adduct analysis.
Within the flavonoid-supplementation group, eighteen subjects, hereafter identified as ‘supplement’ group, completed the dietary protocols and underwent a detailed analysis of dietary information as extracted from diaries. For nine subjects out of the eighteen, blood samples were collected according to proper protocols for RNA extraction, and underwent gene expression analysis (‘GE subgroup’).
Methods
Flavonoid-rich foods were chosen by an expert dietitian. A recipe booklet was given to each participant as a reference and a professional chef taught all the participants how to prepare recipes with the selected foods in order to limit differences in cooking methods and food choice, making the dietary regimen comparable within each group. For each subject, a trained interviewer recorded dietary and smoking habits by a direct interview at enrolment and after 1 month of the diet. Adherence to dietary protocols was assessed through telephone interviews by using 24-h dietary recalls (every 2 d) as well as weekly domestic visits by a dietitian throughout the 4 week intervention.
Average intakes of flavonoids and some micronutrients (vitamins B1, B2, B3, B12, C, A, D, E, folates, Se) were estimated on the basis of daily dietary records.
Urine was collected weekly, as reported in Malaveille et al. Reference Malaveille, Fiorini, Bianchini, Davico, Bertinetti, Allegro, Hautefeuille, Sacerdote and Vineis32 Briefly, urine samples were collected in polyethylene bottles on Friday between 16.00 and 22.00 hours, kept at 4°C in the volunteers' homes and collected weekly by a dietitian. Exfoliated bladder cells were immediately filtered from a part of each sample, while the remaining urine was kept at − 30°C and analysed within 6 months for anti-mutagenicity and chemical content (phenolics, etc).
The amount of total urinary phenolics was measured in a dimethyl sulphoxide (DMSO) solution of urinary extracts. The glucuronides and sulfates contained in a 24 h urine aliquot containing 3 mg creatinine were enzymatically deconjugated and the amount of phenolics was measured by spectrophotometric analysisReference Malaveille, Hautefeuille, Pignatelli, Talaska, Vineis and Bartsch35.
Urine anti-mutagenicity was measured as a decrease in Salmonella Typhimurium revertants/total volume of collected urine equivalent as described in Malaveille et al. Reference Malaveille, Fiorini, Bianchini, Davico, Bertinetti, Allegro, Hautefeuille, Sacerdote and Vineis32.
DNA damage in exfoliated bladder cells was measured as relative adduct labelling × 10− 9 by 32P post labelling techniqueReference Talaska, Schamer, Skipper, Tannenbaum, Caporaso, Unruh, Kadlubar, Bartsch, Malaveille and Vineis36 at three time points: baseline; week 2; week 4 of diet.
Blood collection for RNA extraction was done twice (baseline, week 4). Peripheral blood (2 ml) was directly collected in PAXgene Blood RNA Tubes (PreAnalitiX/QIAGEN, Valencia, CA, USA) and total RNA was isolated using a column affinity procedure (PAXgene Blood RNA Kit; QIAGEN) according to the manufacturer's instructions. A DNA nuclease treatment step was included to prevent genomic DNA carryover. The random primed reverse transcription of 1 μg total RNA to single stranded cDNA was achieved by High-Capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA, USA) according to the manufacturer's instructions, adding 1 U/μl final concentration of RNAse inhibitor (Applied Biosystems).
Primers and probes for the target genes (APEX, ERCC1, ERCC2, ERCC4, MGMT, OGG1, XPA, XPC, XRCC1, XRCC3, AHR, CYP1A1) were purchased as pre-made assays (Assay on Demand) from Applied Biosystems and analyses carried out according to the producer's instructions. As a reference gene, we used β-actin AoD (Hs99999903_m1; Applied Biosystems), which in a preliminary validation assay proved to be suitable for the relative quantitation of mRNA in our samples. Real time PCR analyses were carried out on an ABI Prism 7000 Sequence Detection System (Applied Biosystems). Three replicates for each sample were analysed and a no-template control for each assay was included in all the amplification plates.
Real-time PCR data were collected and analysed by the ABI Prism 7000 SDS Software (Applied Biosystems). A relative quantitation analysis (ΔΔCt method) was performed as described37.
Statistical analysis
Differences in the levels of all the variables analysed before and after diet (considering week 2 and 4 whenever possible) were tested by parametric (t test) and non-parametric methods for paired samples (Wilcoxon and Friedman for two or more groups, respectively). Correlations between variables were tested by Pearson correlation test. All the analyses were performed by the SPSS 13·0 package for personal computers (SPSS Inc., Chicago, IL, USA) and values of P ≤ 0·05 were considered statistically significant.
Results
The mean age of the subjects was: supplement group 52 (sd 5) years (range 43–63 years); GE subgroup 51 (sd 7) years (range 43–63 years).
The diet-related variation in flavonoid intake (μg) as measured at three different time points (baseline, week 2 and week 4) significantly changed as a consequence of the diet (P < 0·001) as shown in Table 1, in agreement with that reported for the overall trialReference Malaveille, Fiorini, Bianchini, Davico, Bertinetti, Allegro, Hautefeuille, Sacerdote and Vineis32.
Table 1 Diet-related variation in flavonoid intake, urinary phenolics and urinary anti-mutagenicity at different time points¶ (Values are means and standard deviations)
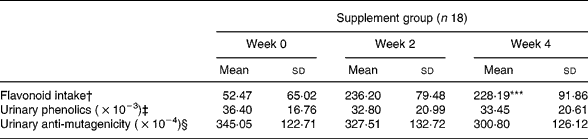
Significant diet related variation (Friedman test): ***P < 0·001.
† Estimated from dietary recordings.
‡ μg/total volume of collected urine equivalent, as described in Malaveille et al. Reference Malaveille, Fiorini, Bianchini, Davico, Bertinetti, Allegro, Hautefeuille, Sacerdote and Vineis32
§ Decrease in revertants/total volume of collected urine equivalent (creatinine corrected), as described in Malaveille et al. Reference Malaveille, Fiorini, Bianchini, Davico, Bertinetti, Allegro, Hautefeuille, Sacerdote and Vineis32
¶ For details of subjects and procedures, see Materials and methods.
Urinary phenolic content and anti-mutagenicity as evaluated at the same three time points did not change during the diet period (Table 1).
The whole group and the GE group showed virtually no difference; thus, results are reported in Table 1 for the whole group.
Flavonoid intake during the diet period showed no correlation with either urinary phenolic levels or urinary anti-mutagenicity.
Urinary anti-mutagenicity showed a significant positive correlation with urinary phenolics during the diet period both in the ‘supplement’ (n 18, week 0: Pearson correlation coefficient (PCC) 0·743, P < 0·001; week 2: PCC 0·745, P < 0·001; week 4: PCC 0·724, P = 0·001) and GE subgroup (n 9, week 0: PCC 0·607, P = 0·083; week 2: PCC 0·774, P = 0·014; week 4: PCC 0·760, P = 0·017).
No significant difference was found in the mean number/d of cigarettes smoked pre- (baseline) and post-diet both in the overall supplement group (n 18; baseline: 21·44 (sd 7·04); week 4: 20·50 (sd 5·85); NS) and in the GE subgroup (n 9; baseline: 20·44 (sd 4·42); week 4: 20·33 (sd 3·08); NS).
During the diet period, a negative correlation between smoking and urine anti-mutagenicity was shown for the whole group, statistically significant when considering the GE subgroup (PCC − 0·693, P = 0·038).
No difference in the mean levels of DNA adducts in exfoliated bladder cells was found during the diet period both in the overall (n 18; baseline: 26·8 (sd 22·2) × 10− 9; week 2: 27·8 (sd 50·3) × 10− 9; week 4: 25·7 (sd 21·2) × 10− 9; NS) and in the GE group (n 9; baseline: 22·0 (sd 23·9) × 10− 9; week 2: 55·4 (sd 78·4) × 10− 9; week 4: 21·9 (sd 29·7) × 10− 9; NS), as computed by both Friedman test and Wilcoxon paired test.
Gene expression analysis
Considering the GE subgroup (n 9), the mean ratio of gene expression level for each of the twelve target genes (pre- v. post-diet) ranged from a 0·813-fold mean variation for AHR gene to 1·71-fold mean variation for XPA (β-actin 1; APEX 1·187; ERCC1 1·113; ERCC2 1·132; ERCC4 1·014; MGMT 1·316; OGG1 1·095; XPA 1·711; XPC 0·971; XRCC1 1·125; XRCC3 1·271; AHR 0·813; CYP1A1 1·181).
The majority of the genes seem to undergo a generalized up regulation after the diet period, statistically significant for XRCC3 gene (P = 0·038 Wilcoxon paired test; P = 0·036 paired t test), AHR gene (P = 0·038 Wilcoxon paired test; P = 0·026 paired t test) and with borderline significance for MGMT gene (P = 0·051 Wilcoxon paired test; P = 0·107 paired t test).
A positive correlation (Pearson correlation test) between smoking and gene expression levels during the month of diet, statistically significant for ERCC1 (P = 0·037) and ERCC2 (P = 0·045), is shown for all the genes except CYP1A1, which shows a negative though not significant correlation with smoking.
We measured the correlation between the variation of expression levels for the twelve genes at the end of the diet period, which all positively correlate (most of them significantly), except the CYP1A1 gene, which shows a negative correlation (although not significant) with all the other genes (see supplementary table, available online).
Food and gene expression
Food consumption at baseline and during the month of diet was recorded and categorized according to nutritional profiles (e.g. cereals, meat, vegetables, fruit, dairy, etc.), specifying as separate categories flavonoid-rich foods (soyabeans and derivatives, onions, red wine, green tea and bilberry juice). Micronutrient consumption (folates, vitamins, minerals) as well as flavonoid intake was estimated from dietary records.
Estimated mean dietary flavonoid intake negatively correlates with variations in expression levels of all the examined genes except CYP1A1 (correlation statistically significant for ERCC2, P = 0·049). Total folates and vitamin B12 levels positively correlate with CYP1A1 gene expression variations (P = 0·006 and P = 0·009 respectively).
Comparing the food intake at baseline and the mean food intake during the diet period (Wilcoxon paired test), we found a significant diet-related change for some food categories both in the overall supplement group and in the GE subgroup as presented in Table 2. However, no statistical significance was found after Bonferroni correction for multiple comparisons.
Table 2 Dietary variations during the trial for selected food categories§ (Values are means and standard deviations)
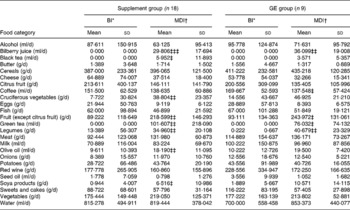
* Baseline intake; food intake the day before the first day of diet.
† Mean dietary intake: food intake abstracted from self-administered daily diaries and averaged over 1 month.
Significant dietary variation (Wilcoxon Z test): ‡P ≤ 0·05; ‡‡P ≤ 0·01; ‡‡‡P ≤ 0·001 – not significant after Bonferroni correction for multiple test.
§ For details of subjects and procedures, see Materials and methods.
According to the present results, some food categories seem to affect gene expression levels in the GE subgroup, shown in Table 3. A non-significant positive correlation between citrus fruits, cruciferous vegetables, red wine and fish intakes and the expression levels of the majority of the genes examined was found, while fruits and vegetables showed a generalized non-significant negative correlation (P>0·05). Flavonoid-rich foods, such as soya products, onions, green tea and bilberry juice, negatively correlate with the expression of all the genes, except CYP1A1 (with soya, onions and green tea) and AHR (with bilberry juice) (P>0·05).
Table 3 Gene expression variations v. food intake variation§

Pearson Correlation Coefficient: *P ≤ 0·05; **P ≤ 0·01.
† Gene expression variation values are reported for quick reference: 1·000, no variation in gene expression after the diet; >1·000, up regulation of gene expression after the diet; < 1·000, down regulation of gene expression after the diet.
Significant gene expression variations (Wilcoxon test) ‡P < 0·05 − not significant after Bonferroni correction for multiple tests.
§ For details of subjects and procedures, see Materials and methods.
To investigate a short-term effect of specific food consumption on gene expression, we compared in the GE subgroup the food intake the day before baseline blood sampling (baseline pre sampling) and the food intake the day before blood sampling after 4 weeks of diet (week 4 pre-sampling; 4-PS). The variation in mean fruit consumption proved to be statistically significant (baseline pre-sampling 93·11 (sd 134·36) g/d v. 4-PS 347·00 (sd 230·44) g/d, P = 0·012), while mean green tea and vegetable intakes were increased, but not significantly (baseline pre-sampling 0·00 ml/d v. 4-PS 50·00 (sd 75·00) ml/d, P = 0·083 and baseline pre-sampling 177·22 (sd 163·14) g/d v. 4-PS 230·44 (sd 113·61) g/d, P = 0·066, respectively).
Considering food categories, a negative correlation between gene expression variations and cheese intake was found for all the genes, statistically significant for ERCC1 (P = 0·021), ERCC2 (P = 0·033), MGMT (P = 0·028) and OGG1 (P = 0·029). Seed oil intake also negatively correlated with gene expression variations in all the genes, significantly for ERCC1 (P = 0·011), ERCC2 (P = 0·024) and OGG1 (P = 0·046), while showing a positive though not significant correlation with CYP1A1 gene expression variations.
CYP1A1 expression positively correlated with coffee intake (P = 0·037), while negatively correlating with alcohol intake (P = 0·047).
Flavonoid intake on 4-PSd negatively correlated with variations in expression levels of all the examined genes and the correlation was statistically significant for ERCC2 (P = 0·049).
Discussion
In the context of a randomized dietary intervention trial to assess the ability of flavonoids to increase urinary anti-mutagenicity and inhibit DNA damage in male heavy smokers, we conducted a pilot study aiming at investigating a possible effect of a flavonoid-rich diet on the expression levels of twelve genes: ten DNA repair genes belonging to different repair pathways (nucleotide excision repair, base excision repair, double strand breaks repair) and two metabolic genes (CYP1A1 and AHR). Flavonoids can be hypothesized to modulate the expression levels of the afore-mentioned genes according to two distinct mechanisms: (i) a down regulation of DNA repair genes could be expected if the antioxidant properties of flavonoids contribute to reduce the burden of reactive oxygen species that can damage DNA; or (ii) a modulation (up regulation or down regulation) of the expression levels could be expected if the diet-derived flavonoids exert a direct effect on gene expression.
The trial demonstrated the expected modification in diet-related flavonoid intake as assessed from dietary diaries, achieved through dedicated instructions given to volunteers by a chef. Although some authors describe urinary excretion of dietary-derived polyphenols and flavonoid aglycones or flavonoid conjugated in human subjects as biomarkers of dietary intakeReference de Vries, Hollman, Meyboom, Buysman, Zock, van Staveren and Katan38, Reference Bourne and Rice-Evance39, the present data do not support a substantial change in urinary phenolics as a consequence of the trial.
Factors influencing bioavailability include the chemical properties of the polyphenols, deconjugation/reconjugation in the intestines, intestinal absorption and enzymes available for metabolismReference Yang, Landau, Huang and Newmark40.
Nevertheless, the strong correlation between urinary phenolic levels, not affected by dietary changes, and urine anti-mutagenicity against 2-amino-1-methyl-6-phenylimidazo[4,5-6]pyridine (PhIP)-induced mutations in vitro, which was shown and discussed previouslyReference Malaveille, Fiorini, Bianchini, Davico, Bertinetti, Allegro, Hautefeuille, Sacerdote and Vineis32, supports a protective role of polyphenols and/or their bacterial metabolites against mutagens, such as heterocyclic amines, contained in tobacco smoke.
Although several studies report a beneficial effect of flavonoids against chronic degenerative diseases and a protective role against cancer through their antioxidant properties, some authors describe a detrimental effect of some dietary bioflavonoids, which were shown to cause a high rate of DNA breaks in the MML gene, whose translocations are involved in the onset of infant leukaemiaReference Strick, Strissel, Borgers, Smith and Rowley33. Interestingly, in our sample of subjects the gene XRCC3 belonging to the double strand break repair pathway is significantly up regulated. XRCC3 participates in DNA double-strand break and cross-link repair through homologous recombination and contributes, as other RAD51-related proteins, to the maintenance of chromosomal stabilityReference Tebbs, Zhao, Tucker, Scheerer, Siciliano, Hwang, Liu, Legerski and Thompson41–Reference Brenneman, Weiss, Nickoloff and Chen43.
A high frequency of chromosomal instability or aberrations induced by tobacco-related carcinogens in bladder cancer is to be expected. Indeed, a number of studies showed aberrations and allelic losses in different chromosomesReference Czerniak, Li, Chaturvedi, Ro, Johnston, Hodges and Benedict44, Reference Hartmann, Rosner, Schlake, Dietmaier, Zaak, Hofstaedter and Knuechel45, so bladder cancer can be considered one of the cancers prone to chromosomal instability. In this context, also, certain flavonoids could apparently produce similar damage, inducing in particular double strand breaks repair through the homologous recombination pathway.
Even though AHR expression seems to be significantly reduced in our sample after the diet period, we found an increase in the expression levels of CYP1A1, whose induction is known to be mediated by polycyclic aromatic hydrocarbon activated AHR.
It was shown that lack of AHR-mediated induction of CYP1A1 conferred protection against benzo[a]pyrene (B[a]P) induced carcinogenesis in AHR knock-out miceReference Jang, Cai and Udeani46, Reference Dertinger, Silverstone and Gasiewicz47, and that a resveratrol-mediated inhibition of CYP1A1 and CYP1B1 expression can reduce the B[a]P adduct formation in human bronchial epithelial cellsReference Berge, Øvrebø, Botnen, Hewer, Phillips, Haugen and Mollerup48.
Given this, an up regulation of CYP1A1 can be supposed to be detrimental. Nevertheless, a flavonoid mediated stimulation/induction of CYP family genes can activate cellular defence mechanisms, allowing a more effective response to future stressors, according to the principles of hormesisReference Bukowski and Lewis49, Reference Kitchin50. It is accepted today that flavonoids can exert their protective effect through a stressor mechanism, predisposing the cells to better respond to damaging agents. Depending on doses and chemical properties, some flavonoids can show both antioxidant and pro-oxidant activityReference Kessler, Ubeaud and Jung51. Quercetin, a flavonoid contained in high doses in onions, can be activated to DNA reactive species, with the formation of alkylating DNA-reactive intermediates and proved to be mutagenetic, at least in vitro, through several mechanisms reviewed in Rietjens et al. Reference Rietjens, Boersma, van der Woude, Jeurissen, Schutte and Alink52. An in vitro AHR mediated induction of CYP1A1 stimulated by quercetin, was also describedReference Ciolino, Daschner and Yeh24. In the present sample, no relationship was found between onion intake and gene expression, suggesting that dietary intakes of onion-derived quercetin may not be enough to elicit a detectable induction of CYP family. Moreover, adduct levels did not significantly change after the diet, indicating no detrimental effect of onion intake on quercetin-induced AHR-mediated carcinogenesis in human subjects, at least in dietary amounts.
However, a possible limitation of the DNA adducts analysis in the present study should be taken into account. Even though exfoliated bladder cells proved to be suitable for biomarker analysisReference Degtyar, Neulander, Zirkin, Yusim, Douvdevani, Mermershtain, Kaneti and Manor53–Reference Dörrenhaus, Muller and Roos58 and the DNA adduct detection in exfoliated bladder cells by Reference Malaveille, Fiorini, Bianchini, Davico, Bertinetti, Allegro, Hautefeuille, Sacerdote and Vineis32P post-labelling is a commonly used analysis whose feasibility is well provenReference Bartsch, Castegnaro, Camus, Schouft, Geneste, Rojas and Alexandrov59–Reference Talaska, Al-Zoughool and Malaveille63, we found no change in exfoliated bladder cell DNA adduct levels after the diet. In addition to advocating no effect of dietary flavonoid intake on the DNA adduction state, this may also be explained considering that exfoliated cells obtained in a urine sample are terminally differentiated and as such likely have much lower metabolic activity than the stem cells from which they arose, so DNA damage is less likely to be repaired.
Even in the context of a generalized up regulation of DNA repair genes, we found an inverse correlation between flavonoid intake and gene expression levels, which may suggest a flavonoid-mediated reduction of smoke-induced DNA damage, that should nevertheless be confirmed in larger studies. Although the present findings are intriguing, it must be remembered that this is a pilot study on a small number of subjects and with a limited power: even if the inter-variability among subjects concerning gene expression levels can be in part corrected by the choice of a relative quantitation assay using a housekeeping gene as an internal reference as described in the Methods section, increasing the number of subjects can overwhelm the occurrence of random variability due to uncontrollable factors (e.g. the subjects do not share exactly the same environment).
Moreover, it would be reductive to ascribe to flavonoids a single protective mechanism. A synergistic effect of several compounds and mechanisms of action resulting in a cumulative beneficial effect against carcinogenesis seems to be a more realistic interpretation. For instance, antioxidant properties of flavonoids are effective in scavenging free radicals, thus reducing oxidative DNA damage and initiation of lipid peroxidation, which also leads to the formation of DNA-damaging reactive speciesReference van Acker, van den Berg, Tromp, Griffioen, van Bennekom, van der Vijgh and Bast64–Reference Sang, Hou, Lambert and Yang66. In addition to having antioxidant properties, polyphenolic compounds, including flavonoids, were found to inhibit the formation of N-nitroso compounds (e.g. from nitrosamine in tobacco products, drugs, nitrite-cured meat), which are a known aetiological factor for tumours in stomach, oesophagus, nasopharynx, urinary bladder and colonReference Bartsch, Pignatelli, Calmels and Ohshima67, Reference Mirvish68. Several other mechanisms of action of subclasses of flavonoids are reported and reviewed in Mariappan et al. Reference Mariappan, Winkler, Parthiban, Doss, Hescheler and Sachinidis69, including modulation of gene expression of several genes involved in cellular processes (proliferation, signalling, immune response transcriptional regulation, apoptosis, etc.) and anti-proliferative properties by induction of apoptosis and inhibition of nuclear transcription factors and signal transduction pathwaysReference Lee, Huang, Hwang, Lee, Ke, Nair, Kanadaswami and Lee70–Reference Rahman, Biswas and Kirkham73.
Despite the growing bulk of literature about flavonoids as putative anti-cancer compounds, the results are often contrasting and much remains to be clarified about the effects of specific categories of flavonoids on human health and the underlying biological processes. The contribution of each single mechanism of action of a specific flavonoid or subclass is far from being completely characterized, and synergisms and cumulative effect should be taken into account. An extension of the present study to a larger sample under more controlled dietary conditions is warranted, including functional tests (e.g. Comet Assay; Host Cell Reactivation Assay) to better understand the impact of a flavonoid/polyphenol-rich diet on DNA repair pathways.
Acknowledgements
Supported by the World Cancer Research Fund, Compagnia di San Paolo (Torino), Italian Association of Cancer Research (AIRC) and a grant of the European Commission for the ECNIS project (FOOD-CT-2005-513943).
We are grateful for the cooperation of the volunteers who participated in the present study. We also thank the cook Giovanni Allegro for teaching the volunteers how to manage with the assigned diet, Ernestina Magnano of the restaurant ‘Ratatui’ for the collaboration in food preparation and Monica Bianchini for assessing dietary intakes from questionnaires.
NoteSupplementary information accompanies this paper on the Journal's website (http://journals.cambridge.org).
Supplementary Table Pre diet vs post diet gene expression correlations.

Pearson Correlation Coefficients are reported: *P ≤ 0.05; **P ≤ 0.01, ***P ≤ 0.001






