Introduction
Functional neurological disorder (FND) is among the most common diagnoses encountered in outpatient neurology practices, accounting for approximately 16% of neurology outpatient visits.Reference Stone, Carson and Duncan1 FND is costly: in an economic evaluation of more than 40 000 emergency department visits and 20 000 admissions annually (2008-2017, limited to the United States), FND-related charges were estimated to exceed $1.2 billion per year.Reference Stephen, Fung, Lungu and Espay2 This expense was similar to expenditures for neurologic conditions, such as anterior horn cell disease and demyelinating disorders, conditions that are fatal and/or highly disabling. The prognosis of FND in children is generally good. However, in adults and children with delayed diagnosis the prognosis of FND is poor, with low remission rates at follow-up,Reference Gelauff and Stone3 and rates of job loss and social dependency in FND are high: patients receive more disability-related support than patients with other conditions.Reference Carson, Stone and Hibberd4 In contrast to the large clinical and economic impact of FND, relatively little attention has been devoted to systematically improving the diagnosis and treatments of patients with FND.
Effectively communicating the FND diagnosis is a fundamental part of caring for patients with functional symptoms.Reference Stone5, Reference La Faver, LaFrance, Price, Rosen and Rapaport6 Although the importance of clear communication in FND has been recognized for decadesReference Shen, Bowman and Markand7, Reference Duncan8 healthcare providers (HCPs) find it challenging to communicate the FND diagnosis.Reference Friedman and LaFrance9, Reference Pohontsch, Zimmermann, Jonas, Lehmann, Löwe and Scherer10 HCPs cited concerns that they might offend patients, or make verbal missteps that would complicate management, as primary reasons for limiting their discussion with FND patients.Reference Barnett, Davis, Mitchell and Tyson11 This fear of using terms that may offend is an understandable concern: when neurology patients reviewed a scenario describing medically unexplained weakness, and were then asked their opinions of seven diagnostic terms that have been applied to FND, each of the terms was offensive to some patients.Reference Ding and Kanaan12 The use of structured education tools (flyers; group discussions) can significantly increase the understanding of FND and improve patient’s perceptions of the clinical encounter,Reference Hall-Patch, Brown and House13, Reference Cope, Smith, Edwards, Holt and Agrawal14 but such practices are not commonly implemented. The FND diagnosis can carry substantial stigma,Reference Klinke, Hjartardóttir, Hauksdóttir, Jónsdóttir, Hjaltason and Andrésdóttir15, Reference MacDuffie, Grubbs and Best16 potentially reducing the likelihood that providers will convey an FND diagnosis using the same clinical skills utilized for patients with less-stigmatized conditions. To this point, when HCPs reviewed clinical scenarios of patients with FND and then made diagnostic decisions, many reported that they would not record the FND diagnosis to avoid social and economic consequences for the patient.Reference Pohontsch, Zimmermann, Jonas, Lehmann, Löwe and Scherer10 Although the importance of communication in FND is well established, and a range of communication strategies helpful in FNDReference Adams, Anderson, Madva, LaFrance and Perez17 are available to HCPs, a more fundamental barrier to caring for FND patients may be at work; HCPs who recognize FND but do not communicate the diagnosis to patients at all. For example, we often receive referral notes stating that the patient had “non-epileptic seizures” or “functional gait,” but the referring clinician never informed the child or family of this diagnosis. We set out to assess the frequency of this noncommunication and identify the reasons why HCPs may communicate an FND diagnosis differently than they would with other disorders.
Observational studies of communication between HCPs and patients with FND demonstrated that HCPs speak differently when conveying an FND diagnosis;Reference Burbaum, Stresing, Fritzsche, Auer, Wirsching and Lucius-Hoene18, Reference Monzoni, Duncan, Grünewald and Reuber19 physicians and psychologists employ different rhetorical strategies, display higher levels of defensiveness, and are more likely to justify the diagnosis based on outside factors rather than on their own expertise. These linguistic assessments begin to reveal why an HCP might recognize FND but not share the diagnosis: talking about FND is more difficult than most other types of clinical encounter.
However, such studies have not identified why clinicians find these FND-related conversations to be more difficult, or how often this difficulty leads to noncommunication of an FND diagnosis. It is possible that observation alone may alter the nature of the FND conversation, as HCPs and patients knew their conversations were recorded for research. Ideally, studies of clinical dialogue in FND would include unobtrusive observation of HCP communication in a manner that would not alter the nature of the conversation and could reveal factors that influence communication style. To the best of our knowledge, such a dataset does not exist for FND; collecting such data would be difficult.
We instead opted to study a form of medical communication that was readily available, objective, quantifiable, and standardized across all U.S. practice sites: the diagnostic codes selected by HCPs for clinical billing and documentation. International Statistical Classification of Diseases and Related Health Problems, 10th revision (ICD-10) codes allow HCPs to describe their perception of a patient’s disease and/or symptoms using a common framework. The study of coding is not a replication of what might be learned from direct observation, but allows multiple advantages for assessing HCPs’ beliefs and behaviors regarding FND: the application of codes is binary, not subject to nuance or varying terminology; comparison of code choices between disorders (FND vs non-FND) can quantify differences in real-world HCP behavior; coding decisions are made in real-time but can be assessed retrospectively without the confounds of recall bias; and coding involves only an HCP and their charge entry system, removing the potential for difficult interpersonal interactions to reduce communication of an FND diagnosis. Insights into HCPs’ beliefs about FND, and the ways that HCPs treat FND differently than other disorders, may help to understand the face-to-face interaction between HCPs and patients with FND.
In this study, we sought to understand how neurologists communicate the diagnosis of FND. To be clear, this is a study of physician behavior, not a study of children or adults with FND. We paired survey and chart review methods to quantify noncommunication—when an HCP diagnoses FND but does not code for it—and to identify factors that lead neurologists to code for FND differently than other disorders commonly encountered in clinical practice. We explored factors such as prior negative experiences, expectation of negative consequences for HCP or patient, methods of establishing the FND diagnosis, concern for nonpayment for care, and demographic features of the HCP, to understand why HCPs might code differently for FND. The magnitude of under-coding we identified may necessitate a reassessment of prior research in FND, as studies that utilized ICD-10 codes as an entry point likely underestimated the true clinical range and impact of FND. The reasons HCPs do not code for or communicate the FND diagnosis suggests that practical reforms to medical education could boost patient engagement and satisfaction and improve outcomes for patients with functional symptoms.
Methods
This study combined chart review and survey data collection, allowing us to compare actual to predicted performance when assigning diagnostic codes for FND-related consultations. Data from our own pediatric neurology faculty included both chart review and survey elements; our large, geographically diverse sample of pediatric neurologists included only survey data. A separate retrospective analysis of reimbursement for all FND-related encounters in our health system included both neurologists and non-neurologists. All research was conducted in accordance with the principles in the Declaration of Helsinki and with oversight and approval by the UT Southwestern Institutional Review Board.
Retrospective review of FND-related consultations
Case identification
We aimed to identify all consultations performed by pediatric neurologists at Children’s Medical Center of Dallas from January 2017 to January 2020, and then to identify patients for whom the supervising pediatric neurologist (the attending physician) stated that the patient’s symptoms were consistent with FND. Starting with all encounters in which our neurology faculty assigned billing codes for inpatient consultations (99251-99255), we limited the encounters to patients between 5 and 18 years of age. Although FND can occur in children younger than 5 years,Reference Park, Lee, Lee, Lee and Lee20 such cases are relatively rare. We eliminated patients older than 18 to focus on pediatric patients. This yielded 1420 independent consultations (ie, none were repeat assessments of the same patient during the same hospitalization). Although some patients initially presented through the Emergency Room, all were admitted for an inpatient hospitalization. Neurology is a consultation service in our hospital with the exception of patients admitted to our Epilepsy Monitoring Unit (EMU). Six patients were admitted to the EMU at the time their FND was diagnosed, and the remaining 51 patients were admitted to other primary services. We then searched each consultation note for predetermined keywords we frequently encounter in FND-related clinical notes (functional, psychogenic, nonorganic, somatization, conversion, astasia abasia, Hoover’s sign, dissociative, somatoform, and non-epileptic) to identify visits for closer scrutiny, yielding 109 consultations. Finally, we reviewed each consultation for a declarative statement by the supervising physician that the patient’s symptoms were consistent with FND. We excluded consultations in which a trainee’s FND diagnosis was not echoed by the supervising neurologist, an FND diagnosis was described in provisional terms (eg, “this may be FND”) or was mentioned only to exclude it, and cases in which a non-FND diagnosis was proposed as the primary diagnosis with FND explaining a minor portion of the patient’s symptoms. This yielded 57 in-hospital visits in which neurologists unambiguously diagnosed FND in the text of their consultation note.
Retrospective data collection
From each of the 57 consultations in which a supervising physician diagnosed FND, we categorized the phenomenology of the FND-related symptom (functional seizures, movements, gait, or sensory symptoms) and identified all ICD-10 diagnostic codes that the neurologist assigned to that consultation (ranging from 1 to 6 diagnostic codes). Note that ICD-10 codes are assigned separately for each day’s clinical encounter; we extracted ICD-10 codes only for the day of consultation (day 1), and only in cases when the neurologist stated the FND-related diagnosis in that day’s note. For patients diagnosed with functional (non-epileptic) seizures, we determined whether an electroencephalography (EEG) was performed as part of that consultation (same day or preceding the encounter) and if the EEG captured the event in question.
Assessment of reimbursement
To assess the likelihood of reimbursement for FND-related diagnosis codes across specialties, we reviewed all encounters for evaluation and management from all members of the Department of Pediatrics (including pediatric neurologists, general pediatricians, and all other pediatric subspecialties) performed from January 2017 to January 2020 in which the provider selected ICD-10 codes F44.0 to F44.9. This search included both inpatient and outpatient claims, and both new- and established-patient visits. Among these encounters we then assessed whether insurers paid the claim and if not, the reasons for nonpayment. In both our clinical practices and in our survey responses, many HCPs expressed a concern that FND-related encounters would only be reimbursed if the provider was in a mental health field; these codes were perceived by some to be “psychiatry codes,” not available for use by HCPs in other specialties. We aimed to assess the validity of these concerns.
National survey of neurologists
Survey design
We developed a survey to address the following question: When a neurologist’s clinical judgement supported the diagnosis of FND, what factors influenced whether the neurologist applied FND-related diagnostic codes to that encounter? Our survey included 11 questions (Supplementary Figure S1): six clinical scenarios, two demographic questions, two questions regarding HCP beliefs and experiences, and a request for HCPs to rate factors that might influence their opinions about FND. Two free text elements were provided: if HCPs indicated that they had experienced negative consequences resulting from an FND-related interaction, we asked that they describe the experience and when asked to rate factors that influenced their FND-related diagnostic decisions, HCPs could add factors beyond the choices we supplied.
Population surveyed
We wished to reach the broadest possible sample of pediatric neurologists practicing in the United States. However, we could not identify an extant, comprehensive list of such physicians. We therefore set a goal of identifying the name, practice site, and email address of every practicing pediatric neurologist in the United States. We began with the membership directories of the Child Neurology Society and the Child Neurology section of the American Academy of Neurology, which reported names and cities of residence. For each listed individual, we performed internet searches to identify other physicians in the same practice or institution. To find individuals outside of the clusters we had already identified, especially small-group and solo practitioners, we queried the medical networking website Doximity. Pediatric neurologists are a small population, making statewide searches for missing physicians a practical option. After identifying our cohort of physicians, we verified that each physician had an active medical license using public search tools provided by each state’s medical board. For physicians who practiced in multiple states, we reached out to both locations to determine the predominant practice site for that individual. Our cohort of U.S.-based pediatric neurologists included 2525 individuals. Notably, this cohort of verified pediatric neurologists is 80% larger than the number of active members of the Child Neurology Society, underscoring that our survey cohort is broadly representative.
We identified viable email addresses for this cohort through several means. Peer-reviewed publications provided email addresses for many subjects, and the institutional convention for those emails (eg, first.last@university.edu) allowed us to derive email addresses for many of their colleagues. For individuals whose email was not discoverable through those means, we emailed their colleagues or phoned their listed practice site and requested permission to contact the physician. With a provisional list of email addresses for most subjects, we sent a brief email notification describing our search; delivered messages confirmed that email addresses were active, while returned messages led us to continue searching for an operational address. We identified 2517 active email addresses of pediatric neurologists (99.7% of our target cohort) representing every U.S. state and Puerto Rico.
Data collection
We distributed survey invitations and collected response data through the REDCap (Research Electronic Data Capture) electronic data capture tools hosted at UT Southwestern.Reference Harris, Taylor, Thielke, Payne, Gonzalez and Conde21 REDCap is a secure, web-based suite of software tools designed to support data capture for research studies, providing (a) an intuitive interface for reliable data capture; (b) audit trails for tracking data manipulation and extraction procedures; and (c) automated export procedures for seamless data transfer to common statistical packages. We excluded data from 65 respondents who started the survey but did not answer all questions.
Statistical assessment
All statistical tests were performed using Stata (StataCorp, 2013, Stata Statistical Software: Release 13. College Station, TX). For tests of simultaneous, independent outcomes (binary choice in two clinical scenarios), both our dependent and explanatory variables were categorical, and our explanatory variables could not be assumed to be independent (ie, years of practice and practice type may covary). Therefore, for these comparisons we utilized multivariate logistic regression. For comparisons between categories of disorder (mean responses for the group of organic disorders vs the group of functional disorders), our dependent variables were continuous and all independent variables were categorical. Therefore, for these tests we utilized ANOVA. In total, we performed six statistical comparisons and therefore corrected for our significance threshold utilizing the Bonferroni method (p adjusted = .05/6 = .0083).
Data availability
Survey results and summary data from our review of clinical encounters are available upon request. The original records from clinical encounters include protected health information and are not available for distribution.
Results
Review of FND-related consultations
We identified all new inpatient consultations in children older than 5 years at Children’s Medical Center of Dallas from January 1, 2017 to January 31, 2020, which yielded 1420 distinct encounters. We screened these consultations by searching for predetermined keywords, followed by manual evaluation of all candidate encounters. We thereby identified 109 consultations in which the supervising physician mentioned a suspicion for an FND-related disorder. Of these, the supervising physician stated a clear, affirmative diagnosis of an FND-related disorder in 57 cases (Figure 1). Note that these 57 (4.0% of all consultations) are an undercount of all FND cases since we did not include patients diagnosed without the involvement of the neurology service, cases that evolved to an FND diagnosis later in the hospitalization, or cases in which the supervising physician expressed that FND was a likely, but not certain, diagnosis. We identified all diagnostic codes assigned by the supervising physician for each of those 57 consultations. Neurologists could choose more than one ICD-10 code to describe the encounter; we included all utilized ICD-10 codes.

Figure 1. Our methods for data acquisition, combining retrospective and survey methods. We assessed the behavior, beliefs, prior experiences, and attitudes of Neurologists regarding patients with functional neurological disorder (FND) through paired assessments. We reviewed all inpatient consultations for a 3-year period to identify cases in which the supervising physician made a diagnosis of FND, and then assessed whether they assigned FND-related diagnostic codes (ICD-10) for that encounter. We conducted a survey of U.S.-based Pediatric Neurologists, including those physicians whose consultations we reviewed, to identify factors that influence a Neurologist’s decision about whether to utilize FND-related diagnostic codes. Finally, we compared physicians’ real-world diagnostic coding decisions with their stated beliefs regarding FND.
The average age of these 57 patients was 14 years, 3 months. This cohort included 41 females and 16 males. The sex ratio for adolescent patients (43 subjects, F:M, 2.6) did not differ from the sex ratio for patients 12 years and younger (14 subjects, F:M, 2.5). Most patients presented with a single FND manifestation (49/57), while a minority of subjects had more than one FND subtype (8/57). Therefore, the total number of FND manifestations exceeded the number of patients, which included 42 cases of NES; 11 with functional gait disorder; 6 with functional sensory loss or change; 3 with functional weakness; two with functional vision loss; 1 with functional tremor; and 1 with functional amnesia.
Neurologists who stated an FND-related diagnosis in their consultation note utilized FND-related diagnostic codes only 22.8% of the time (13/57 consultations). Non-epileptic seizure was the most likely FND manifestation to garner an affirmative diagnosis, at 42/57 consultations, and thus was also the most frequently coded FND manifestation (11/57 consultations). Of consultations that utilized an FND-related diagnosis code, the three most commonly utilized codes were F44.5 (conversion disorder with seizures or convulsions, present in 61.5%), F44.9 (dissociative and conversion disorder, unspecified, 38.5%), and R56.9 (unspecified convulsions, 30.8%). When neurologists diagnosed FND but did not code for it, they instead selected codes that were less specific: six non-FND codes were utilized at least three times across the cohort and each of these codes included the terms “unspecified” or “other.” The three most commonly utilized codes were R56.9 (unspecified convulsions, 36.3%), R41.82 (altered mental status, unspecified, 11.4%), and G40.909 (epilepsy, unspecified, not intractable, without status epilepticus, 9.0%). Each of the physicians who utilized FND-related diagnosis codes also wrote other consultations that diagnosed FND but did not code for it.
Insurance payment for FND-related encounters
From 2017 to 2020, HCPs in our health system utilized FND-related ICD-10 codes 141 times. While we searched for all ICD-10 codes in the F44 family, only F44.4, F44.5, F44.7, and F44.9 were utilized. These visits were paid outright 94% of the time (132/141 encounters). Of the nine encounters that were not paid, all were for administrative issues: past timely filing deadlines, missing claim forms, duplication of services, lack of prior authorization, or lack of provider enrollment with the insurance carrier. No HCP, of any specialty, for any type of evaluation and management service, was denied payment for services based on coding for FND.
Survey of USA-based neurologists
We collected survey data from October 5 to 31, 2020, with 483 completed responses (19% response rate, Figure 1). Although we attempted to invite only pediatric neurologists in independent practice, 14 respondents noted that they were still in training. Nine respondents noted that they do not make billing decisions, either because in their hospital system, HCPs did not choose diagnostic codes, or they had accepted administrative roles and no longer saw patients. We removed these 23 responses. Of the remaining 460 respondents, 24.3% were general neurologists in academic practice, 54.3% were subspecialty neurologists in academic practice, 12.8% were general neurologists in private practice, and 8.5% were subspecialty neurologists in private practice. Physicians practicing for 0 to 3 years made up 17.6% of our cohort; 4 to 10 years, 29.3%; 11 to 25 years, 30.0%; and those in practice for >25 years made up 23.0% of our cohort. More than a third (39.1%) of responding neurologists reported that they had faced a personal negative consequence after making a diagnosis of FND, and many of these respondents detailed their negative experiences in free-text responses (Supplementary Table S1).
View of FND as a diagnosis of exclusion
More than half (51.5%) of our respondents viewed FND as a diagnosis of exclusion (in contrast with the view that FND is recognizable by specific, positive features). We assessed for correlations between the view that FND is a diagnosis of exclusion and features of individual HCPs—demographic factors (site, type and duration of practice), prior negative experiences with patients who had FND, and self-assessed reasons for not coding for FND. The preference to complete diagnostic workup before coding for underlying diseases was positively correlated with the view that FND is a diagnosis of exclusion (odds ratio [OR] = 3.3, z = 4.25, p < 2.1 × 10−5), as was working in private practice (OR 2.0, z = 2.67, p < 7.6 × 10−3). Factors that had no significant correlation with this view included duration of practice, type of practice (general vs subspecialty), and a prior negative experience with FND.
Self-identified reasons neurologists do not utilize FND-related diagnostic codes
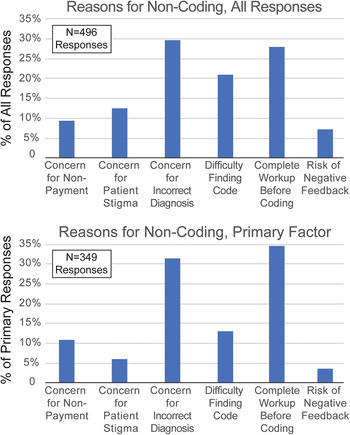
Survey respondents were asked to consider a prior patient with suspected FND for whom the HCP did not utilize FND-related diagnosis codes. Respondents were then provided with potential reasons for not coding and asked to select all that were important reason for them (Figure 2). Respondents then ranked their reasons for nonutilization of FND codes by importance. The three most common reasons for noncoding were concern that the FND diagnosis was incorrect (29.6%), preferring to code for symptoms, not underlying diagnoses, until all diagnostic work-up was complete (28.0%), and difficulty finding the correct FND-related diagnosis code (21.1%). These responses comprised 78.7% of all reasons for noncoding. As respondents were encouraged to select all relevant factors, the number of selected factors is greater than the number of survey participants (N = 496).

Figure 2. Self-identified reasons for not utilizing functional neurological disorders (FND)-related diagnosis codes. Neurologists were asked to recall a patient with suspected FND for which they did not utilize FND-related diagnosis codes. Respondents were asked to select all applicable reasons for not coding (could select none or multiple reasons, upper panel), and to also select the primary (most-important, lower panel) reason for not utilizing FND-related diagnostic codes. Potential responses included the concern for nonpayment (insurance denial); concern that patient would be stigmatized by other healthcare providers; concern for the FND diagnosis being incorrect; difficulty finding the correct FND-related billing code; the practice style of only coding for symptoms, not underlying causes, until diagnostic testing is complete; and concern about negative feedback from patient (including retaliation, bad reviews, or litigation).
We also asked survey participants to rank their responses by the degree of influence each factor had on their decision to not utilize FND-related diagnostic codes. Not all respondents selected factors (some replied that they always utilized these codes, and thus no factor influenced them negatively), and therefore the number of primary factor responses was smaller than the number of respondents (N = 349, Figure 2). Among factors identified as the primary reason for noncoding of FND disorders, the two least-frequent factors combined comprised <10% of respondents: potential stigma against patients from other healthcare providers (6.0%), and potential negative feedback for HCPs (3.7%). We therefore focused subsequent multivariate comparisons on the three factors that were most frequently identified as reasons for not utilizing FND-related codes: the practice style of only billing for symptoms instead of underlying etiologies until diagnostic testing is complete (34.7% of respondents); concern about coding for a diagnosis that later proved to be incorrect (31.5%); and insufficient knowledge of FND codes and their reimbursement status (24.1%). It is notable that only 3.7% of respondents cited negative personal consequences as an influence on their code selection, while 39.1% of respondents stated that they had suffered a negative consequence as a result of making an FND diagnosis. A review of the free-text accounts of those negative consequences (Supplementary Table S1), which detailed job losses, formal censure from their hospital, threatened lawsuits, reduced patient volume as a result of negative online reviews, and intense anger from patients and families, suggests that neurologists may underestimate the impact of these negative experiences on their manner of practice.
Familiarity with the disorders in our survey
In response to our clinical scenarios, respondents had the option to report that they had never seen that disorder. All 460 respondents reporting having previously seen both seizures and non-epileptic seizures. A small number of respondents had never seen other surveyed disorders: pseudotumor cerebri, 2.2%; Guillain-Barre syndrome, 3.0%; and astasia-abasia, 5.9%. For subsequent analyses, we wished to compare factors that influenced decision-making among the clinical scenarios, so considered data only from the 417 individuals who reported having seen all surveyed disorders.
Diagnostic thresholds in organic vs functional disorders
Every diagnostic scenario must reach a threshold of plausibility before HCPs move from a potential to a pragmatic and actionable diagnosis. We considered that threshold to be the point at which the common clinical presentations of the highest-probability disorder are distinguished from the common clinical presentations of other potential diagnoses. Beyond that margin, additional data might change the diagnosis only if presentations are uncommon. For example, a clinical event that has no electrographic correlate on a high-quality EEG is likely non-epileptic. The probability of a small or deep epileptic focus whose dipole is insufficient to be detected by surface EEGReference Waugh, Prabhu, Bourgeois and Kothare22 is nonzero, but is not typically sufficient to necessitate performing more invasive testing, such as PET (positron emission tomography), SPECT (single-photon emission computerized tomography), or intracranial EEG electrodes. We wished to determine if neurologists had differing thresholds of completion for common organic and functional disorders.
When presented with a range of clinical scenarios for which diagnostic testing was incomplete (Figure 3), HCPs employed a more lenient diagnostic threshold for organic disorders than for FND. Considering the three organic disorder scenarios we presented, 81.0% of respondents were willing to code for organic disorders when the workup was explicitly incomplete. For non-epileptic seizure awaiting confirmatory EEG, only 57.3% were willing to utilize FND-specific codes (t = 9.68, p < 3.95 × 10−20). HCPs who identified a general practice style of waiting to code for specific diagnoses until all testing is complete were accurate in their self-assessments—these HCPs were 8.1% to 28.9% less likely to code across the full range of disorders, both organic and functional, than HCPs who did not identify that practice preference. However, these two groups did not differ (23.7 vs 26.4%, p < .67) in their differential probability of coding for organic and functional disorders. That is, all HCPs expressed this higher bar for FND-related disorders, not solely those HCPs who prefer to complete all testing before utilizing diagnosis-specific codes. Lower rates of coding for FND-related disorders therefore cannot be explained by a preference for coding only when the diagnostic workup is complete. Considering individual HCP factors that correlated with this higher diagnostic threshold for FND, the belief that FND is a diagnosis of exclusion was the sole factor that predicted HCPs’ lower utilization of FND-related codes (F = 25.1, p < 7.9 × 10−7).
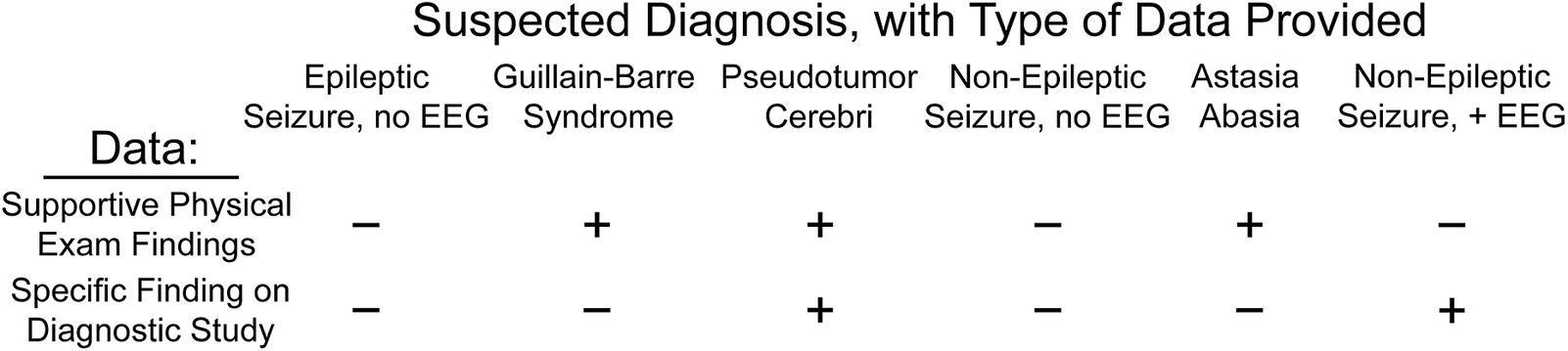
Figure 3. Types of information provided in the clinical scenarios surveyed. Each of the six clinical scenarios we surveyed included a range of diagnostic information, allowing us to assess decision-making under varying levels of clinical surety. Comparison of scenarios with identical types of information (eg, epileptic seizure and non-epileptic seizure, both with supportive history but without electroencephalography [EEG] characterization) allowed us to identify factors associated with differential diagnostic coding between these scenarios.
Trust in the physical exam and probability of coding for FND
Many HCPs reported a hesitancy to code for FND-related disorders out of concern for making an incorrect diagnosis. 31.1% of survey respondents described this concern as the primary or second most important factor leading them to not utilize FND-related codes, the highest frequency response among top two factors. Diagnostic confidence rests in part on supportive physical exam findings, so we assessed whether physical exam findings are weighted differently in organic and functional disorders, and whether underweighting of physical exam findings contributes to undercoding of FND-related disorders.
Our Guillain–Barre syndrome and astasia-abasia scenarios presented identical categories of information (supportive histories and physical exam findings but no additional dispositive diagnostic testing). However, the physical examination in astasia-abasia is specific and diagnostic,Reference Nonnekes, Růžička, Serranová, Reich, Bloem and Hallett23 while rapidly progressive ascending paralysis is suggestive but not diagnostic of Guillain–Barre syndrome. This asymmetry of information conveyed through the physical exam suggests that HCPs presented with these two scenarios should have coded for astasia-abasia more frequently than for Guillain–Barre. Indeed, our respondents were 16.5% more likely to code for astasia-abasia than for Guillain–Barre (75.1% vs 58.5%, respectively). However, when we divided the cohort by concern for misdiagnosis (respondents who ranked this factor highest, N = 103, vs all other respondents) a different pattern emerged. Those respondents concerned by potential misdiagnosis were both more likely to code for Guillain–Barre syndrome and less likely to code for astasia-abasia, reducing the differential utilization between organic and functional disorders to 4.9%. In contrast, HCPs less concerned with misdiagnosis coded for astasia-abasia 20.4% more often than Guillain–Barre (F = 7.1, p < 8.2 × 10−3). For disorders in which the primary source of information was clinical assessment, concern that FND was a misdiagnosis led neurologists to undervalue the physical examination by 3.3-fold.
Business of medicine knowledge as a barrier to diagnostic coding
Two independent reasons for not utilizing FND-related codes can be broadly seen as reflections of a respondent’s knowledge of the business of medicine, at least as pertains to FND. Neurologists cited a fear of nonreimbursement as the primary reason for not coding for FND in 9.7% of cases. As noted above, this belief is false.Reference Mark, Olesiuk, Ali, Sherman, Mutter and Teich24 A similar percentage (11.5%) of respondents had difficulty finding the appropriate FND-related diagnosis codes. These reasons for nonutilization do not have a clear link to a particular type of clinical scenario, so we instead investigated the demographic and practice variables associated with these knowledge gaps. Longer duration of clinical practice was the only associated factor: HCPs who completed training 10 to 25 years (OR: 3.8; p < 5.3 × 10−3) or >25 years (OR: 3.3; p < 0.015, NS) prior were threefold more likely than recently trained respondents to identify these educational deficits as their most important reason for not coding for FND.
Coding for seizures and non-epileptic seizures
In our national survey, we described two clinical scenarios with identical history, physical examination, diagnostic testing, and remaining workup: seizures and non-epileptic seizures. Neurologists reported coding for seizures 72.2% more often than for non-epileptic seizures (89.2% vs 51.8%). When the events concerning for non-epileptic seizures were captured on EEG, self-reported rates of FND-related coding rose to 94.5%. This latter scenario might be considered the gold standard for diagnosing FND, so we assessed what factors were shared among the 23 respondents who would not code for EEG-confirmed non-epileptic seizures. No demographic, experiential, or behavioral factor was a significant predictor of noncoding in this scenario. We performed a post hoc analysis that included all self-reported reasons for noncoding, rather than assessing only the primary factor. The only significant reason why neurologists did not utilize FND-related diagnosis codes for EEG-confirmed non-epileptic seizures was difficulty finding the correct code (OR 4.4, p < 1.0 × 10−3).
Negative consequences after making an FND diagnosis
Survey respondents were provided a free-text option to report any negative consequences they experienced after making an FND-related diagnosis (Supplementary Table S1). Most negative consequences fell into discrete categories, although many neurologists included more than one type of consequence in their response. Therefore, the total number of consequences reported exceeded the number of responses. Anger from the patient and/or family and resistance of the diagnosis was reported by 60.8% of respondents (Figure 4). Specific examples included parents yelling at the physician, becoming “irate,” and yielding “highly contentious” conversations. Situations that were personally or professionally damaging were described by 31.2% of respondents. These included formal complaints to hospital and university leadership, negative reviews on social media and physician rating websites, and threats to sue the physician. Note that these complaints occurred in spite of the fact that the FND diagnosis was correct, as reported by these neurologists. One physician reported that parents angry after an FND diagnosis “drove me out of a job that I was happy with due to bad patient reviews.” One parent reported to “the hospital CEO [this neurologist] was unfit to practice medicine” after the physician diagnosed FND in his child. Another physician reported that negative patient reviews following an FND diagnosis “exacerbates burnout; increasing likelihood I will not keep practicing.”

Figure 4. Negative consequences after making a functional neurological disorders (FND) diagnosis. Physicians who responded that they had suffered negative consequences after making an FND diagnosis were invited to give examples of their experiences. These responses largely fell into a few categories, though some responses fit multiple categories of consequences.
Many patients with FND severed the clinical relationship with their neurologist after hearing an FND diagnosis (19.0% of respondents), although we suspect that this number is an undercount given the frequency with which anger and negative reviews were cited. Some physicians (12.7%) experienced a negative consequence but described ways that system-level failures contributed to this poor outcome. Common responses included the recognition that patients with FND require more time than other patients with neurological diseases, and the dearth of psychiatric support for most neurology practices. One physician stated, “A patient was given diagnosis … without psychiatric support and the patient suicided.” Finally, some physicians expressed regret at either making an incorrect FND diagnosis or fearing that they would make a diagnostic error (10.1% of respondents). Some level of diagnostic error is inevitable in clinical practice, of course, but several physicians reported regret about the “one time” they had misdiagnosed FND in decades of clinical practice. Although these are limited anecdotes, they suggest that physicians feel a greater burden when they misdiagnose FND than when they misdiagnose other neurological disorders.
Comparison of chart review and survey data
We compared the survey responses of our 26 local pediatric neurology faculty with those of all other respondents. No demographic, behavioral, experiential, belief, or coding practice was significantly different between local and all other HCPs. Only one factor would have been significant, but for multiple comparisons correction (p < 0.047, not meeting our Bonferroni-corrected significance threshold): local neurologists were less likely to hold the view that FND is a diagnosis of exclusion (OR 0.35, 95% CI 0.13–0.99). Therefore, we concluded that the results of the chart review limb of our project are generalizable to the larger pool of 460 respondents.
In our 26 local respondents (each of which had both survey and chart review data), we compared the estimates of coding behavior (survey) with those respondents’ actual coding decisions. As our survey was anonymous, respondents were compared at the group level. In the clinical scenario of suspected non-epileptic seizure with spells captured on confirmatory EEG, 96.2% of neurologists predicted that they would utilize an FND-related code. In practice, only 36.7% of EEG-confirmed non-epileptic seizures were coded as such. In the survey scenario of suspected non-epileptic seizure when EEG had not yet captured an episode, 57.7% of local HCPs stated that they would apply FND-related diagnostic codes. In practice, these same HCPs coded for FND in zero of 12 consultations. For FND manifestations that did not resemble seizure, neurologists coded at a rate less than half that utilized for non-epileptic seizures—of the 15 FND consultations for other manifestations, only two utilized FND-related ICD codes (13.3%). It is notable that our survey scenarios were presented as “suspected” diagnoses—likely, but not yet certain—but the majority of HCPs estimated they would code for FND in each of our clinical scenarios. In contrast, in each of the reviewed consultations the physician had already recorded an FND diagnosis—but rates of coding for FND fell by >50% relative to physicians’ predictions of their own behavior.
Discussion
We have demonstrated that pediatric neurologists who made an affirmative diagnosis of FND failed to code for it the majority of the time (77.2% of diagnoses), even when that diagnosis had been confirmed with independent testing. Neurologists applied FND-related diagnosis codes in an uneven distribution among FND manifestations, with NES garnering an FND-related ICD-10 code at double the rate utilized for other forms of FND (26.2% vs 13.3%). Many neurologists held the view that only psychiatrists can be reimbursed for FND-related care. In our health system, zero of 141 clinical encounters by non-psychiatrists were denied payment as a result of coding for FND. Survey data from our own institution were highly similar to those of pediatric neurologists from across the United States, suggesting that the coding discrepancies we identified in our institution are likely to be found nationwide. Given the centrality of the neurological exam to making an accurate FND diagnosis, and the fact that education regarding FND varies widely among and within clinical specialties,Reference Barnett, Davis, Mitchell and Tyson11, Reference Lehn, Bullock-Saxton, Newcombe, Carson and Stone25 we think it plausible that non-neurologists code for FND even less frequently than the low rates we identified. ICD-10 codes are the entry point for many types of investigation in FND, including epidemiologic, economic, and prognostic assessments.Reference Altalib, Galluzzo and Argraves26, Reference Nemade, Shivkumar, Ferguson, Singh and Shah27 Our findings suggest that prior research may markedly underestimate the true frequency and cost of FND, an undercount predicted by Stephen et al,Reference Stephen, Fung, Lungu and Espay2 which skews our understanding of the natural history and impact of FND. If our nationwide sample of 460 neurologists accurately represents the opinions and practice patterns implemented in the care of children and adults with FND, total US healthcare expenditures for FND likely exceed $5.3 billion per year.Reference Stephen, Fung, Lungu and Espay2 FND is a common, debilitating, and very expensive neurological disease. Improving care for children and adults with FND will likely require both fundamental research in the neurophysiology of functional symptoms and pragmatic research to improve clinical care delivery.
The primary motivation for this study was to identify reasons why HCPs who recognize FND do not explicitly communicate the diagnosis to their patients (Figure 5), a phenomenon we have observed in neurologists and non-neurologists. We found the same phenomenon in coding: when neurologists diagnosed FND but did not code for it, they selected ICD-10 codes that were less precise. We can imagine scenarios in which a sense of (misinformed) beneficence would drive a reduction in diagnostic coding without negatively affecting HCP-patient communication (eg, concern regarding stigma from other HCPs, or nonreimbursement). Such scenarios are unusual; 6.0% and 10.9% of our survey respondents, respectively, noted these as their top reasons for noncoding. For the majority of neurologists (69.9%), however, the primary factors that influenced noncoding are also plausible drivers of noncommunication: fear of making an incorrect diagnosis, mistrust of their physical exam, holding FND to a higher diagnostic threshold than other neurological disorders, and avoidance of difficult conversations and/or negative ratings.

Figure 5. Factors associated with noncoding for functional neurological disorders (FND), and educational aims to counteract them. We assessed factors that were the primary drivers of noncoding when a neurologist diagnosed FND and identified specific educational interventions that may positively influence coding behaviors. We hypothesize that factors negatively associated with coding behaviors will also negatively influence clinician–patient communication in FND.
One-third of our respondents reported that fear of misdiagnosing FND was their primary or second most important reason for not coding for it. The actual rate of misdiagnosis in FND is approximately 4%,Reference Stone, Smyth and Carson28 similar to the rate of misdiagnosis in other neurological disorders. In clinical scenarios that depended solely on physical exam findings for diagnosis, concern for misdiagnosis drove noncoding higher, by 3.3-fold. More than half of neurologists (51.5%) endorsed the view that FND is a diagnosis of exclusion, and this factor was the strongest predictor that a neurologist would employ a higher diagnostic threshold for FND than for other neurological disorders. This outdated framing of FND persists among many neurologists, despite the fact that Diagnostic and Statistical Manual of Mental Disorders, fifth edition (DSM-5) eliminated the requirement that HCPs “exclude” other disorders in 2013. Neurologists frequently reported having had personal negative consequences after making an FND diagnosis (39.1%), and their descriptions of these negative experiences (Supplementary Table S1 and Figure 4) suggest that many HCPs altered their interaction with FND patients as a result. One such HCP reported, “I think it has altered my practice in that I honestly try a little less hard to connect with the PNES patients and help them. I spent hours over several visits with this particular patient… Now I just give them a diagnosis but I am much less involved in follow up.”
This study has several important limitations. First, we assessed only pediatric neurologists, and their training or practice environments might lead them to code differently than neurologists who exclusively treat adults. However, pediatric neurologists in the United States spend one-third of their residency training embedded with adult neurologists. Pediatric neurologists in private practice commonly share on-call responsibilities for adult patients, and pediatric neurologists in U.S.-based academic practices are often embedded within or affiliated with adult Neurology departments. We were unable to identify any factor that would lead a pediatric neurologist to communicate less effectively, or to treat patients with FND with less professionalism, than their colleagues in adult neurology. Second, the study was not designed to study HCP–patient communication directly, and we thus cannot establish which of the factors that negatively influence coding behaviors also contribute to lower-quality communication, delayed diagnosis, and poor prognosis in FND. Third, since each insurance carrier produces a unique payment policy, it is possible that some companies might refuse payment for FND-related care. However, our findings concur with those of Mark et al, who found that non-psychiatry HCPs were reliably reimbursed for visits that utilized mental health codes, and in fact, non-psychiatry HCPs were reimbursed at higher rates than psychiatrists for in-network services.Reference Mark, Olesiuk, Ali, Sherman, Mutter and Teich24 Finally, we identified a bias in reporting by neurologists that led some forms of FND to be relatively overrepresented (non-epileptic seizures vs. all other forms). Readers should therefore recognize that our observations may generalize less-well to forms of FND that are underrepresented in this dataset.
In spite of these limitations, this study identified knowledge gaps and practice patterns that are a reasonable starting point for targeted educational interventions. For some drivers of noncoding, it may be sufficient to dispel single clinical myths. For example, there is no evidence that insurers refuse payment for FND-related care. Our findings support the clinical best practice that HCPs should code for the diagnoses they think most relevant to patient care, independent of payment policies. Likewise, an FND diagnosis must rest on positive diagnostic symptoms and signs.Reference Stone, LaFrance, Brown, Spiegel, Levenson and Sharpe29, Reference Demartini, D’Agostino and Gambini30 Since 2013, when FND was defined as a new entity in DSM-5, clinicians have been free to utilize their physical exam skills and clinical expertise to diagnose the disorder; FND has never been a “diagnosis of exclusion.” This change in perspective—framing FND as a positive diagnosis rather than the final disorder remaining after eliminating all other possibilities—is transformative for the clinician–patient interaction and has been adopted by modern research in FND mechanisms, epidemiology, and treatment.Reference Daum, Gheorghita and Spatola31-Reference Cock and Edwards36
Boosting confidence in a provider’s clinical skillset may improve their ability to communicate an FND diagnosis. Specific training in neurological exam findings with high specificity for FND,Reference Nonnekes, Růžička, Serranová, Reich, Bloem and Hallett23, Reference Hallett37, Reference Lidstone and Lang38 and facility with dispositive maneuvers in cases of suspected FND,Reference Perez, Hunt, Sharma, Flaherty, Caplan and Schmahmann39 have the potential to increase the accuracy of and confidence in FND diagnoses. Likewise, incorporating longitudinal observation into the diagnostic conversation—for example, “This physical exam feature makes me confident that you have FND. But I owe it to you to keep checking.”—allows the clinician to build certainty and mutual understanding over time. Indeed, establishing a professional relationship with one’s patient is associated with less disability and increased confidence by cliniciansReference Barnett, Davis, Mitchell and Tyson11—which runs counter to a culture in which neurologists diagnose FND but do not follow patients over time. Given the therapeutic value of educating a patient using those FND-specific exam findings during the clinical assessment,Reference Cock and Edwards36 ensuring that clinical training (and continuing education curricula for established practitioners) includes training in neurologic exam skills that aid the diagnosis of FND may shorten the diagnostic odyssey and speed recovery in FND.
Improving outcome in FND may require cultural shifts in the way that individual providers, and health systems, are oriented toward patients with this disorder. As noted by Kozlowska et al,Reference Kozlowska, Sawchuk and Waugh40 HCPs caring for patients with FND have absorbed many clinical lessons that can undermine the clinical relationship. Improving the prognosis for FND will likely require that providers assess, at the individual and group levels, where care delivery falls short and where biases against this group of patients contribute to poor outcomes. However, it may be difficult for HCPs to assess the impact of these factors on their clinical decisions: neurologists markedly overestimated their willingness to code for functional disorders, even for the “gold standard” for diagnosing FND, NES with events characterized by contemporaneous EEG (self-estimate: 96.2% and actual performance: 36.7%). One starting point is the recognition that patients with FND suffer substantial stigma.Reference Klinke, Hjartardóttir, Hauksdóttir, Jónsdóttir, Hjaltason and Andrésdóttir15, Reference MacDuffie, Grubbs and Best16, Reference Perez, Edwards, Nielsen, Kozlowska, Hallett and LaFrance41 Stigma against specific diagnoses and demographic groups is associated with substantial negative health consequences for both patientsReference Link, Struening, Rahav, Phelan and Nuttbrock42, Reference Hatzenbuehler, Phelan and Link43 and their caregivers,Reference Song, Mailick and Greenberg44 suggesting that efforts to reduce the stigmatization of FND will improve health outcomes.
It is important to recognize that clinicians are also harmed by the current culture surrounding FND. Neurologists related prior negative experiences with FND that were professionally damaging and emotionally bruising, including scenarios that led them to alter their future treatment of all patients with FND (Supplementary Table S1 and Figure 4). This is not the fault of a few angry patients—a system that reliably produces difficult encounters is a system designed to produce difficult encounters. Clinical visits are generally not designed to meet the complex needs of FND patients, leaving HCPs, patients and families to fill any gaps. Proactively addressing these difficult clinical scenarios with FND-specific training, additional evaluation time, and ancillary support (eg, patient and family counseling, social work, workplace, and/or educational advocates) has the potential to improve the lives of clinicians, as well as speeding recovery and reducing healthcare expenditures for patients with FND.
Conclusions
Our study demonstrates that many neurologists utilize higher diagnostic thresholds, and express greater concern for making an error, for those suffering with FND relative to patients with other neurological disorders. Whether as a result of out-of-date medical knowledge regarding functional disorders, the absence of training opportunities to hone examination and discussion skills specific to functional symptoms, or reticence arising from difficult prior encounters, it is essential to acknowledge that we treat patients with FND differently than we treat patients with other brain-based symptoms. Clinicians spend years developing the ability to forge effective therapeutic relationships; we speculate that the knowledge gaps, cognitive biases, and personal discomfort described here may disrupt this hard-fought skill. Future research on the impact of clinician mindset on the therapeutic relationship will be necessary to optimize care for children and adults with FND. We propose that improved FND-specific education, proactive efforts to reduce stigma against FND, and future research to understand why neurologists practice differently with FND than with other neurological disorders, will improve clinician–patient communication and healthcare outcomes in FND.
Abbreviations
- FND
-
functional neurological disorder
- HCPs
-
healthcare providers
- ICD-10
-
International Classification of Diseases, 10th revision
Supplementary Materials
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S1092852921000833.
Author Contributions
Conceptualization: A.W.-S., L.D.H., and J.L.W.; Methodology: A.W.-S. and J.L.W.; Investigation: A.A.O.H., R.K., and J.L.W.; Data curation: L.D.H. and J.L.W.; Investigation: L.D.H.; Formal analysis: J.L.W.; Project administration: J.L.W.; Resources: J.L.W.; Software: J.L.W.; Supervision: J.L.W.; Validation: J.L.W.; Visualization: J.L.W.; Writing – original draft: L.D.H. and J.L.W.; Writing – review & editing: A.W.-S., A.A.O.H., L.D.H., R.K., and J.L.W.
Disclosures
Lorena DoVal Herbert, Rachel Kim, Asim Hassan, Alison Wilkinson-Smith, and Jeff Waugh report no competing interests, and no fiduciary, employment, or familial conflicts that affected the design, execution, or description of this research.
Funding
This project was supported by the American Academy of Neurology, Career Development Award (JW) and the CTSA NIH Grant UL1-RR024982, which supported the UT Southwestern REDCap database. These funding agencies did not contribute to the design or execution of this study.
Data availability
Survey results and summary data from our review of clinical encounters are available upon request. The original records from clinical encounters include protected health information and are not available for distribution.