Introduction
Surgical resection of insular tumors was pioneered in the 1990s by Yaşargil and Reeves Reference Yaşargil and Reeves1 and is a subspecialization within neuro-oncologic surgery requiring detailed knowledge of surgical anatomy, microsurgical technique, and intraoperative mapping to maximize extent of resection and minimize morbidities. Support for aggressive insular glioma surgery is reinforced by regional tumor biology, symptom improvement (i.e., seizures), and increased survival associated with extent of resection. Reference Sanai, Polley and Berger2,Reference Skrap, Mondani and Tomasino3
Methods
A literature search using PubMed was conducted using the term “insular glioma” as a screening tool with the authors selecting appropriate articles to cover the main goals of this article regarding the pathogenesis, incidence, clinical presentation, genetics, anatomy, and surgical management strategies/outcomes for insular gliomas. Two hundred and forty-seven articles were screened. The largest clinical series in the literature were used to discuss surgical management strategies and patient outcomes. Inclusion of senior neurosurgeon series with an established reputation in insular glioma surgery was targeted in this review under guidance of the senior author. Reporting of outcome variables including seizure control, extent of resection, and patient survival varied and are summarized in Table 1.
Table 1. Summary of the largest published insular glioma surgical series ranging from 2002 to 2017

* ESTR, extensive subtotal resection (>90% tumor); GTR, gross total resection; STR, subtotal resection (>70% tumor).
Incidence, Grade, and Clinical Presentation of Insular Gliomas
Gliomas are disproportionately represented in the insula, which makes up 2% of the total cortical area, yet is affected in 25% of low-grade gliomas and up to 10% of high-grade gliomas. Reference Yaşargil and Reeves1,Reference Duffau and Capelle4 Cell migration routes and proximity to subventricular and subgranular stem cell zones, the purported cell of origin in gliomas, may account for these observations. Reference Mandonnet, Capelle and Duffau5,Reference Kalani, Kalani, Gwinn, Keogh and Tse6 In terms of tumor grade, a series of 218 insular glioma patients found the majority of insular lesions are low grade (grade II (54.3%), grade III (34.1%), and grade IV (11.6%)). Reference Yaşargil and Reeves1,Reference Hervey-Jumper, Li and Osorio7–Reference Duffau9 The natural history of grade II insular lesions is transformation to a higher malignant grade with a median latency of 7–8 years, Reference Sanai, Polley and Berger2,Reference Cavaliere, Lopes and Schiff10 although it is interesting to note the median time to malignant progression is 3–6 years Reference Smith, Chang and Lamborn11–Reference Chaichana, McGirt, Laterra, Olivi and Quiñones-Hinojosa15 in grade II gliomas in other regions, suggesting a more indolent course for insular lesions. The typical clinical presentation is seizures in patients younger than 40 years of age. Reference Duffau9 Patients can also experience other psycho-emotional changes, anxiety about being in public places, fear of venturing into new tasks that may be more demanding mentally, and limiting exposure to social stimuli. Patients often describe postoperative relief of these symptoms. Reference Yaşargil, Krisht, Türe, Al-Mefty and Yaşargil16
Molecular Genetics and Histological Classification of Insular Gliomas
As the genetic classification of brain tumors evolves, distinct IDH1/IDH2 (isocitrate dehydrogenase) mutation profiles emerged in purely insular vs. paralimbic World Health Organization (WHO) Grade II gliomas. Reference Gozé, Mansour, Rigau and Duffau17 In a study of 11 insular tumors, IDH 1/2 mutations were present in all purely insular lesions in contrast to IDH wild-type lesions with more widespread paralimbic involvement (only 20/36 (55%) of tumors were IDH 1/2 mutant). Reference Gozé, Mansour, Rigau and Duffau17 IDH wild-type tumors also demonstrated a more malignant clinical course despite similar extent of resection, highlighting a relationship between genetics and outcome. The dominant lineage of low-grade insular gliomas (i.e., IDH mutant) is unresolved. Most authors report 1p/19q codeletion is relatively uncommon among IDH mutant insular gliomas, thus making them diffuse infiltrating astrocytomas per WHO classification (tumors likely harboring ATRX loss and TP53 mutation). Reference Hervey-Jumper, Li and Osorio7,Reference Gozé, Mansour, Rigau and Duffau17–Reference Gozé, Rigau, Gibert, Maudelonde and Duffau19 However, this finding has not been universal and some have reported that 1p/19q codeleted tumors (i.e., oligodendrogliomas) actually make up the majority of insular gliomas. Reference Wu, Aldape and Lang20,Reference Eseonu, ReFaey, Garcia, Raghuraman and Quinones-Hinojosa21
Anatomy of the Insula
Surface Anatomy
A triangular depression on the lateral temporal region defines the insular cortex at 3 months gestation. The surrounding cerebral lobes rapidly engulf this depression, becoming the opercular neocortex and forming the sylvian fissure. Reference Kalani, Kalani, Gwinn, Keogh and Tse6
The sylvian fissure is a deep divide between the frontal/parietal and temporal lobes beginning at the anterior perforated substance and extending posteriorly to the supramarginal gyrus (Figure 1). Reference Yaşargil and Reeves1 The anterior stem trifurcates at the sylvian point into the horizontal, ascending, and posterior rami, which define the pars orbitalis, pars triangularis, and pars opercularis. The posterior ramus extends from the sylvian point to the supramarginal gyrus and forms the sylvian line.
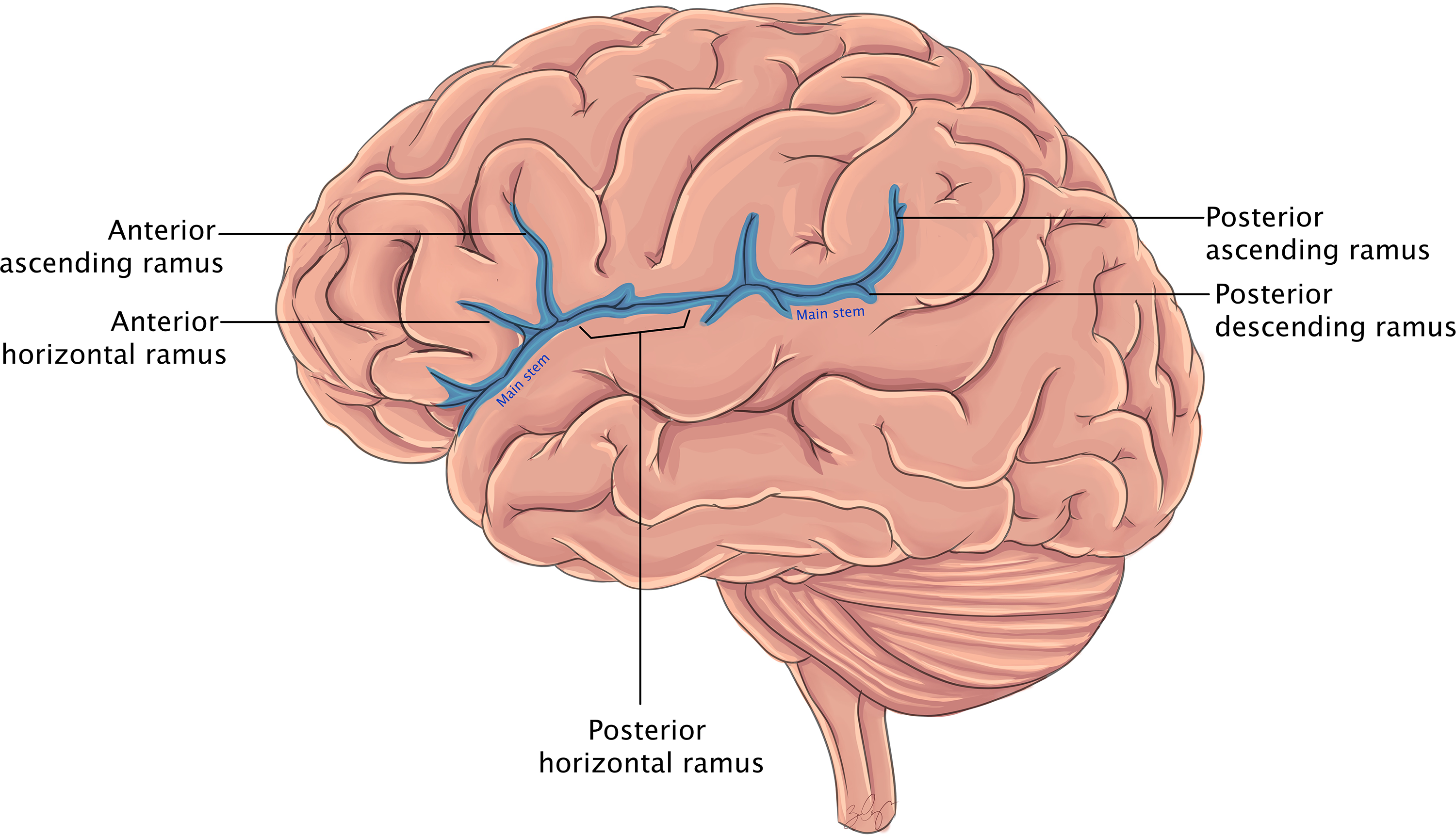
Figure 1. Cartoon rendering of the lateral cortical surface and sylvian fissure components labeled, within the contents of the insula remain hidden.
The insula is surrounded by a perimeter called the limiting sulcus (with anterior, superior, and inferior limbs; Figure 2). Tumors extending above the superior limiting sulcus can involve language (i.e., impaired perception and conduction aphasia), motor paresis, and short-term memory deficits. Reference Duffau, Gatignol, Mandonnet, Capelle and Taillandier22 Anatomically, these finding are explained by the corticospinal tract within the corona radiata, and, laterally, the superior longitudinal fasciculus and the horizontal portion of the arcuate fasciculus. The inferior limiting sulcus is an important landmark to anticipate the white matter tracts involved in speech, language, and visual functions. A directionality change from anterior to posterior marks the deep posterior portion of the lateral geniculate body, aiding in identification and preservation of the optic radiations. Reference Ribas, Yagmurlu, Wen and Rhoton23

Figure 2. Cartoon rendering of wide sylvian fissure split revealing the insula, which is bounded from the surrounding cortical surfaces by the anterior, superior, and inferior limiting sulci.
Overlaying the insular surface are superiorly the frontoorbital and frontoparietal opercula and inferiorly the temporal opercula. The frontoorbital operculum is composed of the orbital gyrus and the pars orbitalis. The frontoparietal operculum is composed of the pars triangularis, pars opercularis (i.e., speech motor), the inferior portions of the precentral and postcentral gyri (i.e., face motor), and the superior portion of the supramarginal gyrus. The temporal operculum is composed of the superior temporal gyrus, temporal pole, and the inferior portion of the supramarginal gyrus. The entorhinal sulcus separates the uncus and temporal operculum from the anterior perforated substance, which exploration is generally avoided to preserve the lenticulostriate arteries. Reference Türe, Yaşargil, Al-Mefty and Yaşargil24
The central insular sulcus is parallel to the rolandic sulcus and divides anterior insula from posterior. The accessory and transverse gyri form the anterior insular pole. On average, three short insular gyri (anterior, middle, and posterior) compose the remaining anterior insula. The posterior insula is formed consistently by anterior and posterior long gyri.
The anterior insula is connected to the limbic system, amygdala, cingulate and posterior orbito-frontal cortex serving autonomic, olfactory, and emotional function. Reference Michaud and Duffau25 The mid-portion is involved in memory and language. The dorsal zone is involved in gustatory, viscerosensory, and somatosensory functions.
White Matter Anatomy
In the 19th century, Broca observed that aphasic patients harbored a caudal inferior frontal gyrus lesion; however, one patient had an insular and gyri continuous with the inferior frontal gyrus lesion suggesting a connective pathway to transmit speech information yet to be described. Reference Dronkers, Plaisant, Iba-Zizen and Cabanis26
The insula is surrounded by the superior longitudinal (arcuate) fasciculus, which connects the temporal region to the parietal and frontal regions, and results in phonemic paraphasia if stimulated. The extreme capsule consists of the subcortical white matter of the insula and unites the frontal and temporal opercula. The external capsule is between the claustrum and the putamen and is comprised of the uncinate fasciculus, inferior frontooccipital fasciculus (IFOF), and claustrocortical fibers which pass under the limiting sulci. Reference Ribas, Yagmurlu, Wen and Rhoton23 The uncinate fasciculus is anterior to the IFOF in the region of the limen insula (Figure 3). It supplies the motor interconnection between limbic and paralimbic structures (temporal pole and orbital gyri) and interconnects the limbic structures (the amygdala/uncus to the subcallosal area/gyrus rectus). Reference Türe, Yaşargil, Al-Mefty and Yaşargil24 If one makes an insular incision just posterior to the anterior perforated substance, the uncinate fasciculus will be violated, but if the incision is limited to 6 mm the IFOF and optic radiations should be spared and language disturbances avoided. Reference Duffau, Gatignol, Mandonnet, Capelle and Taillandier22,Reference Ribas, Yagmurlu, Wen and Rhoton23

Figure 3. Enhanced axial and coronal T1 magnetic resonance imaging (MRI) of the brain demonstrating with the region of the limen insula highlighted in red (theoretical tumor) queried for white matter tracts passing through this region. The ventral language stream consisting of the IFOF and uncinate fasciculus are appreciated in green (anterior−posterior fiber orientation) and blue (ventral−dorsal fiber orientation) and comprise the external capsule as seen on the coronal image. The image demonstrates there is no natural separation of these fibers with the uncinate fasciculus situated ventral and anterior to the IFOF as the blue region highlights the uncinate fibers path ventral−dorsal to connect the interior frontal to the anterior temporal lobe. The IFOF originates in the inferior frontal gyrus and terminates widely in the posterior temporal/parietal/occipital lobes. Intraoperative stimulation of the ventral language pathways results in semantic paraphasias (e.g., substitution of words in a similar category like orange when shown an apple).
The IFOF, carrying semantic language information, originates in the inferior frontal gyrus, passes beneath the anterior limiting sulcus, travels posterior and superior to the uncinate fasciculus (noting there is no natural separation between these fiber tracts), and then continues posteriorly to terminate within the temporal, parietal, and occipital lobes.
Work by Hickok and Poeppel describes ventral and dorsal main streams of white matter relating to language function, and Daffau has translated these tenants as a neurosurgeon in insular glioma surgery. Reference Duffau, Gatignol, Mandonnet, Capelle and Taillandier22,Reference Duffau27,Reference Hickok and Poeppel28 The ventral stream is composed of the uncinate fasciculus and the IFOF with intraoperative stimulation producing semantic paraphasias (Figure 3). The IFOF participates in reading, attention, visual processing, and language bilaterally. Reference Duffau27,Reference Houston, Allendorfer, Nenert, Goodman and Szaflarski29 The dorsal stream is composed of the superior longitudinal fasciculus and the arcuate fasciculus. Intraoperatively, stimulation induces phonemic paraphasias, apraxia, and deficits in repetition. Reference Duffau, Gatignol, Mandonnet, Capelle and Taillandier22
Lenticulostriate Artery Position
The lenticulostriate arteries (vary from 1 to 15) course through the anterior perforated substance and are difficult to identify and avoid during insular glioma surgery. Reference Lang, Olansen and DeMonte30 The mean distance from the insular apex to the most lateral lenticulostriate artery is less than 1.5 cm in the normal brain (may vary with tumor size/morphology) and if injured can cause postoperative hemiparesis. Reference Signorelli, Guyotat, Elisevich and Barbagallo31,Reference Türe, Yaşargil, Al-Mefty and Yaşargil32 Tumor infiltration in the anterior perforated substance can be identified on sagittal magnetic resonance imaging (MRI) as T2 hyperintense signal in the anterior/ventral portion of the insula. Reference Velasquez, Caballero and Vazquez-Barquero33
Preoperative angiograms demonstrate the lenticulostriate arteries are displaced medially by tumor bulk in 80% but are surrounded by more diffuse tumor in 20%. Reference Moshel, Marcus, Parker and Kelly34 Similar information can typically be obtained from T2-weighted MRI scans. Strategies to identify the lenticulostriate arteries are to follow the M1 until the first perforating arteries are encountered to establish a boundary for resection; however, this dismisses the distal artery course. Another method is to use the claustrum as a limit for resection; however, this may be infiltrated with tumor making intraoperative identification difficult. A third method is the use of 3-T time-of-flight MRI. Reference Saito, Kumabe and Inoue35,Reference Ghali36 Several influential surgeons advocate for an adopted approach to intentionally leave a small residual in the anterior perforated substance with an emphasis on minimizing morbidity. Reference Yaşargil and Reeves1,Reference Duffau9,Reference Ebeling and Kothbauer37 Perhaps heeding this advice, permanent complication rates have decreased over the past two decades in operative series. Reference Skrap, Mondani and Tomasino3
The M2 branch over the central insular sulcus often terminates as the rolandic artery, which is useful knowledge to avoid injury to this vessel. Reference Benet, Hervey-Jumper, González Sánchez, Lawton and Berger38 Lang and Duffau advise to exercise caution with large posterior M2 perforating branches as they may supply the corona radiata with injury an alternative explanation for postoperative hemiparesis aside from a lenticulostriate stroke involving the internal capsule. Reference Michaud and Duffau25,Reference Hentschel and Lang39
Surgical Approaches and Considerations for Insular Gliomas
Evidence concludes extent of resection in gliomas impacts prognosis (i.e., overall survival) and morbidity (i.e., seizure control), which motivates surgeons to aggressively resect insular lesions. Reference Sanai, Polley and Berger2,Reference Skrap, Mondani and Tomasino3,Reference Duffau9,Reference Eseonu, ReFaey, Garcia, Raghuraman and Quinones-Hinojosa21 Two dominant insular surgical approaches are transcortical and transsylvian. In a cadaveric evaluation comparing the two approaches, the transcortical approach provided larger exposures suggesting an opportunity for greater extent of resection. Reference Benet, Hervey-Jumper, González Sánchez, Lawton and Berger38 Another retrospective study had equivalent outcomes with the exception of a transcortical approach offering greater safety to the superior−posterior insular quadrant. Reference Przybylowski, Baranoski, So, Wilson and Sanai40 Ultimately, the approach is a multifaceted decision and involves the experience/comfort of the individual surgeon as well as patient anatomy and neurological function (including results of cortical mapping).
Specific intraoperative tools to consider in insular glioma surgery include somatosensory evoked potential neuromonitoring and bipolar or unipolar cortical and subcortical stimulation speech/motor mapping, which are detailed below. Also, surgeons routinely use intraoperative neuroimaging (navigation systems, ultrasound, or MRI) to assess local anatomy and progress of resection. At the time of writing, ultrasound and intraoperative MRI have the ability to provide updated anatomic information considering brain shift after surgical exposure and tumor debulking. Intraoperative MRI images also allow the opportunity to be sent to the neuronavigation system to reregister for updated navigation. Papaverine can be used topically on the insular vasculature to help prevent/treat vasospasm. Microdoppler can help to identify major blood vessels and confirm flow. Microclips may be needed in the case of vascular injury. Lastly, tools to remove tumor tissue can vary from general microsurgical technique with dissector instruments and suction, which may allow development for natural planes and is gentle on the tissue, to the use of ultrasonic aspirators, which may allow for efficient and precise tissue removal even in firm lesions with limited traction on adjacent structures.
Transcortical
The transcortical approach emphasizes a subpial resection of the frontal and temporal noneloquent opercula (Figure 4). Identification and preservation of motor and speech cortical and subcortical structures is critical to this approach. Accordingly, awake craniotomies facilitate speech mapping in the dominant hemisphere.

Figure 4. (A–D) Axial fluid-attenuated inversion recovery (FLAIR) MRI preoperative images representative of the typical appearance of an insular glioma with extension into the frontal and temporal regions. (E–H) Coronal T1 with contrast MRI preoperative images demonstrating the lesion is hypointense and non-enhancing consistent with a low-grade glioma. (I–L) Axial FLAIR MRI postoperative images demonstrating a near total resection of the lesion (M–P) Coronal T1 with contrast MRI postoperative images confirming the extent of resection.
Berger et al. Reference Sanai, Polley and Berger2 reported a series of insular tumors approached transcortically and divided the insula into four zones to organize surgically relevant insular function and to correlate with achievable extent of resection. Zone I, anterior to the foramen of Monroe and superior to the sylvian fissure, was amendable to the most extensive resections, correlated with patient survival, was the most frequently involved region of the insula harboring tumors, and was also the most frequent location for complications. Reference Sanai, Polley and Berger2,Reference Hervey-Jumper, Li and Osorio7 Zone I can be entered through an inferior frontal gyrus window as the main motor speech output center can be posterior to this region. Reference Duffau, Gatignol, Mandonnet, Capelle and Taillandier22 Zone II is superior to the sylvian fissure, posterior to foramen of Monroe. In zone II, the opercular segment of the precentral/postcentral gyrus is encountered, accounting for contralateral motor/sensation control of the face. Face function may recover after resection of this region. Reference Sanai, Polley and Berger2,Reference Duffau9,Reference LeRoux, Berger, Haglund, Pilcher and Ojemann41 Entering zone II is more difficult as the overlying operculum is often eloquent. Zone III is inferior-posterior including the posterior temporal operculum, and zone IV is inferior−anterior including the anterior temporal operculum. Reference Sanai, Polley and Berger2 In zone III, Heschl’s gyrus is encountered; however, complete resection of Heschl’s gyrus limited to one hemisphere is not thought to contribute to loss of hearing. Reference Michaud and Duffau25 Posterior to this the precise location of language (i.e., Wernicke’s area) is highly variable and often cortical, and subcortical intraoperative mapping is utilized for identification and protection of this eloquent cortex.
Transsylvian
The transsylvian approach was developed in an effort to minimize resection of unaffected brain. Reference Lang, Olansen and DeMonte30,Reference Hentschel and Lang39,Reference Wang, Wu, Chen, Xu, Yang and Cai42 The anatomical boundaries for the insular lesion are the peri-insular sulcus and basal ganglia. The sylvian fissure is first split widely. As the M2/M3 segments and veins cover the insular cortex, tumor removal is piecemeal through windows created between vascular structures. Central debulking of tumor (depth 1–2 cm) is followed by a deliberate sequential stepwise process to work around the circumference of the lesion.
Perils of the transsylvian approach published by Lang et al. Reference Lang, Olansen and DeMonte30 include a wide split of the sylvian fissure, conscious identification of the periinsular limiting sulci to delineate superior and inferior resection planes, a conscious effort to identify the most lateral lenticulostriate artery to define the medial resection plane, and to work in a subpial plane to protect large perforating arteries (arising from posterior M2 branches).
Laser Interstitial Thermal Therapy
Laser interstitial thermal therapy is a minimally invasive alternative for cytoreductive treatment of insular gliomas with use in small lesions, residual lesions as an adjunct, and larger lesions (may require multiple catheters). Safety and efficacy have yet to be reported in large series. As its advocates note, avoiding key vascular structures is critical and may ultimately limit the efficacy of this technique for this indication. Additionally, insular sulci may limit thermal spread and can be compensated with multiple laser fibers.
Trajectories proposed for ablation of an insular lesion include an entry point off midline passing through the superior or middle frontal gyrus and adjacent to the lateral ventricles to the inferior aspect of the lesion (Figure 5). An alternative described trajectory is a supraorbital entry point, 2 cm superior and 1 cm lateral to the medial orbital rim. Reference Baydin, Gungor, Holanda, Tanriover and Danish43 A single trajectory and laser ablation session is ideal; however, if the lesion is larger than 3 cm in diameter multiple trajectories may be necessary. Even staged procedures to treat larger lesions (avoiding excessive edema) have been described. Reference Hafez, Liekweg and Leuthardt44

Figure 5. (A) Axial and coronal contrast enhanced magnetic resonance imaging of the brain demonstrating a right insular enhancing lesion, biopsy proven glioblastoma. (B) Axial and coronal contrast enhanced magnetic resonance imaging of the brain of the same patient post-treatment with laser interstitial therapy with probe trajectory demonstrated.
Intraoperative Mapping
A retrospective review of over 8000 patients who underwent glioma resection concluded that intraoperative mapping was associated with fewer late severe neurologic deficits and more extensive resection. Reference De Witt Hamer, Robles, Zwinderman, Duffau and Berger45
The techniques for intraoperative monitoring are varied and can include continuous motor evoked potentials, electromyography, electrocorticography, direct electrical stimulation of the cortex, and subcortical stimulation. Several reviews on intraoperative technique can be references for further details. Reference Hervey-Jumper, Li and Lau46–Reference Rey-Dios and Cohen-Gadol49
Motor evoked potential monitoring is performed under general anesthesia and can detect the corticospinal tract, monitoring its integrity. Motor evoked potentials are also sensitive to ischemic changes and can be an early warning sign to halt resection. A change in amplitude of 50% is used as a marker for severe ischemic changes. Reference Alimohamadi, Shirani and Shariat Moharari47
Electrocorticography can be used to detect seizures/after-discharges to prompt rapid treatment of seizures with iced saline/anticonvulsants and also to determine the stimulation thresholds for motor and speech responses elsewhere. In addition to cortical mapping of motor and speech function, if direct cortical stimulation at the end of resection produces the same responses at the same intensity before resection this can reassure the surgeon the patient will go on to make a complete recovery from an immediate postoperative deficit. Reference Duffau, Capelle, Lopes, Faillot, Sichez and Fohanno50,Reference Duffau, Capelle and Sichez51
Subcortical mapping can aid in the detection of functional white matter tracts involved in speech/language and motor function. Reference Bello, Gallucci and Fava52 Identification and preservation of the IFOF will indirectly aid in preserving the anterior perforated substances, which lies deep to it. Additionally, for a lesion occupying the anterior insular lobule intraoperatively, the frontal horn of the lateral ventricle can be a navigation point to halt resection; however, in this region injury to the subcallosal fasciculus (connecting the supplementary motor area to the head of the caudate) can result in impairment of spontaneous speech similar to a transcortical motor aphasia. Reference Signorelli, Guyotat, Elisevich and Barbagallo31 Some surgeons advocate the routine use of continuous intraoperative neuropsychological tasks complementary to subcortical stimulation to monitor language function. Reference Skrap, Mondani and Tomasino3
Patient Outcomes
Median extent of resection averages 80% in most major series. Reference Skrap, Mondani and Tomasino3,Reference Duffau9,Reference Eseonu, ReFaey, Garcia, Raghuraman and Quinones-Hinojosa21,Reference Lang, Olansen and DeMonte30,Reference Sanai, Polley, McDermott, Parsa and Berger53–Reference Vanaclocha, Sáiz-Sapena and García-Casasola55 Morbidity rates vary across series with a notable trend of transient speech and motor deficits, which improve with rehabilitation. Causes of morbidity include encounters with the lenticulostriate arteries, excessive opercular retraction, manipulation of the middle cerebral artery, injury to the long M2 perforating vessels, or interruption of the corona radiata. Reference Lang, Olansen and DeMonte30 The rate of permanent morbidity is less than 15% in major series. Reference Yaşargil and Reeves1,Reference Duffau9,Reference Hentschel and Lang39,Reference De Witt Hamer, Robles, Zwinderman, Duffau and Berger45,Reference Zentner, Meyer, Stangl and Schramm54–Reference Simon, Neuloh, Von Lehe, Meyer and Schramm57 Major series are presented in Table 1.
Utilizing the transsylvian approach, Yasargil et al. Reference Yaşargil, Krisht, Türe, Al-Mefty and Yaşargil16 reported his series of 429 patients with limbic and paralimbic gliomas. Gross total resection was achieved in 80% of patients with pure insular tumors. All 191 patients experienced intractable seizure improvement with 30% of patients off medications, 50% at reduced doses, and 20% with reduced seizure frequency. Similar seizure reduction rates of 89% Reference Zentner, Meyer, Stangl and Schramm54 and 78% Reference Duffau9 in other series confirm these results. Hemiparesis was observed in 2/191 patients with imaging suggesting lenticulostriate injury. In 50% of patients, a transient hemi-motor syndrome sometimes with aphasia was evident. The majority of patients returned to their former lifestyles. Lang et al. Reference Lang, Olansen and DeMonte30 also reported a series of 22 patients utilizing a transsylvian approach in which 36% developed a language or motor deficit postoperatively, however, at 3-month follow-up all but 9% (2 patients) enjoyed a recovery.
Morbidity in a series of 129 procedures Reference Hervey-Jumper, Li and Osorio7 utilizing the transcortical approach had a short-term complication rate of 26.4%. The most common deficits were motor (7.8%), facial weakness (9.3%), and aphasia (16.3%). At 3-month follow-up, 99.2% of the face motor deficits resolved, 1.6% had a persistent motor deficit, and 0.8% had a persistent speech deficit. In his series of 51 cases of WHO II insular gliomas, Duffau reported a transient deficit rate of 59% (hemiplegia in 2/3), which 96% ultimately enjoyed a return to baseline or improved. Reference Duffau9 A notable deficit, predictable from preoperative imaging is Foix−Chavany−Marie syndrome (i.e., paralysis of chewing/swallowing) with removal of the insula and rolandic operculum and an athymhormic syndrome in nondominant insular lesions. Reference Duffau9 In Berger’s series of 115 procedures using transcortical windows reported a postop morbidity profile of 4.8% dysarthria, 7.7% facial droop, 1.9% hemiparesis, of which all but two patients enjoyed full or partial recovery. Reference Sanai, Polley and Berger2 Alfredo Quinones−Hinojosa published on 74 patients and similarly reported 10.8% motor deficit, 4.1% sensory deficit, 6.8% aphasia, all of which but 2.7% recovered by 6 months. Reference Eseonu, ReFaey, Garcia, Raghuraman and Quinones-Hinojosa21 In an Italian cohort by Skrap et al. describing 66 nonenhancing insular gliomas, postop neurological worsening was seen in 33.4% of patients: 16.7% hemiplegia, 16.7% aphasia, all of which but two patients made full recoveries. Reference Skrap, Mondani and Tomasino3
There are less data on patient survival outcomes in these insular series as reporting widely varied. Reporting was also nonuniform and captured overall survival, progression-free survival, and survival rates at the average follow-up interval. Further, the heterogeneity of tumor grade/genetics within this anatomic location adds to the complexity of the interpretation of survival outcomes. The largest cohort reporting survival outcomes details for insular gliomas confirmed well-established evidence that extent of resection has a positive impact on glioma patient survival. Reference Eseonu, ReFaey, Garcia, Raghuraman and Quinones-Hinojosa21 At 5 years, low-grade glioma patients (n = 23) with an extent of resection greater than 90% had a 100% survival compared to a less than 90% extent of resection had a 80% survival. In patients with high-grade gliomas at 2 years, survival was 84% with a greater than 90% extent of resection and 44% with less than 90% resection. Extent of resection was predictive of both overall survival and progression-free survival in both high- and low-grade gliomas.
Conclusions
Surgical resection of insular gliomas offers important benefits of extended survival and improved quality of life and is gaining popularity, though represents a formidable surgical task. Immediate postoperative deficits occur in greater than 20% of cases though often transient, highlighting an opportunity for preoperative informed discussions with patients. Reference Duffau9,Reference Hentschel and Lang39,Reference Hervey-Jumper and Berger58 Detailed knowledge of the surgical anatomy, approaches, tumor pathology, advanced operative skill sets, and intraoperative tools/adjuncts remains within the purvue of neurosurgical oncology as a unique subspecialty to provide the ability to offer a meaningful intervention for a malignant disease.
Acknowledgements
We would like to thank Denise Sprinkle for her invaluable technical support generating the white matter tracts in Figure 3.
Conflict of Interest
Jaclyn J. Renfrow, MD – None; Bao-Quynh Julian, MD – None; Desmond A. Brown, MD, PhD – None; Stephen B. Tatter, MD, PhD – Clinical trial research grants from Monteris Medical, Inc. and Arbor Pharmaceuticals, Inc., Unrelated patents issues to Wake Forest School of Medicine, Former members of DSMBs for Bristol Myers Squibb; Adrian W. Laxton, MD – consulting fees and participation on the data safety monitoring board/advisory board for Moneris Medical; Glenn J. Lesser, MD – Grants/Contracts from Novocure, Oblato, Denoco Biopharma (Site PI of clinical trial with payments to institution), Consulting fees from BTG, Monteris, SDP Oncology, Participation on the DSMB for Agios, Incysus (IN8bio), and ONO Pharma; Roy E. Strowd, MD – Grants from the National Institutes of Health (R21CA248106, R21CA229027), Department of Defense (BC181274), Jazz Pharmaceuticals (VT10820), American Society for Clinical Oncology (Career Development Award – 2018), American Board of Psychiatry and Neurology (Research Award – 2021), Royalties/Licenses with Lecturio and Elsevier, Consulting fees from Monteris Medical and Novocure, membership on the North Carolina Medical Society Foundation Board of Trustees; Ian F. Parney, MD, PhD – Consulting fees from AbbVie Pharmaceuticals (paid to institution).
Author Contribution
JJR: Conceptualization; Software; Visualization; Writing – original draft; Writing – review and editing. BJ: Software; Visualization; DAB: Software; Visualization; Writing – review and editing; SBT: Conceptualization; Data curation; Writing – review and editing; AWL: Writing – review and editing; GJL: Methodology; Writing – review and editing; RES: Methodology; Writing – review and editing; IFP: Conceptualization; Methodology; Supervision; Writing – review and editing.
Abbreviations
ESTR, extensive subtotal resection; FLAIR, fluid-attenuated inversion recovery; GTR, gross total resection; IDH1/IDH2, Isocitrate Dehydrogenase; IFOF, inferior frontooccipital fasciculus; LITT, laser interstitial thermal therapy; MRI, magnetic resonance imaging; SSEP, somatosensory evoked potential; STR, subtotal resection; WHO, World Health Organization.








