INTRODUCTION
Newcastle disease (ND), a viral disease caused by the avian paramyxovirus serotype 1 (APMV-1), is one of the most important poultry diseases worldwide [1]. The disease probably emerged more than 150 years ago in wild birds in Scotland [Reference Macpherson2, Reference Kuiken3]. It was first identified in poultry around the 1920s in Indonesia and England and today is endemic in many countries [Reference Awan4]. Epidemics are observed mainly in Central and South America, Africa, and Asia [Reference Alexander5]. The threat of ND is still present in Europe where sporadic outbreaks occur [Reference Alexander6]. Known also as pseudo-fowl plague, ND is widespread, primarily affecting avian species [7]. The virus may affect more than 8000 bird species [Reference Roy8, Reference Zeng9], including most domestic species whether vaccinated or not [Reference Kuiken10–Reference Zhu15]. Domestic species usually appear to be more susceptible to the virus, and suffer from a higher mortality from it than wild species. The role of the specific diversity and composition of avian communities in the epidemiology of the disease is still largely unexplored. In particular, the reservoirs of the various ND virus (NDV) are not well identified and the variation in pathogenicity in NDV strains and host species combinations has only been partially described [Reference Kuiken10–Reference Zhu15]. Nonetheless, due to the high pathogenicity and strong dispersion capacity of some strains [16], the OIE recognized ND as a list A disease alongside avian influenza [Reference Leighton, Heckert, Thomas, Hunter and Atkinson17].
Since ND first emerged on the African continent in the 1930s and 1940s, the disease has become endemic in many countries [Reference Awan4, Reference Alexander5]. Despite stringent control efforts, ND continues to be detected with high serological prevalence rates in African production systems [Reference Bleich18]. In African rural communities, livestock production is an important economic asset. Chickens, representing the species with the smallest economic value per unit, are widely used, notably by the poorest members of a community, as a source of protein and income [Reference Mekonnen19]. Each year about 18% of the African poultry population die from diverse infections [20, Reference Sayila21]. In some countries, ND alone is responsible for nearly 80% of poultry mortality in village systems [Reference Spradbrow22]. Such mortality rates have a huge impact on the economies of some developing countries and the food security of their inhabitants [Reference Khalafalla, Abdel Aziz and El Hassan23, Reference Alders and Pym24]. Although the spread of the disease in rural production systems and backyard chicken flocks does not appear to be as rapid as in industrial systems, with the disease taking weeks to contaminate a flock and months to reach adjacent villages [Reference Awan4], the disease burden in rural poultry production systems can seriously damage livelihoods. The study and control of ND is therefore a substantial challenge for development over the coming years.
Much remains unknown regarding the ecology of NDV and the epidemiology of this avian pest in African ecosystems. The sparse available data are scattered throughout individual studies and are inconclusive. Mortality can reach 100% when a non-immunized chicken population is infected by a highly pathogenic virus strain [Reference Spradbrow12], the incubation and excretion periods can vary from 2 to 21 days for domestic poultry [Reference Kaleta, Baldauf and Alexander25] and finally the survival of the virus in the environment varies from 21 days to several years depending on the substrate. All these data are synthesized in Figure 1.

Fig. 1. Newcastle disease and virus eco-epidemiological characteristics: survival of the virus for different substrates, temperatures and pHs; incubation periods and transmission modes. (a) [Reference Leighton, Heckert, Thomas, Hunter and Atkinson17], (b) [Reference Barre and Delor95], (c) [Reference Roy8], (d) [Reference Martin and Spradbrow96], (e) [Reference Olesiuk97], (f) [1], (g) [Reference Awan4].
Although some country-wide studies have been conducted [Reference Njagi26], integrative analyses that might reveal ecological and phylogeographical patterns of ND at the continental level are to our knowledge still missing. The present study, a systematic review of the literature produced over the past 30 years involving ND in Africa, addresses two questions. (1) What are the phylogenetic relationships in NDV strains isolated in backyard chickens in Africa? For this first question, particular emphasis is placed on the relationships between virulent and avirulent NDV strains. (2) How is ND distributed geographically and seasonally in Africa? For this second question, meta-analyses of ND serological prevalence variation according to biogeographical factors and of the association between the occurrence of ND epidemics and seasonal climatic characteristics are undertaken.
MATERIAL AND METHODS
Phylogeographical analyses of NDV strains on the African continent from 1994 to 2007
The objective of the analysis was to describe gene and nucleotide diversity, to study lineage stability, and to depict the evolution of strains at the African continental level.
The maximum amount of information on NDV strains characterized from a molecular point of view in Africa up to 2009 was obtained from Genbank. Six European fusion protein F sequences and 133 African sequences isolated from a variety of hosts between 1994 and 2007 were downloaded (see Supplementary Table S1 for the origin and accession numbers of strains). The nucleotide sequences were aligned with ClustalW [Reference Thompson27] as implemented in BioEdit software [Reference Hall28]. A segment around the protein F fusion site of 89 bp length was found to be common to all sequences. Genetic polymorphism measures were estimated using DNAsp 4·0 [Reference Rozas29]. We drew a maximum-likelihood tree using Kimura two-parameter distance with 1000 bootstraps using MEGA (see Supplementary Fig. S1). A median-joining network was constructed using the program Network 4·5 [30]. The gene and nucleotide diversity (π) were computed by lineage following notation proposed by Aldous et al. [Reference Aldous31]. Finally, to detect signatures of population expansion, Tajima's D test [Reference Tajima32] and Fu's Fs test [Reference Fu33] were used. Calculations and tests were performed using Arlequin v. 3.1 [Reference Schneider, Roessli and Excoffier34].
Systematic review
To undertake a systematic review, we followed the four-step protocol described by Moher et al. [Reference Moher35]. This process is presented along with the search results covering documents published between 1980 and 2009 (see Supplementary Fig. S2 for the systematic review synthesis). We searched for information on ND in African rural backyard systems in scientific articles, conference proceedings, PhD theses, and expert reports. An initial set of 2686 documents obtained by consulting databases with Boolean search expressions, was reduced to 201 documents after reading the summaries of each document and selecting those that provided quantitative information on the epidemiology of ND in Africa. Finally 88 documents were selected for inclusion in the study. These documents were carefully examined to establish a list of ND risk factors supported by scientific evidence.
Meta-analysis
Patterns of occurrence of ND in backyard chickens in rural areas of Africa
The objective of the analysis was to depict the biogeographical and seasonal patterns of occurrence of ND in African rural backyard systems and to identify some of the factors underpinning this pattern.
Geographical study
For the meta-analysis of geographical patterns, the list of documents used was further reduced to those reporting antibody prevalence estimates (hereafter referred to as ![]() i) obtained from random sampling protocols in African backyard chicken populations outside outbreak periods (i.e. prevalences estimated following the declaration of an outbreak were excluded). In all these studies, NDV antibodies had been titrated using the haemagglutination inhibition (HI) test or the enzyme-linked immunosorbent assay (ELISA) as recommended by the OIE [1]. The 14 documents that were retained reported prevalence estimates for 12 countries from 1984 to 2005 (Benin, Cameroon, Ethiopia, Madagascar, Morocco, Mauritania, Niger, Nigeria, Democratic Republic of Congo, Tanzania, Zambia, Zimbabwe; see Fig. 2 and Supplementary Table S2 for documents used for the prevalence meta-analysis in the geographical study). Three of the 14 documents reported the results of longitudinal studies (estimates from a same population at different dates). For each of these studies, the prevalence estimates provided were pooled into one single average prevalence estimate that was considered as a single statistical unit in the meta-analysis.
i) obtained from random sampling protocols in African backyard chicken populations outside outbreak periods (i.e. prevalences estimated following the declaration of an outbreak were excluded). In all these studies, NDV antibodies had been titrated using the haemagglutination inhibition (HI) test or the enzyme-linked immunosorbent assay (ELISA) as recommended by the OIE [1]. The 14 documents that were retained reported prevalence estimates for 12 countries from 1984 to 2005 (Benin, Cameroon, Ethiopia, Madagascar, Morocco, Mauritania, Niger, Nigeria, Democratic Republic of Congo, Tanzania, Zambia, Zimbabwe; see Fig. 2 and Supplementary Table S2 for documents used for the prevalence meta-analysis in the geographical study). Three of the 14 documents reported the results of longitudinal studies (estimates from a same population at different dates). For each of these studies, the prevalence estimates provided were pooled into one single average prevalence estimate that was considered as a single statistical unit in the meta-analysis.
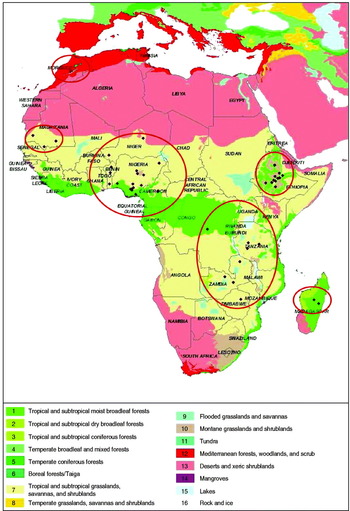
Fig. 2. Locations of Newcastle serological prevalence studies selected for the meta-analyses on a biome map (adapted from [Reference Olson39]).
Eight other documents reported results from investigations of spatial variation in prevalence (estimates from distinct populations obtained over a short period). The prevalence estimates reported in these studies were treated as distinct statistical units in the meta-analysis. Sixty-five population prevalence estimates (hereafter referred to as primary estimates) were eventually considered for the meta-analysis and are depicted in Figure 2.
The location of each prevalence estimate was provided in the source publication [36]. The altitude, poultry [37] and human [38] densities, and biome [Reference Olson39] for the location of each prevalence estimate were considered as potential explanatory variables. The year was also considered as a potential explanatory variable. The biome classification presented in Olson et al. [Reference Olson39] is established according to the assemblage of natural plant and animal communities and species. The biome reflects biotic conditions, such as vegetation type, as well as physical conditions, such as rainfall and temperature, which could influence the survival and spread of NDV in natural and domestic host compartments. Biome was used here as an integrative index of these environmental conditions. Altitude was included in the set of potential explanatory variables for the same reason. Poultry and human densities were considered as proxies for the density and the connectivity of the poultry production network. These characteristics are obviously important for the ecology of NDV because they influence the number of potential hosts and their contact rates. Biome, altitude, human density and poultry density are likely to be inter-related. To account simultaneously for the effect of these explanatory variables, they were combined using a multiple correspondence analysis (hereafter referred to as MCA). For the MCA, continuous variables such as altitude, population density and poultry density were transformed into categorical variables (Table 1). MCA then permitted the identification of groups of localities where prevalence had been estimated and that were relatively similar with regard to the set of explanatory variables considered. MCA was performed using the dudi.acm procedure of the ADE4 package [Reference Chessel40, Reference Dray and Dufour41] of R software [42].
Table 1. Explanatory variables for the meta-analysis

BI, Biome; AL, altitude; Y, year; HD; human density; PD, poultry density.
Meta-analysis allowed the testing of differences in prevalence in the study categories defined with MCA. The variable used as the effect size in the meta-analysis was the logit of the prevalence estimate: logit(![]() i) = ln(
i) = ln(![]() i/1 –
i/1 – ![]() i) [Reference Chang43]. A standard error (s.e.) was associated with each prevalence estimate, which was computed as follows: s.e.(
i) [Reference Chang43]. A standard error (s.e.) was associated with each prevalence estimate, which was computed as follows: s.e.(![]() i) = √[(
i) = √[(![]() i/1 –
i/1 – ![]() i)/ni], where ni is the size of the sample from which prevalence was estimated. The standard error of logit(
i)/ni], where ni is the size of the sample from which prevalence was estimated. The standard error of logit(![]() i) was computed using the delta method: s.e.[logit (
i) was computed using the delta method: s.e.[logit (![]() i)] = 1/
i)] = 1/![]() i(1 –
i(1 – ![]() i)s.e. (pi).
i)s.e. (pi).
The meta-analysis allows each of the MCA categories to estimate the mean and the standard error of the logit of prevalence, while accounting for the fact that the sample consists of estimates, rather than direct measurements, of the focal variable, each of which bears a certain uncertainty (quantified by the standard errors, s.e.[logit(![]() i)], of the primary estimates). In addition, a heterogeneity test [Reference Sutton and Higgins44] can be performed within each category. The null hypothesis tested is that the logit of prevalence is homogeneous across the studies in a category. If this hypothesis is rejected, it can be concluded that prevalence varies in studies within the category considered, in which case the use of a random-effects model is justified. The package META of R software, which is devoted to meta-analytical methods, was used to compute the means of the logit (prevalence) distributions. In a second step, estimates of logit-transformed prevalence means were compared between categories using Z tests [Reference Crawley45]. For these tests, a Bonferroni correction was applied to the threshold P value in order to prevent any inflation of the risk of a type I error (i.e. the risk of erroneously concluding that two means differ) because of multiple tests [Reference Crawley45].
i)], of the primary estimates). In addition, a heterogeneity test [Reference Sutton and Higgins44] can be performed within each category. The null hypothesis tested is that the logit of prevalence is homogeneous across the studies in a category. If this hypothesis is rejected, it can be concluded that prevalence varies in studies within the category considered, in which case the use of a random-effects model is justified. The package META of R software, which is devoted to meta-analytical methods, was used to compute the means of the logit (prevalence) distributions. In a second step, estimates of logit-transformed prevalence means were compared between categories using Z tests [Reference Crawley45]. For these tests, a Bonferroni correction was applied to the threshold P value in order to prevent any inflation of the risk of a type I error (i.e. the risk of erroneously concluding that two means differ) because of multiple tests [Reference Crawley45].
Temporal study
Among the set of 201 documents gathered through the systematic review, 25 reported the seasonal timing of ND epidemics (often along with recommendations about the optimal timing of vaccination). From these 25 documents, we were able to establish the months when distinct types of epidemiological periods (three types were defined: no epidemic, epidemic and epidemic peak) occurred in 19 African countries. All information on seasonality gathered from the literature is synthesized in Table 2. For each of the countries for which data on epidemiological periods were available, the timing of the rainy, dry-cold and dry-hot seasons was determined based on the monthly averages of rainfall and temperature over the last 100 years at three locations with contrasting latitudes (North, Central, South) in the country. These climate data were obtained from the World Climate website (http://www.worldclimate.com/). The epidemiological period and the climatic season data were cross-tabulated. For each of the nine possible combinations of type of epidemiological season × type of climatic season category, the corresponding contingency table cell was filled with the number of country × months reported in the 25 documents (Table 3). An independent χ2 test was computed from this contingency table. This test evaluates the null hypothesis H0: the type of epidemiological season to which a given month in a given country belongs is independent from the type of climatic season to which it belongs.
Table 2. Epidemic periods indicated by (+) and vaccination sessions recommended (hatched cells) per country in Africa according to seasons (rainy, dry-cold, dry-hot)
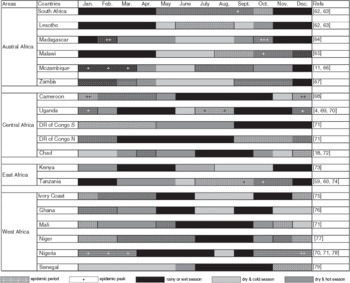
Table 3. Contingency table crossing seasons and epidemic periods according to data found in the literature (see Table 2)

Independence test χ4df2 = 50·7967, d.f. = 4, P < 0·0001.
RESULTS
Phylogeography
The phylogeographical analysis reveals the regionalization of NDV strains, their potential to evolve towards a higher pathogenicity from a local viral pool and suggests a risk for vaccine strains to provide new wild strains.
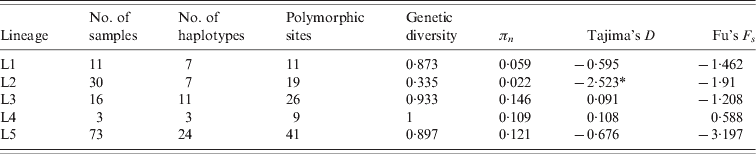
We obtained a segment of 89 bp length in which 55 variables sites, with 66 mutations that defined 53 haplotypes, were observed. Following the notation proposed by Aldous et al. [Reference Aldous31] and previous studies [Reference Abolnik46–Reference Herczeg, Bragg, Travassos Dias, Hadjiev, Werner and Lomniczi50], lineages 1–5, known to be found in Africa, were represented. The main haplotype is represented by II_1, followed by haplotypes V_2 and V_1 (corresponding to Aldous et al.'s classification [Reference Aldous31, Reference Aldous and Alexander51]) mainly found in southern Africa. Gene diversity (Hd) ranged from 0·335 in lineage 2 (L2) to 1 in L4. Low πn values were observed for both L1 (0·059) and L2 (0·022), whereas πn values were >0·1 for L3, L4 and L5. Only L2 showed a significant Tajima's D test (Table 4).
Table 4. Polymorphic measures, Tajimas's D and Fu's Fs test of the major lineages in the African Newcastle strains
* P < 0·001.
Similar strain groupings were obtained using the maximum-likelihood approach (see Supplementary Fig. S1) and median-joining network (Fig. 3). The median-joining network showed that L1 is linked to L2 and that both lineages are clearly separated from the others (Figs 3, 4). L1 and L2 were found across the African continent. A regional distribution of the other lineages was observed. L4 was only found in two countries, Tanzania and Nigeria, from which haplotypes are genetically distant from each other. Within L3, the South African haplotypes are not directly linked to the L3 haplotypes from Tanzania. For L5, three branches are observed. One branch involves haplotypes found in southern countries, whereas the two remaining branches were from Western countries. The former were more genetically different than the others and appear to link haplotypes from lineages 3 and 4. The predominantly represented haplotype II_1 seems to be a central haplotype with a star-like topology while the two other haplotypes (V_2 and V_1) possess ‘pearl collar’ branches with inter-connected haplotypes.

Fig. 3. A median-joining network depicting the relationships between African Newcastle strains. Node size is proportional to haplotype frequencies in the dataset. Colours refer to countries. Red spots indicate a hypothetical missing haplotype and length of lines between nodes are proportional to the genetic distance between nodes.

Fig. 4. Spatial distribution of the Newcastle strains in Africa.
Identification of risk factors for ND in Africa
A large number of risk factors were reported in the selected documents (Table 5). They included biological factors, such as poor physiological condition of the poultry or presence of different types of healthy carriers in the local domestic and wild avian community; environmental factors such as presence of surface water or of pollutants; and socioeconomic factors such as the occurrence of cultural or religious events that result in increased density of human and poultry contact networks. Because multi-disciplinary approaches are not common in epidemiology and risk factors are rarely quantified, it was difficult to rank these different risk factors.
Table 5. Newcastle disease risk factors for backyard chicken identified in the literature

ND, Newcastle disease.
Pattern of occurence
Biogeographical patterns
The two first factorial components of the MCA accounted for 44% of the total variation. The projection of the variables on the factorial plane defined by these two components (see Supplementary Figs S3 and S4) revealed three distinct combinations of conditions: tropical or subtropical moist broadleaf forest regions were associated with studies covering the period 1995–2000 and low altitudes and high human and poultry densities (this combination of conditions hereafter will be referred to as category C1); tropical or subtropical grasslands and savannas and Mediterranean-type forests and shrublands were associated with studies covering the period prior to 1995, intermediate altitudes, and low human and poultry densities (hereafter referred to as C2); mountain grasslands and shrublands were associated with studies covering the period after 2000 and with high altitudes and moderate human and poultry densities (hereafter referred to as C3). Each statistical unit was associated with one of these combinations according to the position of its projection on the factorial plane defined by the two first factorial components.
The heterogeneity test was highly significant within each of the three categories of the classification generated with MCA (C1: Q = 241·52, d.f. = 20, P < 0·0001; C3: Q = 23·45, d.f. = 8, P = 0·0028; C2: Q = 561·55, d.f. = 34, P < 0·0001). The logit of prevalence thus varied strongly within study categories (Fig. 5). A random-effects model therefore was used to compute means of the logit of seroprevalence for each category that accounted for the sampling uncertainty associated with each primary estimate and for the heterogeneity in estimates. The estimates of the mean prevalence (obtained after an inverse logit transformation of the estimate obtained from the meta-analytical random-effects model) were equal to 0·67 [95% confidence interval (CI) 0·58–0·75]; 0·36 (95% CI 0·30–0·41) and 0·27 (95% CI 0·19–0·38) for categories C1, C2 and C3, respectively. The mean prevalence for category C1 was significantly higher than for the other two categories (Z C1vs.C2 = 5·76, P < 0·0001; Z C1vs.C3 = 5·35, P < 0·0001; Bonferroni-corrected threshold P = 0·015; Fig. 5), whereas the mean prevalence for category C2 did not differ significantly from that of C3 (Z C2vs.C3 = 1·38, P = 0·31; Fig. 5).
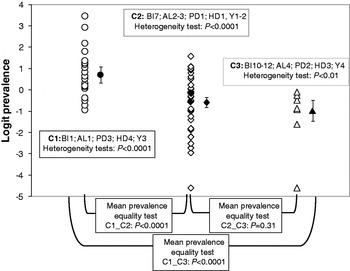
Fig. 5. Individual study serological prevalences (open symbols) and estimates of mean serological prevalences (filled symbols) with associated 95% confidence interval for Newcastle disease in three biogeographical categories defined according to biome (BI), altitude (AL), poultry density (PD), human density (HD) and year (Y).
Seasonal patterns
The independence of type of climatic season and type of epidemic period was strongly rejected (χ4df2 = 50·7967, d.f. = 4, P < 0·0001). The epidemic period coincided with the dry season (either cold or hot) more often than expected under the independence hypothesis, and the non-epidemic period coincided with the rainy season more often than expected under the independence hypothesis.
DISCUSSION
The epidemiology of an infectious disease depends on the ecology and evolution of its causative pathogen and hosts as well as on the biotic and abiotic environment in which they occur. Meta-analytical approaches allow the role of such factors to be tested at a large regional scale, thereby benefitting from the sum of results of different studies, with different designs and locations. Meta-analyses need to be conducted carefully with regard to differences between approaches and the reliability of the estimates extracted from the literature. However, if cautiously conducted meta-analyses are able to reveal association patterns that are relevant for the management and control of an infectious disease that could not be identified by individual studies alone.
Although the results of our phylogeographical analysis should be interpreted with caution due to the small length of the sequence analysed, with 55 variable sites out of 89, these sequences were informative enough to detect interesting phylogeographical patterns which were compatible with the main lineages previously described by Aldous et al. [Reference Aldous31]. Avirulent strains lineages (L1 and L 2) were found across the continent, while L3 and L4 were only found in three countries: South Africa–Tanzania and Nigeria–Tanzania, respectively. The number of branches observed in L5 raises questions regarding its homogeneity with at least one branch which appears to be more connected to L3 and L4 than to L5. Therefore if we consider that L5 is only represented by the branches including haplotypes V-2 and V-1 then this lineage is mainly present in southern Africa.
The virulent strain lineages L3, L4 and L5 appear to be ancient lineages that have reached a certain equilibrium as suggested by the ‘pearl collar’ patterns of their haplotypes and their high gene and nucleotide diversity values. By contrast, the star-like topology, low gene and nucleotide diversity values and significant Tajima's D test obtained for the avirulent strains of lineage L2 (Table 4, Fig. 4) highlight their recent expansion, an expansion that might have been facilitated by their genetic proximity to inoculate vaccines. Indeed, Snoeck et al. [Reference Snoeck48] proposed the hypothesis that lineages L1, L4, and L5 are ‘wild’ strains because vaccines of this type currently do not exist. In contrast, the central haplotype II_1 (L2) has the same genetic characteristics as the ‘La Sota’ vaccine frequently used in Africa to control ND [Reference Snoeck48]. A possible explanation is that the vaccine used during vaccination campaigns might have played a role in the maintenance, dispersion, and diffusion of avirulent viral strains on the continent. Vaccination could even have adverse consequences because through a few simple point mutations virulent strains could emerge from such avirulent strains [Reference Alexander5]. Moreover, the genetic proximity of La Sota virus with the widely distributed avirulent haplotype II_1 (L2) implies that the La Sota vaccine is probably extremely well adapted to avirulent strains that circulate in the environment. Under this hypothesis, we should question the efficacy of vaccination campaigns using vaccines that are primarily efficient against avirulent strains frequently circulating in the environment and for which hosts are likely to have developed natural immunity. Although numerous vaccines exist to control the disease on the continent, each with different antigenic properties depending on the region in which they are used (see Supplementary Table S3 for vaccines used in different African countries), the genetic characteristics of vaccines such as La Sota raise the question of how well vaccines are adapted to the circulating viral strains.
The results of our geographical meta-analysis should be considered with caution because the temporal distribution of the samplings that produced the serological prevalence estimates found in the literature was not homogeneous over the three sets of geographical characteristics defined in our analysis. Consequently, the geographical prevalence pattern revealed by our analysis could be confounded by a temporal pattern. Moreover, since our explanatory variables were tightly interconnected, the interpretation of their respective effects is complicated. We can, however, discuss the potential role of each of them on the epidemiology of ND. Our results suggest that areas that are the most favourable for the persistence and spread of NDV in backyard poultry are characterized by low-altitude, tropical wet forest biomes and high poultry and human densities. This pattern is consistent with empirical evidence [Reference Gilbert52, Reference Tiensin53] and theoretical results [Reference McCallum54] that have demonstrated better spread of density-dependent infectious disease such as ND and avian influenza when host densities are high. It is also in agreement with the fact that humid conditions are conducive for ND outbreaks because they enhance virus survival in the environment and viral transmission through the faecal–oral route [Reference Alexander5, Reference Hugh-Jones55]. Another factor that could explain high serological prevalence rates in wet forest biomes is the increasing disruption of these sylvatic ecosystems for logging and other purposes and the associated colonization by humans and their domestic species (i.e. backyard and livestock) of areas at the edge of wild ecosystems. Such colonization creates novel interactions among host species and between hosts and pathogens that could promote the transmission and maintenance of emerging NDV strains [Reference Despommier, Ellis and Wilcox56].
Our meta-analysis allowed establishing at the continental level that ND epizootics occurred most often during dry seasons. The association between dry seasons and the occurrence of infectious diseases in Africa has already been observed for ND in poultry [Reference Awan4] and avian influenza in wild birds [Reference Cappelle57, Reference Caron58]. This seasonal pattern could at first appear to be contradictory to the geographical association between high ND serological prevalence and wet biomes. However, although wet conditions are likely to be optimal for the survival of the virus in the environment [Reference Alexander5, Reference Hugh-Jones55], the dry season might be the time of year when coincidence of favourable conditions, in terms of virus presence in the environment, susceptibility of the poultry population, and transmissibility all occur. Indeed, during the dry seasons, non-permanent water points disappear, which results in the gathering of wild and domestic birds in high densities at the few remaining available perennial water points. It may then be possible that infected poultry, wild birds or other animals either transmit NDV by the respiratory route through close contact or contaminate a common water source (e.g. communal ponds in villages), which then becomes a source of infection through the faecal–oral route for other birds [Reference Awan4, Reference Cappelle57, Reference Caron58]. Moreover, the dry seasons in Africa are particularly harsh and put wild and domestic animals under severe stress. Temperatures can be >30 °C for long periods and food availability, an important factor for free-ranging backyard scavenging chickens, is decreasing as the dry season is progressing. These environmental conditions might weaken immunity and increase poultry susceptibility to ND [Reference Mavale11, Reference Msami, Wambura and Minga59, Reference Yongolo, Maeda Machangu and Minga60].
Other potentially important risk factors for the epidemiology of ND in Africa could only be explored qualitatively in our study (Table 5). In particular, socioeconomic factors such as cultural practices, trading modalities, as well as poultry-rearing and disease-management practices (e.g. vaccination) can play an important role in the transmission and maintenance of ND in rural poultry production systems. Multi-disciplinary quantitative approaches are needed to rank these different risk factors.
Recently, avian influenza has received more attention worldwide than ND. Africa has not been spared by the highly pathogenic avian influenza pandemic even if ND still caused the death of more poultry on the continent during the same period [Reference Alexander and Brown61]. Interestingly, ND and avian influenza share epidemiological traits: mortality and morbidity patterns, non-pathognomonic symptoms and transmission modes. Some of the risk factors identified in the present study also support this similarity (e.g. relationship with chicken and human density, seasonal profiles). It is therefore recommended that epidemiological investigation of both diseases should be combined. First, this could improve diagnosis, as excluding one pathogen almost confirms the other one. Second, as both pathogens share epidemiological traits, competition for hosts between them can be an important epidemiological factor.
Our study brings new knowledge on eco-epidemiological research with the use of appropriate statistical tools for integrating large-scale data and depicting global patterns. This type of study could be very useful in improving surveillance strategies, in particular for neglected diseases, in areas where data are lacking. We believe that animal disease management with more insight on ecological processes, leading to the mapping of risk areas, period and strain evolution may provide some innovative solutions to veterinary and public health sciences.
SUPPLEMENTARY MATERIAL
For supplementary material accompanying this paper visit http://dx.doi.org/10.1017/S0950268812002610.
ACKNOWLEDGEMENTS
This study was supported by the GRIPAVI project, financed by the French Ministry of Foreign and European Affairs and managed by Cirad. We thank all of members of the AGIRs Research Unit at CIRAD, especially Camille Danes, Marie Gely, Flavie Goutard and Marisa Peyre. We also thank Grace Delobel for assistance with English language version of the paper.
DECLARATION OF INTEREST
None.












