Introduction
Cervical cancer is the fourth most common cancer among women, with 527 000 new cases worldwide each year, causing 265 000 deaths annually [Reference Bruni1]. In Brazil, it is estimated that 16 340 new cases of cervical cancer are diagnosed each year. Cervical cancer is the second most incident cancer in the Northeast region of Brazil, with an estimated risk of 19.49/100 000 women. In the state of Sergipe, Northeastern Brazil, the incidence rate of cervical cancer is 20.17/100 000 women, one of the highest in Brazil [2]. Infection with the human papillomavirus (HPV) is considered the main risk factor for cervical cancer development [Reference Burd3, Reference Natphopsuk4]. However, different HPV types are related to diverse clinical outcomes. Consequently, it is very important to understand which HPV types are circulating in different regions of the world [Reference Rocha5].
Several reports demonstrate that HPV 16 is the most prevalent worldwide [Reference Bruni1, Reference Bruni6–Reference Srivastava, Gupta and Roy10]. However, the prevalence of other HPV types exhibits a wide geographical variability. Therefore, it is important to highlight that the knowledge of the HPV-type distribution is essential to support the development of novel diagnostic methods for HPV identification, along with the evaluation of vaccine impacts in different geographical regions of the world [Reference Vaccarella11].
Vaccination is an important strategy in reducing cervical cancer mortality rate [Reference Lowy and Schiller12]. However, as the HPV distribution varies with geographic location, vaccines that protect against the most prevalent HPV types for that geographic region should be preferred. Therefore, epidemiological studies with HPV genotyping are relevant for monitoring potential increases in the frequency of different HPV types, which is important to assess the impact of a vaccine [Reference Pomfret, Gagnon and Gilchrist13].
In this context, understanding the prevalence of HPV types in different geographical regions is relevant in assessing the impact of a vaccine. In Brazil, the tetravalent vaccine is available to the public. However, knowledge on the prevalence of HPV types in some geographical regions, as the state of Sergipe, Northeastern Brazil, is still scarce. Therefore, the main purpose of this study was to assess the HPV genetic diversity in women with cervical lesions from Sergipe state, Northeastern Brazil, which is important in order to evaluate the efficacy of the vaccine against cervical cancer in that region.
Methods
Patients and clinical samples
This is a cross-sectional study carried out between March and December 2014. The study was conducted at the Center of Integral Attention to Women's Health (CAISM), in the state of Sergipe, Northeastern Brazil. Sergipe is the smallest state of Brazil with 21 915 116 km2 and 2 068 017 inhabitants. This study included women with abnormal cervical cytology, which means that there was at least the presence of atypical squamous cells in the cytological examination. In addition, patients with normal cytology but presented abnormal transformation zone in colposcopy or pathological results in biopsy were also included in the study. HIV-positive patients and those who did not consent to participate were excluded from the study. Socio-economic data and risk factors were obtained: age, contraception usage, tobacco usage, STD history, alcohol usage, marital status, area of residence, formal education, number of sexual partners, age at first pregnancy, number of pregnancies, childbirth, abortion history, age at sexual debut, and age at first cytological examination (Supplementary Table S1).
The sample size was calculated taking into account confidence (95%) and absolute sample error (5%) for a finite population of women in the state of Sergipe. In the calculation, an estimated general prevalence of genital infection by any HPV type of 25% was used, based on Brazilian women of age 18 to 60 years old, which lives in the urban area, with abnormal cervical cytology. We also used other references to compare the results, including studies with general women population. Based on all these references, the HPV prevalence varied from 18% to 34.2%, which gave us a mean prevalence of 25% [Reference Santos Filho14–Reference Silva16]. Therefore, for this population, it was estimated a sample size of 352 women.
Biological material was collected from the cervix with cytobrush, placed into PBS solution (pH 7.0), and stored at −20 °C. We have obtained an informed consent from every subject enrolled in the study. This study has been approved by the Federal University of Sergipe Ethics Committee (CAAE: 23374014100005545).
HPV detection
Genomic DNA of the samples was extracted using Genomic DNA Purification kit (Promega), according to the manufacturer's specifications. The obtained DNA was submitted to amplification by PCR of the human beta-globin gene for the assessment of DNA quality, in order to avoid possible false negative results. Nested PCR reactions were used for HPV DNA detection using MY09/11 and GP5+/6+ primers [Reference Manos17, Reference de Roda Husman18]. All amplification reactions were performed in a final volume of 25 µl using PCR Master Mix kit (Promega), according to the manufacturer's protocol. Reference HPV 16 genome cloned into a pGEM-T vector (Promega) was used as positive control. Each PCR reaction was performed with negative specimens as a control.
PCR conditions for MY09/11 primers were: initial denaturation at 95 °C for 5 min; 35 cycles of denaturation at 94 °C for 1 min, annealing at 50 °C for 1 min, and extension at 72 °C for 2 min; followed by a final extension at 72 °C for 10 min. PCR cycles for GP5+/6+ primers were: initial denaturation at 95 °C for 5 min; 44 cycles of denaturation at 95 °C for 45 s, annealing at 47, 7°C for 45 s, and extension at 72 °C for 1 min; followed by a final extension at 72 °C for 7 min. The amplified product for each primer pair was visualised in 1.5% agarose gel electrophoresis.
HPV genotyping
PCR products were purified using Wizard SV Gel and PCR Clean-Up System kit (Promega). HPV genotyping was carried out by sequencing the PCR amplified genomic regions using ABI PRISM BigDye Terminator Cycle Sequencing v.3.1 kit (Applied Biosystems). Sequencing quality and assembly of contigs were carried out by using PreGap4 and Gap4 programs, incorporated in Staden package [Reference Staden19]. BLAST was used for sequence identity determination [Reference Altschul20].
Co-infection assessment
Samples that presented HPV types other than HPV 16 or HPV 18 were subjected to the co-infection assessment with specific primers. One primer pair specific for HPV-16 detection (HPV-16L1 F: 5′-CACTATTTTGGAGGACTGGAAT-3′; HPV-16L1 R: 5′-GATGAGGTGGTGGGTGTAGC-3′) and another specific for HPV-18 detection (HPV-18L1 F: 5′-GCCCCTGCCTCTACACAGTA-3′; HPV-18L1 R: 5′-ATCCTGCTTATTGCCACCAC-3′) were used in these samples.
PCR reactions were performed as follows: initial denaturation at 95 °C for 2 min, followed by 40 cycles of 95 °C for 30 s, 65 °C for 50 s and 72 °C for 10 s and a final extension at 72 °C for 5 min. The amplified product for each primer pair was visualised in 2% agarose gel electrophoresis. Reference HPV 16 and HPV 18 genomes cloned into a pGEM-T vector (Promega) were used as positive control.
Statistical analysis
Epidemiological data were described by simple frequencies and percentages when they were categorical and by mean and standard deviation when they were continuous or discrete. Fisher's χ 2 test was used to evaluate possible associations between categorical variables. We used the Mann–Whitney test to assess differences in means between two groups, the Kruskal–Wallis test for three or more groups and the Dunn–Bonferroni test for multiple comparisons. A significance level of 5% was used. All statistical tests were performed in R Core Team 2016 program (Vienna, Austria).
Results
The studied population was composed of women with clinical aspects of the abnormal cervix. The samples of the patients were tested for cytological and histological alterations. There was a predominance of women between 25 and 35 years old (34.4%). Only 49% of the patients reported the use of contraceptive methods, of which 58.18% reported using condoms for contraception, and 35.15% reported using a hormonal method. Most of the patients were either illiterate or only completed middle school (53.4%). The average age of the first sexual intercourse was 16.9 years of age (s.d. = 3.54) and the average age of the first pregnancy was 19.1 years old (s.d. = 4.73) (Supplementary Table S1).
This study was carried out with 432 women with cervical lesions, of which 337 (78%) tested positive for HPV DNA. The studied population was composed of patients not vaccinated for HPV. Eighteen different HPV types were detected, which shows a great genetic diversity in the Northeast region of Brazil. Among all women with cervical lesions from this study, high-risk HPV types were detected in 69.2% of the patients. HPV 16 (63.4%) was the most prevalent HPV type found in this study, followed by HPV 66 (4.6%), HPV 18 (1.6%) and HPV 45 (1.4%).
From the samples that presented HPV types other than HPV 16 or HPV 18 (n = 56), 14 have presented more than one HPV type. The results indicated that HPV 16/66 co-infection was the most frequent (35.7%), followed by HPV 16/45 (28.6%), HPV 16/18/66 (21.4%), HPV 16/31 (7.1%) and HPV16/58 (7.1%).
When we assess only the HPV-positive samples (n = 337), the majority of patients tested positive for CIN 1 (n = 207), of which 90% presented at least one high-risk HPV type. High-grade lesions (CIN 3 and invasive cancer) were mostly present in patients with high-risk HPV (n = 41). However, three patients with high-grade lesions presented a possibly carcinogenic HPV type. High-risk HPV types were also present in all age groups. However, patients aged between 25 and 35 years old had the highest detection rates of HPV (Table 1).
Table 1. Association between epidemiological characteristics and HPV risk group among the 337 HPV-positive samples in the studied population
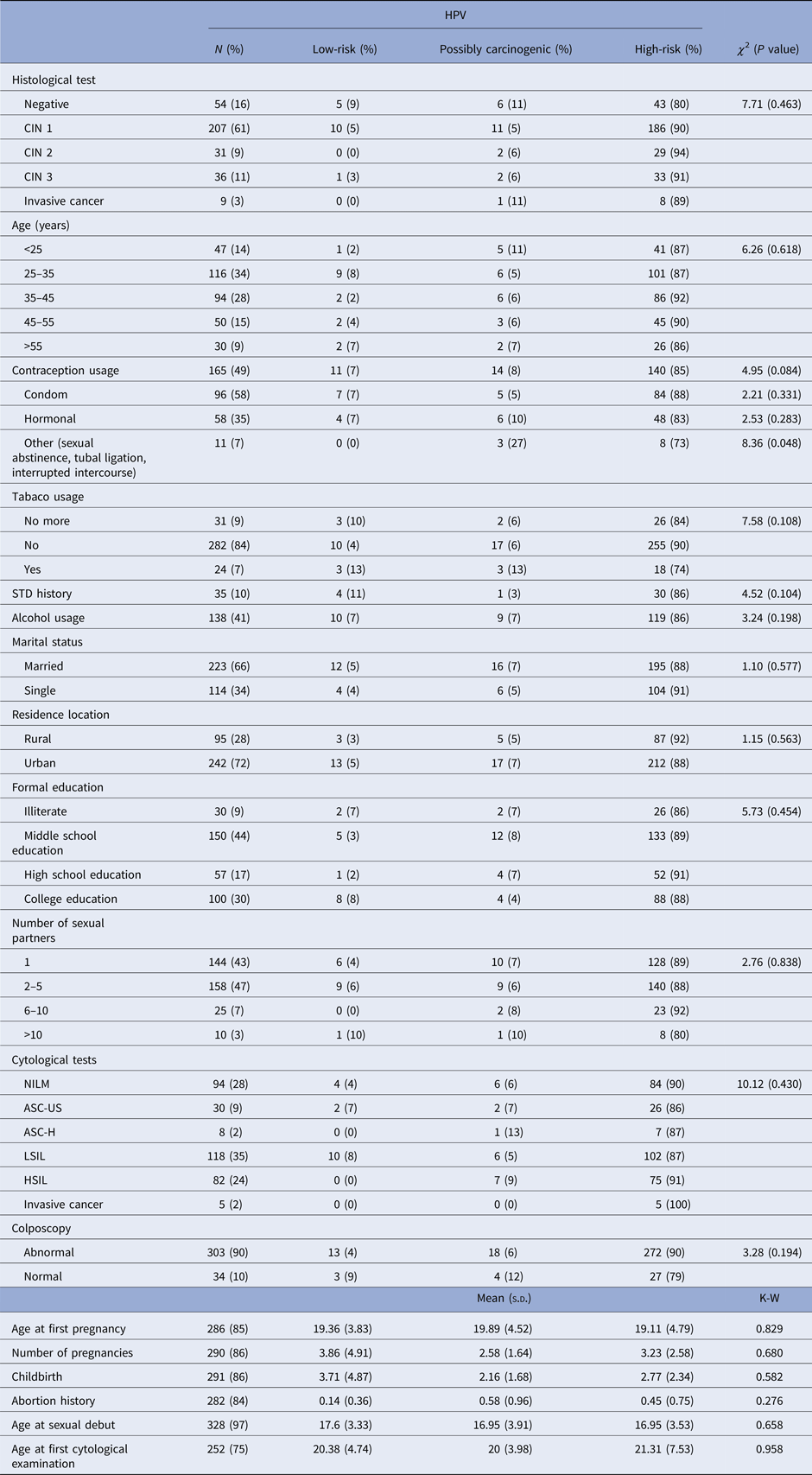
N, The overall HPV prevalence, being the denominator for the calculation of % for each diagnosis; χ 2, Chi-squared test; K–W, Kruskal–Wallis test; s.d., standard deviation; STD, sexually transmitted disease; CIN, cervical intraepithelial neoplasia; NILM, negative for intraepithelial lesions or malignancy; ASC-US, atypical squamous cells of undetermined significance; ASC-H, atypical squamous cells in which a high-grade squamous intraepithelial lesion cannot be excluded; LSIL, low-grade squamous intraepithelial lesions; HSIL, high-grade squamous intraepithelial lesions.
HPV-positive patients aged between 25 and 35 years old accounted for most of the abnormal cytological results. They presented 36% of low-grade alterations and 21% of high-grade alterations. This group also presented abnormal colposcopy in 95% of cases. The group of women older than 55 years old presented the highest frequency of cytological diagnosis of cervical cancer (Table 2).
Table 2. Association between epidemiological characteristics and cytological outcomes among the studied population
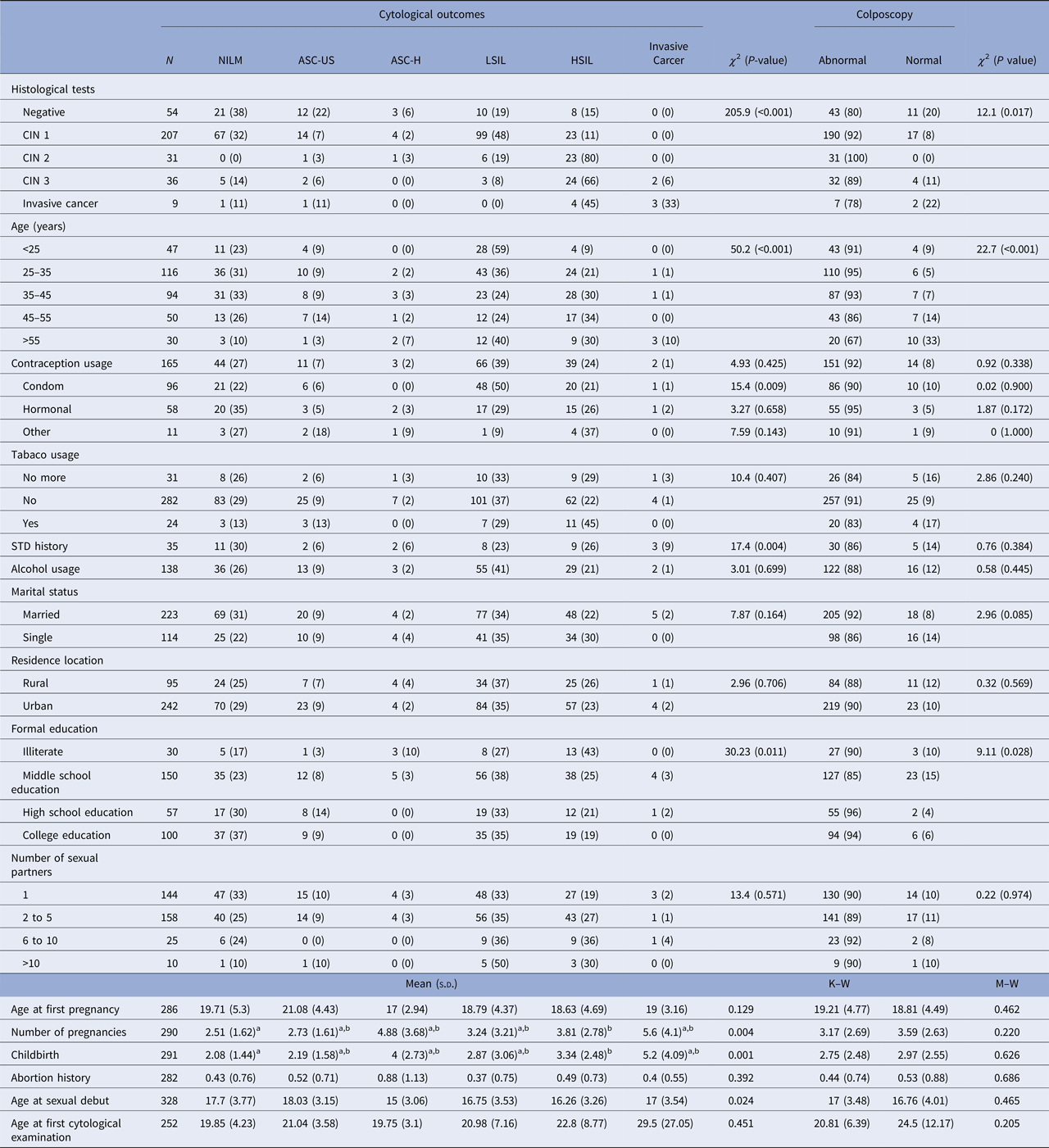
N, The denominator for the calculation of % for each diagnosis; χ 2, Chi-squared test; M–W, Mann–Whitney test; K–W, Kruskal–Wallis test; s.d., standard deviation; STD, sexually transmitted disease; a,b, sub-groups differ from each other at 5% significance level for Dunn–Bonferroni Test; CIN, cervical intraepithelial neoplasia; NILM, negative for intraepithelial lesions or malignancy; ASC-US, atypical squamous cells of undetermined significance; ASC-H, atypical squamous cells in which a high-grade squamous intraepithelial lesion cannot be excluded; LSIL, low-grade squamous intraepithelial lesions; HSIL, high-grade squamous intraepithelial lesions.
Among the patients that tested positive for HPV, HPV 16 was the most common high-risk type detected in ASC-US, LSIL, ASC-H, HSIL or cervical cancer. HPV 16 was present in 76.8% of cases with high-grade cytological lesions and in 100% of invasive carcinomas. In patients with cytological diagnosis of ASC-US, the presence of HPV 16 was detected in 80% of cases and in 78% of patients with low-grade lesions. HPV 66 was the second most prevalent HPV type and it has also been detected in patients with different types of cytological alterations. HPV 66 was detected in 12.5% of patients that presented ASC-H cytology and in 6.1% of patients with high-grade cytology lesions (Table 3).
Table 3. Distribution of HPV types and the association with cytological diagnosis among the 337 HPV-positive samples in the studied population
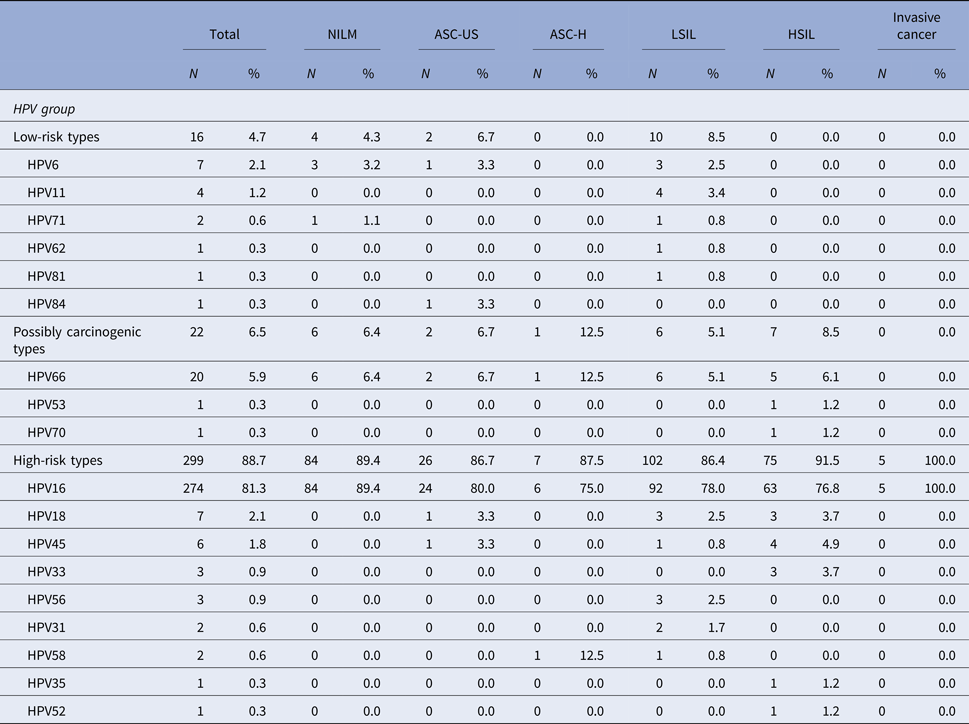
NILM, Negative for intraepithelial lesions or malignancy; ASC-US, atypical squamous cells of undetermined significance; ASC-H, atypical squamous cells in which a high-grade squamous intraepithelial lesion cannot be excluded; LSIL, low-grade squamous intraepithelial lesions; HSIL, high-grade squamous intraepithelial lesions.
The denominator for the calculation of % for each diagnosis was 337, the number of samples that tested positive for HPV.
When it comes to the histological diagnosis, among the HPV-positive patients, HPV 16 was present in 82.6% of women with a diagnosis of CIN 1, in 80.6% of CIN 2 cases, in 83.3% of CIN 3 cases and in 66.7% of invasive carcinoma cases. HPV 66, the second most prevalent HPV type in this population, was detected in 5.3% of CIN 1 cases, in 6.5% of CIN 2 cases, in 2.8% of CIN 3 cases, and also in 11.1% of invasive carcinoma (Table 4).
Table 4. Distribution of HPV types and the association with histological diagnosis among the 337 HPV-positive samples in the studied population
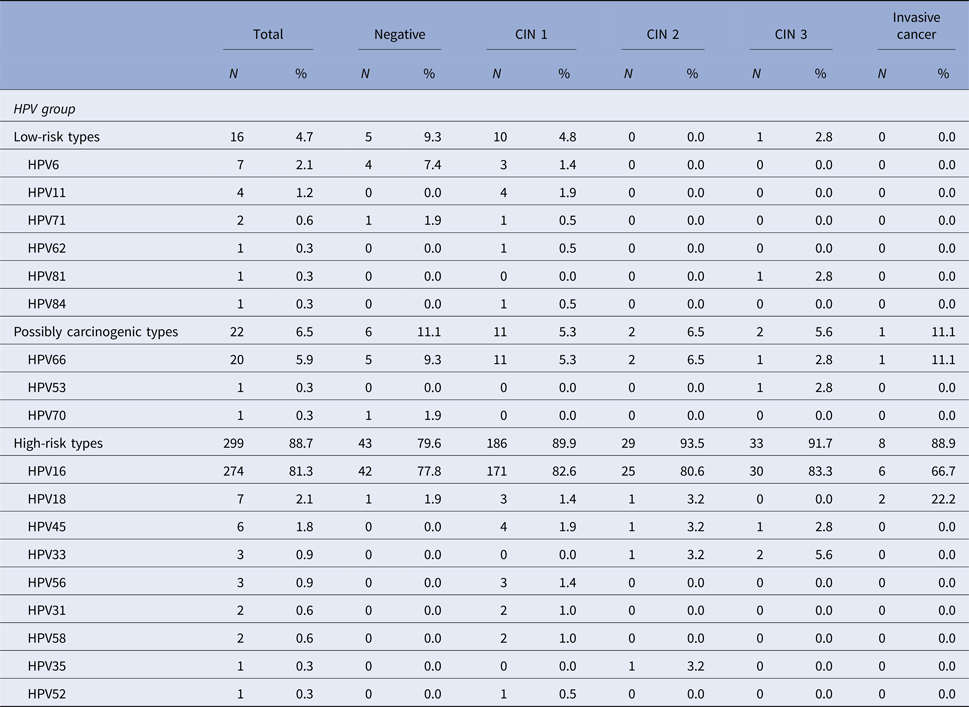
CIN, Cervical intraepithelial neoplasia. The denominator for the calculation of % for each diagnosis was 337, the number of samples that tested positive for HPV.
Discussion
In this study, we have found a large genetic diversity in HPV types infecting women with cervical lesions in Sergipe, Northeastern Brazil, with a predominance of high-risk HPV types. The state of Sergipe is the smallest state of Brazil, and the reference centre (CAISM) is responsible for the public gynaecological health services in the state. During one year, we have followed the cases of women with clinical alterations of the cervix in CAISM. All women with abnormal cervical cytology and the ones who had normal cytology but presented abnormal transformation zone in colposcopy or pathological results in biopsy were used in this study, which makes the study population representative.
This is the first study in the state of Sergipe that assessed the HPV prevalence and the HPV-type distribution in such a scale. HPV 16 was the most prevalent in all patients that presented clinical characteristics of cervical lesions, in accordance with other regions of Brazil and also the world [Reference de Sanjosé21–Reference Oliveira26]. Surprisingly, we detected a relatively high frequency of HPV 66 in women with cytological or pathological abnormalities, the second most prevalent in this study, which is a possibly carcinogenic HPV type, and the currently used vaccines do not protect against this type [Reference Nayereh and Khadem27]. In addition, this is the first time that HPV 71 has been found in this Brazilian region.
Differences on the prevalence and distribution of individual HPV types may be explained by the use of different methods of viral detection, variability in clinical specimens, the age of the participants and geographical variations [Reference Raiol28, Reference Ribeiro29]. The prevalence of HPV types varies across different geographical regions, but HPV types 16, 18, 31, 52 and 58 are consistently found on every continent [Reference Bruni6]. In this study, we observed the presence of all these HPV types. In addition, we were able to detect HPV 66 in several samples, which are not covered by the vaccine.
In Northeastern Brazil, a number of papers have been published on HPV prevalence and type distribution among women with cytological and/or histological alterations [Reference Santos Filho14, Reference Chagas30–Reference Batista35]. However, to the best of our knowledge, HPV prevalence among women with cervical lesions in the state of Sergipe, located in this region, has never been assessed.
Although HPV 16 was expected to be the most prevalent viral type among women with cytological and/or pathological abnormalities, this viral type was present in high rates in this study. In low-income countries, the prevalence of HPV 16 was estimated to be around 18.3% in LSIL and 38.8% in HSIL [Reference Bruni6, Reference Bruni36]. In this study, HPV 16 was present in 76.8% of patients with HSIL and in 83.3% of patients with CIN3. Other studies in Brazil showed a prevalence of 42.4% for HPV 16 found in HSIL, 63.1% of cases of CIN3 and, in the rest of world, the prevalence of HPV 16 found in HSIL was around 45% [Reference Ribeiro29, Reference Bruni36, Reference Bruno37]. In the agreement, other studies in Northeastern Brazil have also found HPV 16 to be the most prevalent HPV type in women with cervical lesions [Reference Santos Filho14, Reference Nunes31–Reference Oliveira34]. In contrast to our data, another study carried out in the Northeast region of Brazil found the HPV 31 to be the most prevalent among women with normal and abnormal cytological outcomes [Reference Chagas30]. However, most of the samples were collected from another geographical region, with another HPV-type distribution pattern. In the state of Maranhão, in the same region of Brazil, HPV 68 was the most prevalent, but only women from quilombo communities with cytological abnormalities were assessed [Reference Batista35].
These results highlight the importance of assessing the prevalence of HPV types in other populations from this Brazillian region, which is associated with a high rate of cervical cancer incidence [2, Reference Castellsagué38]. Therefore, to the best of our knowledge, this is the first study on HPV prevalence and HPV-type distribution in the state of Sergipe, which extends the knowledge on the prevalence of HPV types in cervical lesions in this region.
While the prevalence of HPV 16 is high across the world, the second most prevalent HPV type varies according to the geographic region [Reference Clifford39]. In this study, HPV 66 was the second most prevalent HPV type among women with cytological and/or histological alterations. This HPV type has been found in other studies with women that presented cervical lesions conducted in Brazil [Reference Chagas30, Reference Nunes31, Reference Bruno37, Reference Castro40, Reference Camara41], but its frequency was very low. HPV 66 has an increased prevalence among women with cytological and/or pathological abnormalities in the state of Sergipe, which highlights the importance of the efficacy surveillance of the vaccine implemented by the Brazilian government for the entire country. To the best of our knowledge, just a few studies have shown HPV 66 as the second most frequent HPV type in women with cervical alterations around the world, one in Trinidad and one in Bangladesh [Reference Andall-Brereton42, Reference Nahar43].
Although no study has provided strong evidence for carcinogenicity of HPV66, and its single detection is rare in cervical cancer, knowledge on the natural history of HPV 66 in cervical lesion development is still incipient and needs to be investigated. In other regions of Brazil, HPV 58, 31, 33 and 56 were the second most prevalent HPV types among women with cervical abnormalities [Reference Rocha5, Reference Bruno37, Reference Fernandes44–Reference Ayres and Silva47]. In the Northeast region, HPV 58, 31, 74, 18 and 16 were the second most prevalent HPV types in abnormal cervical samples [Reference Santos Filho14, Reference Chagas30–Reference Batista35]. Therefore, based on our observations, the state of Sergipe is the only state in Brazil that presented HPV 66 as the second most prevalent HPV type in women with cervical alterations.
HPV 66 is phylogenetically related to HPV 56, which is a high-risk type, and classified in the genus Alphapapillomavirus, species group α-6. Although this phylogenetic proximity to high-risk HPV types could shed light on the possibility of HPV 66 to be associated with cervical cancer development, it is known that different HPV types in Alphapapillomavirus genus, in the clade that contains the carcinogenic types, have different carcinogenic potential. Some of these HPV types were reported to cause precancerous lesions up to CIN3, but they are rarely found in invasive cancer. However, knowledge of the biological mechanisms that could explain this clinical diversity is still scarce.
In this study, HPV 66 was detected in women with normal cytological outcomes, in women with ASCU-US/LSIL, and in women with ASC-H/HSIL. It is relevant to highlight the presence of HPV 66 in one patient with a histological diagnosis of invasive cancer. All women with HPV 66 and high-grade lesions or invasive cancer also tested negative for HPV 16 and 18. Since we have not tested for co-infection with high-risk HPV types other than HPV16/18, it is possible that these samples might have one of these other high-risk HPV types. However, although low-risk and possibly carcinogenic HPV types in high-grade lesions and cervical cancer is rare, this situation has already been reported previously [Reference Burd3, Reference Cornall48, Reference Salehi-Vaziri49]. Therefore, our results cannot confirm if this is a case of single infection with a low-risk type or co-infection, they also cannot exclude this hypothesis. These results pointed to the epidemiological importance of HPV 66 in this population. Although these results are very relevant to provide novel data on the importance of HPV 66 in cancer development, other epidemiological studies with different populations should be carried out.
In Brazil, the quadrivalent vaccine was implemented by the government, and it does not protect against HPV 66, the second most prevalent HPV type in this study. In European countries and the United States, a non-avalent vaccine has been licensed in 2014 [Reference Markowitz50]. However, the non-avalent vaccine cannot protect against HPV 66 either. Although the authors think that more studies should address the relevance of HPV 66 in the development of novel vaccines that could protect against a broader group of viral types, there is not enough evidence for the consideration of HPV 66 as an important cause of cervical cancer until now.
The highest prevalence of high-risk HPV was observed in women with lower levels of education, which cannot be considered to be a well-defined risk factor, but this group could have less access to health care, which is implicated in higher levels of infectious disease disorders [Reference Fernandes44]. In accordance to other studies, the epidemiological factors found to be significantly associated with HPV infection were high parity, young age at first sexual intercourse and history of other sexually transmissible diseases [Reference Vaccarella11, Reference de Mendonça45, Reference Castellsagué51, 52]. In addition, patients with higher number of pregnancies had the highest rates of high-grade lesions, which could possibly be an important risk factor [Reference de Mendonça45, Reference Rosa53].
The main limitation of this study was the low number of invasive cancer cases assessed, which limited us to make stronger assumptions about the progression of HPV66 infection. These few cancer cases could be explained because the reference centre (CAISM), in which the study was carried out, do not treat the patients with cervical cancer and we did not have access to most of these patients. However, we have never detected HPV66 in high-grade lesions in this Brazilian region before, which might point out the possible role of HPV66 in these lesions. Although no study has provided strong evidence for carcinogenicity of HPV66, and its single detection is rare in cervical cancer, knowledge on the natural history of HPV 66 in cervical lesion development is still incipient and needs to be investigated.
The identification of HPV-type distribution in a particular region or population is important to predict the real impact of the vaccines and to monitor the prevalence of viral types not covered by the vaccine. Our results have shown a high prevalence of high-risk HPV types in the studied population. In addition, we have observed a high prevalence of HPV 66, which is a possible carcinogenic HPV type not protected against by available vaccines. Therefore, it is important that further studies could assess the prevalence of HPV 66, and other HPV types, in different geographical regions in order to assess the necessity of developing novel vaccines that protect against them.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S095026881800105X.
Acknowledgements
The authors acknowledge the Platform of Sequencing-LABCEN/CCB/UFPE for the use of its facilities.
Financial support
This work was supported by the Brazilian Institutes FAPITEC/SE (grant number 8444.UNI332.22122.17102013/2013-02 (PPSUS Sergipe)), CNPq (grant number 446425/2014-1) and CAPES (grant number 23038.010050/2013-04).
Declaration of interest
None.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. This study has been approved by the Federal University of Sergipe Ethics Committee (CAAE: 23374014100005545).







