Late-life depression may have an organic basis (Reference Baldwin and O'BrienBaldwin & O’Brien, 2002). Neuroimaging abnormality, executive dysfunction and altered depressive symptomatology are reported (Reference KrishnanKrishnan, 2002). Research into neurological signs is limited. Simpson et al (Reference Simpson, Baldwin and Jackson1998) found an association between adverse outcomes in late-life depression and extrapyramidal signs, abnormal tendon reflexes and a grasp reflex. In contrast, ‘soft neurological signs’ in schizophrenia have been studied and a specific assessment tool developed to assess them: the Neurological Evaluation Scale (NES; Reference Buchanan and HeinrichsBuchanan & Heinrichs, 1989). The NES distinguished patients with schizophrenia from control subjects. It incorporates a number of measures that reflect subcortical–cortical neurological function, which may be disordered in late-life depression (Reference Baldwin and O'BrienBaldwin & O’Brien, 2002). We compared neurological findings in patients with late-onset depressive disorder with a group of control subjects. The hypothesis was that, compared with a control group, patients with late-onset depressive disorder have more subcortical central nervous system (CNS) signs but no differences in cortical signs.
METHOD
The cohort described is different from that in an earlier report from this centre (Reference Simpson, Baldwin and JacksonSimpson et al, 1998). Findings on this new cohort for the relationship between treatment outcome and neuropsychological variables have been reported (Reference Baldwin, Jeffries and JacksonBaldwin et al, 2004).
Recruitment
Subjects were recruited from four health districts in Greater Manchester, in the North of England. Three-quarters of the subjects with depression were referred from local psychiatric out-patient services and the remainder from primary care physicians within the same locality. All control subjects came from the same geographical area as the patients. Some were spouses or partners and some were recruited via advertisement at day centres. All participants gave informed consent. The research was approved by the relevant local research ethics committees.
Inclusion criteria
For patients:
-
(a) Diagnostic criteria for DSM–IV major depressive disorder (American Psychiatric Association, 1994) met within the past 24 months (chosen pragmatically to minimise loss of eligible subjects who had recovered recently and not over-relying on distant memory).
-
(b) Age over 60 years at the time of assessment.
-
(c) First episode of depressive disorder aged 50 years or above.
-
(d) Patients could be depressed or in remission at the time of recruitment.
-
(e) Stable medication regime for 2 weeks minimum.
For control subjects:
-
(a) No previous history of psychiatric disturbance.
-
(b) Stable medical health.
Exclusion criteria
-
(a) Past diagnosis of a mood disorder before the age of 50 years.
-
(b) History or neurological evidence of stroke, space-occupying lesion or neurodegenerative disorders, including idiopathic Parkinson's disease, Huntington's chorea or a clinical diagnosis of dementia (DSM–IV).
-
(c) Past history of head injury with loss of consciousness.
-
(d) Mini-Mental State Examination (MMSE; Reference Folstein, Folstein and McHughFolstein et al, 1975) score below 21 at the time of assessment (used pragmatically to avoid exclusion of patients with depression-associated cognitive impairment).
-
(e) History of bipolar disorder, psychosis, alcohol dependence syndrome or Korsakoff's psychosis.
-
(f) Inability to cooperate with the test schedules.
Demographic information
Age, gender, civil status, social class and years of education were recorded. The National Adult Reading Test (NART; Reference NelsonNelson, 1991) was administered to assess premorbid intellectual level. Information was collected about prescribed psychotropic medication.
Psychiatric measures
All subjects were administered the following:
-
(a) Schedule for Affective Disorders and Schizophrenia – Lifetime version (SADS–L; Reference Spitzer and EndicottSpitzer & Endicott, 1979) for past psychiatric history and caseness for DSM–IV major depressive disorder within past 24 months.
-
(b) The Mini-Mental State Examination (MMSE; Reference Folstein, Folstein and McHughFolstein et al, 1975).
-
(c) The Hamilton Rating Scale for Depression, 17-item (Reference HamiltonHamilton, 1960).
-
(d) A neuropsychological test battery designed to assess attention and concentration, verbal and visual memory, primary and secondary memory, visuo-spatial functioning, language and semantic function and executive function (described in Reference Baldwin, Jeffries and JacksonBaldwin et al, 2004).
Physical measures
General measures
Burvill physical illness scale. This scale rates acute and chronic physical illness for the dimensions of severity (‘mild’, ‘moderate’ or ‘severe’) and disability (‘not at all’, ‘little’, ‘some’, ‘great deal’) for eight body systems (Reference Burvill, Mowry and HallBurvill et al, 1990). The higher the score, the greater the problem.
Framingham stroke risk factor score ( Reference Wolf, D'Agostino and Belanger Wolf et al, 1990 ). Computed for each patient based on the history and physical findings, this score comprises a weighted composite measure of the following factors: age, systolic blood pressure, treatment with antihypertensives, diabetes, cigarette consumption, evidence of cardiovascular disease, atrial fibrillation and left ventricular hypertrophy. The score gives a likelihood of stroke within the next 10 years, expressed as a percentage. Separate scores are provided for males and females. Although not strictly linear, the higher the score, the greater the risk.
Specific neurological measures
Structured central nervous system examination. A research psychiatrist (S.J.) received training in a protocol developed at the Manchester Cerebral Function Unit (Reference NearyNeary, 1999). This encompassed cranial nerves, speech articulation, tone and power in upper and lower limbs, peripheral sensation, tactile localisation, rapid alternating movements, hand posture, presence of tremor in limbs and plantar responses. Ratings were either present/absent or normal/abnormal. The maximum score was 30, with a higher score suggesting more neurological impairment. The scale was subdivided into items that were mainly upper motor neuron, pyramidal and/or cortical in nature (21 items) and those that were mainly subcortical (9 items).
The ten-item Webster evaluation scale for parkinsonism ( Reference Webster Webster, 1968 ). This incorporates bradykinesia, rigidity, posture, upper extremity swing, gait, tremor, facies, seborrhoea, balance and rising from a chair. Each item is rated on a four-point scale from ‘0’ (no abnormality/not present) to ‘3’ (severe difficulty or deficit).
The Neurological Evaluation Scale (NES; Reference Buchanan and Heinrichs Buchanan & Heinrichs, 1989 ). This comprises four subgroups: sensory integration (stereognosis, graphaesthesia, extinction, right/left confusion); motor coordination (tandem walk, rapid alternating movements, finger–thumb opposition, finger–nose test); sequencing of complex motor tasks (fist–ring test, fist–edge–palm test, Ozeretski test of rapid alternating movements, rhythm tapping); and ‘other’ (Romberg sign, tremor, mirror movements, synkinesis, convergence, gaze impersistence, grasp, snout and suck reflexes). In all there are 28 items and each is scored on a three-point scale (0=no abnormality; 1=mild but definite impairment; 2=marked impairment) except for the snout and suck reflexes, which are scored as either ‘0’ or ‘2’.
Participants were asked not to disclose whether they were patients or control subjects and were asked to avoid giving details about their health.
Neuroimaging evaluation
This was conducted using a 1.5T Phillips Gyroscan scanner (Phillips Medical Systems, Best, NL). The imaging protocol used was the axial FLAIR (fluid-attenuated inversion recovery) sequence. Slices were 3.0 mm thick with no interslice gap. Imaging parameters were TR 11000, TE 140, TI 2600, matrix 256×256 and field of view 230 mm2. Axial T1-weighted inversion recovery images were matched in anatomical location to the FLAIR sequence. Images were reconstructed to produce ‘real’ rather than modulus images.
Volumetric analysis was performed with the ‘TINA’ software package (a free open-source image analysis software package: http://www.niac.man.ac.uk/Tina/) using an automated algorithm for assessment of the severity and pattern of cerebral atrophy (Reference Thacker, Varma and BathgateThacker et al, 2002). The analysis was performed on T1-weighted inversion recovery images. The method is designed to allow detection of subtle degrees of atrophy in the prosencephalon without prior knowledge of the location. Atrophy measures were obtained for the left and right sides and for the whole brain. Higher scores indicate more atrophy.
White matter lesions were assessed on a PC workstation using EFilm viewstation software (EFilm Medical Ltd, Toronto, Ontario, Canada). The assessment was performed on matched T1-weighted inversion recovery and T2-weighted FLAIR images using the Scheltens scale (Reference Scheltens, Barkhof and LeysScheltens et al, 1993). All ratings were conducted by an experienced neuroradiologist (A.J.) who was masked to patient group. Inter- and intra-observer variation for this scale had been established previously in a group of 60 elderly patients comprising a mixture of subjects: normal subjects and those with Alzheimer's disease, frontotemporal dementia and vascular dementia. These trials indicated weighted Cohen's κ values in the range 0.52–0.89 (good to excellent) for all components of the scale.
Statistical analysis
No data for this population could be found to conduct a power calculation based on neurological signs. It was not thought valid to base a power calculation on neuroimaging findings, as in our previous report (Reference Baldwin, Jeffries and JacksonBaldwin et al, 2004). Data were entered into a Statistical Package for the Social Sciences database (Version 11.5). Normally distributed data were analysed using t-tests and non-normally distributed data with Mann–Whitney testing. For group differences significant at P<0.05 the data were re-analysed after exclusion of patients prescribed major tranquillisers and/or meeting the criteria for major depression at the time of neurological evaluation (n=13). A Bonferroni correction was made with respect to the main neurological measures (nine in all, as in Table 2).
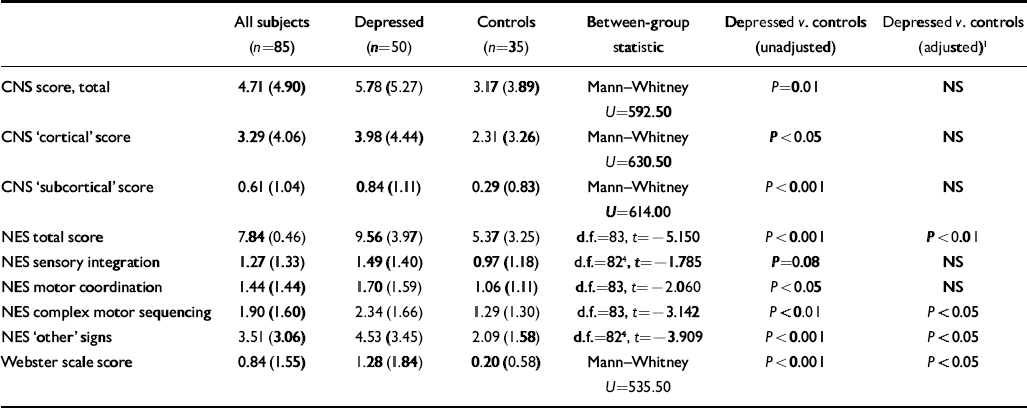
Table 2 Late-onset depressive disorder and neurological signs: neurological findings (t-tests for normally distributed data; Mann—Whitney for non-normally distributed data; χ2 for categorical data; standard deviations in parentheses)
| All subjects (n=85) | Depressed (n=50) | Controls (n=35) | Between-group statistic | Depressed v. controls (unadjusted) | Depressed v. controls (adjusted)1 | |
|---|---|---|---|---|---|---|
| CNS score, total | 4.71 (4.90) | 5.78 (5.27) | 3.17 (3.89) | Mann—Whitney U=592.50 | P=0.01 | NS |
| CNS ‘cortical’ score | 3.29 (4.06) | 3.98 (4.44) | 2.31 (3.26) | Mann—Whitney U=630.50 | P<0.05 | NS |
| CNS ‘subcortical’ score | 0.61 (1.04) | 0.84 (1.11) | 0.29 (0.83) | Mann—Whitney U=614.00 | P<0.001 | NS |
| NES total score | 7.84 (0.46) | 9.56 (3.97) | 5.37 (3.25) | d.f.=83, t=-5.150 | P<0.001 | P<0.01 |
| NES sensory integration | 1.27 (1.33) | 1.49 (1.40) | 0.97 (1.18) | d.f.=824, t=-1.785 | P=0.08 | NS |
| NES motor coordination | 1.44 (1.44) | 1.70 (1.59) | 1.06 (1.11) | d.f.=83, t=-2.060 | P<0.05 | NS |
| NES complex motor sequencing | 1.90 (1.60) | 2.34 (1.66) | 1.29 (1.30) | d.f.=83, t=-3.142 | P<0.01 | P<0.05 |
| NES ‘other’ signs | 3.51 (3.06) | 4.53 (3.45) | 2.09 (1.58) | d.f.=824, t=-3.909 | P<0.001 | P<0.05 |
| Webster scale score | 0.84 (1.55) | 1.28 (1.84) | 0.20 (0.58) | Mann—Whitney U=535.50 | P<0.001 | P<0.05 |
Logistic regression with forward stepwise selection of variables was used to predict variables significantly associated with group membership. The variables chosen were those that were significant in univariate analysis and included the NES, CNS and Webster total scores. Neuropsychological test results showing significant results from the previous study with the same sample (Reference Baldwin, Jeffries and JacksonBaldwin et al, 2004) were included (Rey Auditory Verbal Learning Test Trial I (Reference ReyRey, 1964), verbal fluency, Haylings test for dysexecutive disorder (Reference Burgess and ShalliceBurgess & Shallice, 1997), the Rey copy figure and logical memory test), along with other potential predictors (gender, age and scores on the NART, MMSE, Scheltens and Framingham scales).
RESULTS
Demographic and general physical findings
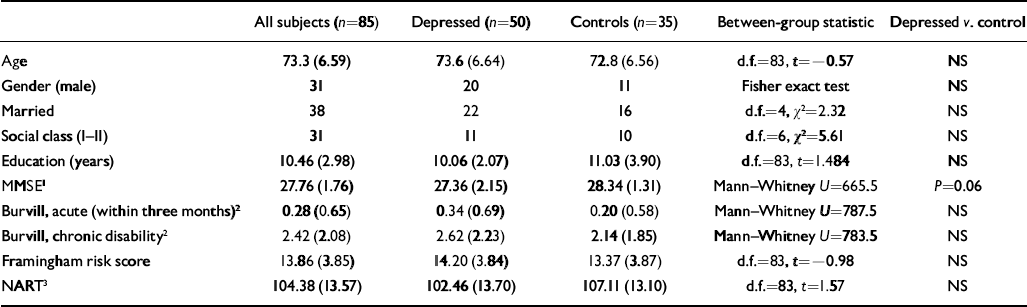
There were no significant differences between the two groups on general measures (Table 1).
Table 1 Late-onset depressive disorder and neurological signs: general findings (t-tests for normally distributed data; Mann—Whitney for non-normally distributed data; χ2 for categorical data; standard deviations in parentheses)
| All subjects (n=85) | Depressed (n=50) | Controls (n=35) | Between-group statistic | Depressed v. control | |
|---|---|---|---|---|---|
| Age | 73.3 (6.59) | 73.6 (6.64) | 72.8 (6.56) | d.f.=83, t=-0.57 | NS |
| Gender (male) | 31 | 20 | 11 | Fisher exact test | NS |
| Married | 38 | 22 | 16 | d.f.=4, χ2=2.32 | NS |
| Social class (1-11) | 31 | 11 | 10 | d.f.=6, χ2=5.61 | NS |
| Education (years) | 10.46 (2.98) | 10.06 (2.07) | 11.03 (3.90) | d.f.=83, t=1.484 | NS |
| MMSE1 | 27.76 (1.76) | 27.36 (2.15) | 28.34 (1.31) | Mann—Whitney U=665.5 | P=0.06 |
| Burvill, acute (within three months)2 | 0.28 (0.65) | 0.34 (0.69) | 0.20 (0.58) | Mann—Whitney U=787.5 | NS |
| Burvill, chronic disability2 | 2.42 (2.08) | 2.62 (2.23) | 2.14 (1.85) | Mann—Whitney U=783.5 | NS |
| Framingham risk score | 13.86 (3.85) | 14.20 (3.84) | 13.37 (3.87) | d.f.=83, t=-0.98 | NS |
| NART3 | 104.38 (13.57) | 102.46 (13.70) | 107.11 (13.10) | d.f.=83, t=1.57 | NS |
Stroke risk on the Framingham scale differs for men and women (Reference Wolf, D'Agostino and BelangerWolf et al, 1990). In this study: for women, the lowest score was 5, equating to a 2.4% 10-year risk; for men the scores equated to a 4.7% 10-year risk. The median score was 14, equating to a 13.3% 10-year risk for women and a 17% 10-year risk for men (Reference Wolf, D'Agostino and BelangerWolf et al, 1990). There were no significant group × gender differences in the Framingham scores.
Neurological findings
The unadjusted P values are presented in the penultimate column of Table 2 and the last column shows the P values following adjustment. As outlined in the Method section, the adjustment took account of: (i) the prescription of major tranquillisers (cases censored); (ii) whether the patient was suffering major depression at the time of evaluation (cases censored); 13 cases meeting both (i) and (ii) were censored; (iii) a Bonferroni correction for multiple comparisons. After these adjustments, scores on the NES (total), the NES complex motor sequencing sub-scale, the NES ‘other’ signs sub-scale and the Webster scale remained significant at P<0.05 or less (Table 2).
To check for the possibility that patients from the depression group who were in remission had sufficient symptoms to affect neurological function, the mean Hamilton score for those subjects who had major depression at the time of the investigation (n=8) was compared with the score for those who were not depressed (n=42). The values were 16.1 and 5.1, respectively. Although the residual scores in the remitted group seemed too low to influence the results, the analysis was repeated using the alternative strategy of including all subjects with depression but covarying using the Hamilton score. After Bonferroni correction the results from this analysis were almost identical to the previous ones (NES total score: F=13.25, P<0.01; NES complex motor sequence: F=7.31, P=0.04; NES ‘other’: F=8.05, P<0.05; Webster: F=5.96, P<0.05).
No laterality effects were observed with the NES (data not shown, all non-significant).
Of patients prescribed antidepressants (n=39) at the time of evaluation, two-thirds were taking selective serotonin reuptake inhibitors (SSRIs) or newer antidepressants and the remainder were on tricyclics, including lofepramine. Because SSRIs may precipitate or aggravate parkinsonism (Reference LaneLane, 1998), the mean Webster score for those on tricyclics was compared with that of patients prescribed SSRIs. The scores were 1.87 (s.d.=2.06) and 1.21 (s.d.=1.89), respectively. The total NES scores were very similar (9.67, tricyclics; 9.83, SSRIs).
Neuroimaging
Complete brain volumes were available for 58 of the 85 subjects. Using the TINA software program, there was significantly greater atrophy among subjects with depression than the controls, after covarying for skull size and age at examination (depressed, 196 515 mm; controls, 175 397 mm; F=5.161, P=0.03). The total Scheltens score of white matter hyperintensity (available for all 85 subjects) showed higher scores (more white matter lesions) for the depressed group than controls, but this difference was not statistically significant (depressed, 11.54; controls, 9.03; F=1.585, P=0.21).
Logistic regression
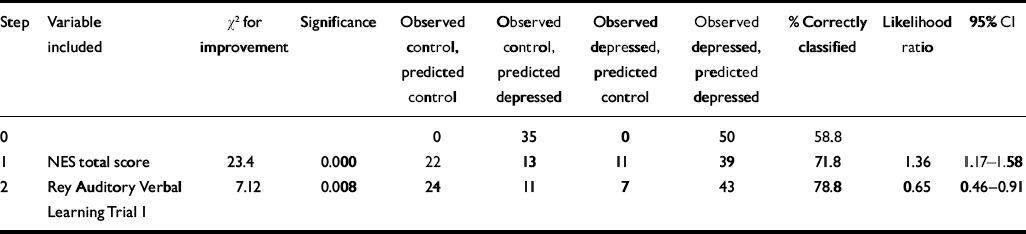
The NES total score alone accurately predicted 71.8% of group membership (CI 1.17–1.58). One further variable, the Rey Auditory Verbal Learning Test Trial I, was also significant, improving the prediction to almost 80% (Table 3). Brain volume data were only available for 58 subjects. The logistic regression was repeated (data not shown), adding the whole, left and right brain volumes using this smaller data set. The NES alone predicted 72.4% correct membership. The Webster score was the second variable selected, increasing predictive accuracy to 79.3%. No other variables were selected.
Table 3 Final model for predictor variables using forward stepwise logistic regression
| Step | Variable included | χ2 for improvement | Significance | Observed control, predicted control | Observed control, predicted depressed | Observed depressed, predicted control | Observed depressed, predicted depressed | % Correctly classified | Likelihood ratio | 95% CI |
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 0 | 35 | 0 | 50 | 58.8 | |||||
| 1 | NES total score | 23.4 | 0.000 | 22 | 13 | 11 | 39 | 71.8 | 1.36 | 1.17-1.58 |
| 2 | Rey Auditory Verbal Learning Trial I | 7.12 | 0.008 | 24 | 11 | 7 | 43 | 78.8 | 0.65 | 0.46-0.91 |
DISCUSSION
Main findings
The principal finding of the study was the significant difference in the presence and type of neurological signs between patients with depression and control subjects. Several differences ceased to be significant after adjusting for major depression and the use of major tranquillisers but motor sequencing and the ‘other’ sub-scales of the NES remained significantly different. Motor sequencing is dependent on intact frontal–striatal brain function (Reference NearyNeary, 1999), and a number of the NES ‘other’ measures (such as gaze impersistence and primitive reflexes) rely on subcortical–frontal integrity. Scores on the Webster scale also remained significantly higher in the depressed group after statistical adjustment, which probably reflects subcortical function. There were no significant differences in physical health burden or vascular risk factors that might account for the findings. Using logistic regression with a range of variables, the NES was the strongest predictor of group membership.
The primary hypothesis was supported: that neurological signs consistent with subcortical–frontal dysfunction are present in late-onset depression.
Limitations
It was not possible to guarantee that the physician carrying out the neurological examination was blind to the participant's group. Although subjects were asked not to reveal which group they were in, an experienced physician might guess correctly. This difficulty is no different from that faced by the originators of the NES. Buchanan & Heinrichs (Reference Buchanan and Heinrichs1989) comment that there is no easy solution to this but that a consistency of findings across research centres (as has happened with the NES) helps to mitigate this criticism. Replication of our findings in subjects with depression from other centres is therefore indicated.
Further limitations include the possibility that patients differed from controls in their medical comorbidity, although this was not reflected in either the composite measure of physical morbidity (Burvill physical illness scale) or the stroke risk factor score. The study was probably underpowered, leading to the possibility of type II errors, although there is a consistent pattern in the results. The most commonly used class of antidepressant in this study, SSRIs, has been implicated in causing extrapyramidal side-effects (Reference LaneLane, 1998). However, the Webster score was (non-significantly) lower among those on SSRIs compared with tricyclics, making antidepressants an unlikely cause for the findings. Although most of those in the depressed group were in remission, a prospective study is needed to address whether neurological abnormality in depressive disorder represents a state or trait. Lastly, the subjects all had late-onset depression (age of onset after 50 years) and it may not be valid to extrapolate the findings to depression in later life with an early onset.
Relevance to current literature
There is little research involving neurological signs in depression. Parker and colleagues developed a sign-based system, ‘CORE’, which demonstrated greater psychomotor dysfunction in melancholic compared with non-melancholic major depression, including in later life (Reference Parker, Snowden and ParkerParker et al, 2003). Some of the CORE-rated items overlap with the Webster scale used in this study.
However, age and the presence of white matter lesions are two factors that might confound the findings of neurological abnormality in late-life depression. Extrapyramidal signs occur in the absence of detectable neurological disease and increase with age (Reference PrettymanPrettyman, 1998). In control subjects white matter lesions increase with age and are associated with demonstrable gait impairment (Reference Whitman, Tang and LinWhitman et al, 2001). Primitive reflexes (such as the snout and grasp reflex) are also reported in subjects at risk of stroke (Reference Rao, Jackson and HowardRao et al, 1999). In that study, the control subjects were of similar age to the patients with depression and had similar amounts of white matter lesions, suggesting that these factors themselves are insufficient to explain the neurological findings.
Relevance to vascular depression
Strong links exist between depression and vascular disease (Reference Thomas, Kalaria and O'BrienThomas et al, 2004). Late-onset depression is associated with a high level of white matter lesions (Reference O'Brien, Ames and SchwietzerO’Brien et al, 1996; Reference KrishnanKrishnan, 2002), and white matter lesions, as visualised by magnetic resonance imaging, are associated with cerebrovascular risk factors such as hypertension, cardiac disease and diabetes mellitus (Reference Longstreth, Diehr and ManolioLongstreth et al, 2001). Depression in association with cerebrovascular risk factors and white matter lesions is increasingly referred to as ‘vascular depression’ (Reference Alexopoulos, Meyers and YoungAlexopoulos et al, 1997; Reference Baldwin and O'BrienBaldwin & O’Brien, 2002). Abnormal neurological signs in late-life depression may be particularly relevant to vascular depression because they could represent a further manifestation of vascular brain disease.
We were surprised, then, that no significant group differences emerged either on the Framingham measure, which incorporates several common cerebrovascular risk factors, or the Scheltens measure of white matter lesion burden. With respect to risk factors for cerebrovascular disease it is increasingly recognised that traditional ‘bedside’ measures may overlook important mechanisms leading to vascular damage. An example is blood pressure. Alterations in circadian blood pressure rhythms (Reference Sander, Winbeck and KlingelhoferSander et al, 2000) and blood pressure at the upper level of normal (Reference Goldstein, Bartzokis and GuthrieGoldstein et al, 2002) and not merely one-off resting readings are implicated in cerebral damage. Altered cerebrovascular reactivity is important in the genesis of white matter lesions (Reference Cupini, Diomedi and PlacidiCupini et al, 2001). In this study there was a trend for higher Scheltens white matter lesion scores in the depressed group compared with controls. This was not statistically significant, possibly due to the small numbers, and our study does not rule out a vascular basis for the neurological signs reported.
Lloyd et al (Reference Lloyd, Ferrier and Barber2004) reported that patients with late-onset depression had more hippocampal atrophy compared with patients with early-onset depression and controls, but had similar amounts of white matter lesions. Of interest is the finding in this study that the depressed group had higher scores on a measure of brain atrophy. Kumar et al (Reference Kumar, Bilker and Jin2000), using statistical modelling, have proposed that atrophy and white matter lesions may represent separate pathways to late-life depression, based on neurodegeneration and vascular disease, respectively. Numbers in this study were small, thus necessitating caution, but it is possible that neurodegeneration represents another explanation for altered neurological signs in late-life depression. Suggested mechanisms, besides age, include hypercortisolaemia (Reference Baldwin and O'BrienBaldwin & O’Brien, 2002), inflammation (Reference Penninx, Britchevsky and YaffePenninx et al, 2003) and altered homocysteine metabolism (Reference Naismith, Hickie and WardNaismith et al, 2002). It would be fruitful to explore the role of neurodegeneration in the neurology of late-life depression.
The NES emerged as a significant predictor of whether a participant was from the depressed group or was a control subject. Future studies might explore whether the NES has the potential to predict outcomes, including symptomatic recovery and reversible neuropsychological deficits.
Clinical Implications and Limitations
CLINICAL IMPLICATIONS
-
▪ Late-onset depression is associated with mild neurological abnormality, notably involving subcortical signs.
-
▪ Neurodegeneration or subtle vascular damage may underlie this.
-
▪ The Neurological Evaluation Scale may be a useful tool in detecting ‘soft neurological signs’ in depressive disorder as well as schizophrenia.
LIMITATIONS
-
▪ Most subjects were in remission, but this does not adequately address the issue of whether the findings represent trait or state.
-
▪ It is difficult to maintain rater masking in studies of this sort.
-
▪ It may not be valid to extrapolate the findings to early-onset depression in later life.
Acknowledgement
Our thanks to Barbara Tomenson, School of Psychiatry, University of. Manchester, for statistical advice.






eLetters
No eLetters have been published for this article.