Several observational studies reveal that eating late in the day, for example immediately before bedtime, is associated with increased overall daily energy intake( Reference de Castro 1 ), poor weight management( Reference Tholin, Lindroos and Tynelius 2 ), and increased CVD risk( Reference Lennernäs, Akerstedt and Hambraeus 3 ). Consistent with this notion, physiological data exist to demonstrate that energy intake in the hours immediately leading up to bedtime results in a lower acute diet-induced thermogenesis( Reference Romon, Edme and Boulenguez 4 ) and a reduced feeling of satiation compared with energy intake in the morning or afternoon( Reference de Castro 1 ). Thus, it is intuitive that over a chronic time period, a dietary pattern in which energy intake is prioritised close to bedtime may promote a positive energy balance and weight gain.
Conversely, there are emerging data from acute metabolic studies indicating that consumption of lower energy and single macronutrient snacks 30 min before bedtime may confer favourable outcomes with regards to whole-body metabolism and appetite( Reference Kinsey and Ormsbee 5 ). Collectively, these bedtime snack studies have focused primarily on comparing the impact of acute ingestion of the individual macronutrient constituents of milk (whey protein, casein protein and carbohydrate) on next morning RMR, substrate utilisation and appetite( Reference Kinsey, Eddy and Madzima 6 – Reference Ormsbee, Gorman and Miller 10 ). For instance, the consumption of 30 g of whey protein, 30 g of casein protein or 33 g of carbohydrate (equivalent to an energy intake of 586–627 kJ) 30 min before bedtime was reported to increase next morning RMR in active young men( Reference Madzima, Panton and Fretti 7 ). In addition, next morning fat oxidation rates were increased with bedtime casein ingestion compared with whey and carbohydrate ingestion( Reference Madzima, Panton and Fretti 7 ). The authors speculated that the lower insulin response elicited by casein ingestion( Reference Pennings, Boirie and Senden 11 ) may explain the higher rates of fat oxidation the next morning in the bedtime casein snack condition( Reference Madzima, Panton and Fretti 7 ). Furthermore, a subsequent study in overweight and obese females reported an increased next morning satiety and decreased desire to eat with bedtime whey, casein, or carbohydrate ingestion compared with the omission of a bedtime snack( Reference Kinsey, Eddy and Madzima 6 ). Interestingly, this study reported elevated fasting blood insulin concentrations the following morning in the bedtime snack conditions, despite no changes in fasting glucose concentrations. Whereas these data in overweight and obese females imply that an acute bedtime snack, irrespective of content, may increase next morning cardiometabolic risk by increasing insulin resistance( Reference Kinsey, Eddy and Madzima 6 ), the same increase in fasting blood insulin concentrations was not observed in obese males( Reference Kinsey, Cappadona and Panton 8 ). Hence, a growing body of scientific evidence from acute metabolic studies supports the notion that a low energy snack (approximately 586–627 kJ) before bedtime may be beneficial for weight management, but with unclear impact on the acute cardiometabolic risk the following morning in overweight and obese individuals.
Casein protein is commonly perceived to be an ideal bedtime snack given its slower digestion properties that allows for a sustained elevation in plasma amino acid concentrations for the duration of sleep( Reference Kinsey and Ormsbee 5 , Reference Trommelen and van Loon 12 ). Nonetheless, based on findings from acute studies, whey protein and carbohydrate also appear to be important components of a bedtime snack as an increase in next morning RMR has been shown to be comparable to casein protein in active young men( Reference Madzima, Panton and Fretti 7 ). Given that milk is a protein-dense foodstuff, consisting of 80 % casein and 20 % whey protein, and contains carbohydrate( Reference Pereira 13 ), in theory milk, may be considered an ideal bedtime snack for increasing next morning RMR because of its macronutrient composition. Readily available in both fluid and powder form, milk provides a more practical and economically viable bedtime snack compared with an isolated (or hydrolysed) whey or casein protein supplement( 14 ). Moreover, within an acute study setting, the provision of milk as a mid-day snack, or as part of a standardised breakfast, has been shown to be effective in decreasing perceived appetite when compared with ingestion of water and beverages comprised primarily of carbohydrate( Reference Dougkas, Minihane and Givens 15 , Reference Dove, Hodgson and Puddey 16 ). However, to our knowledge, only a single study to date in female athletes has examined the impact of bedtime milk ingestion, administered in chocolate milk form, compared with a non-energetic placebo and observed an increase in RMR and reduction in appetite the following morning( Reference Ormsbee, Gorman and Miller 10 ). A logical follow-up study is to investigate the impact of bedtime consumption of a mixed macronutrient food source such as milk on next morning RMR, substrate utilisation, and appetite in healthy, overweight adults.
Evidence regarding the optimal bedtime protein dose required to effectively modulate RMR and appetite the following morning also remains unknown. Interestingly, the intake of ~30 g of protein during the day has been shown to induce greater diet-induced thermogenesis, modulate appetite, and enhance satiety( Reference Halton and Hu 17 , Reference Phillips, Chevalier and Leidy 18 ). However, to date, no acute study has examined whether a protein dose less than 30 g confers a similar increase in RMR and modulatory effect on appetite the following morning.
Accordingly, the primary aim of this acute metabolic study was to compare the impact of bedtime skimmed milk ingestion to a non-energetic placebo on next morning RMR, substrate utilisation, subjective appetite ratings, subsequent energy intake, and insulin and glucose responses in healthy, mildly overweight young men. The secondary aim was to determine the dose–response relationship between bedtime milk ingestion and next morning RMR, substrate utilisation, appetite and energy intake. We hypothesised that ingestion of the bedtime milk beverage containing 30 g of protein would increase next morning RMR, reduce appetite, and increase fat oxidation rates to a greater extent than a milk beverage containing 10 g of protein or a non-energetic control. We also hypothesised that next morning fasting insulin and glucose responses would be similar between bedtime snack conditions in this cohort of healthy, mildly overweight males.
Methods
Participants and ethics approval
In all, twelve healthy, mildly overweight but weight stable, and active but untrained males participated in the present study. A priori, we conducted a power calculation (GPower version 3 software) of appropriate sample size based on previous published data( Reference Ormsbee, Gorman and Miller 10 ) that measured, on average, a 5 % higher RMR the following morning after bedtime ingestion of chocolate milk v. placebo using the same indirect calorimetry technique conducted in the present study. By setting statistical power (1-ß err prob) at 0·8, α error probability at 0·05 and effect size (Cohen’s d) at 1·4 (based on previous data( Reference Ormsbee, Gorman and Miller 10 )), our power calculation revealed a minimum sample size of ten participants (using a cross-over research design) would be necessary to detect a statistical difference in RMR between milk and placebo treatment conditions. Exclusion criteria included any known diagnosis of CVD, stroke, diabetes mellitus and thyroid or kidney dysfunction. Participants taking medications that may affect appetite, taste and smell were excluded. Smokers and those with lactose intolerance or a dislike of dairy products also were excluded. Baseline anthropometric parameters including age, height, weight, BMI, waist and hip circumferences, and the sum of five skinfolds (triceps, biceps, subscapular, iliac crest, calf) were measured before the start of experimental trials (Table 1). The present study was conducted according to guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the University of Stirling, Faculty of Health Sciences and Sport Research Ethics Committee. Written informed consent and health questionnaires were obtained from all participants before participation.
Table 1 Participant characteristics (Mean values with their standard errors; n 12)
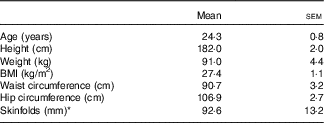
* Sum of skinfolds included triceps, biceps, subscapular, iliac crest and calf.
Protocol overview
Each experimental trial was conducted over 2 d (see Fig. 1 for protocol overview). Consistent with similar previous studies( Reference Kinsey, Eddy and Madzima 6 , Reference Madzima, Panton and Fretti 7 , Reference Ormsbee, Kinsey and Eddy 9 ), a washout period of ≥4 d was standardised between trials. On day 1, participants consumed a standardised evening meal at 19.30 hours and then a bedtime snack at 22.30 hours on the night before the morning laboratory visit. Subjective appetite and thirst were assessed before and after the bedtime snack and before the standardised bedtime at 23.00 hours. Overnight, participants wore actigraphy devices on their wrists for the assessment of sleep quality.
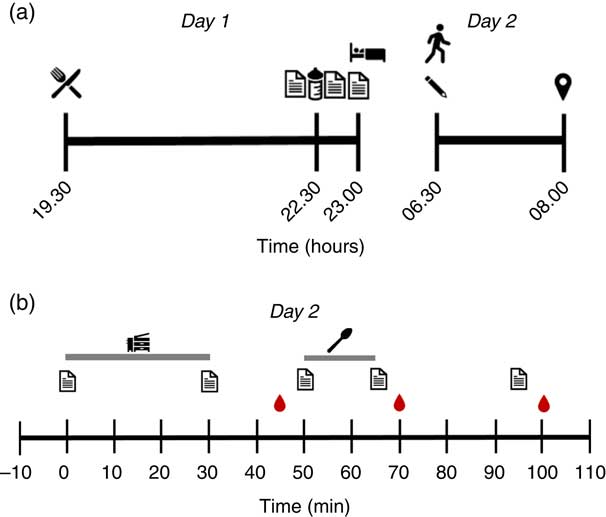
Fig. 1 Schematic diagram of study protocol on (a) day 1 and day 2 before arriving at the laboratory and (b) during the trial on day 2. A standardised dinner was consumed at 19.30 hours, followed by the bedtime snack at 22.30 hours. Participants went to sleep at 23.00 hours and woke up at 06.30 hours the next day. Participants arrived at the laboratory at 08.00 hours and rested in the supine position for 10 min. Metabolic measurements were completed via indirect calorimetry for 20 min, which was proceeded by the 15-min ad libitum breakfast. The appetite and thirst questionnaire was completed before and after both the metabolic measurements and breakfast. The first and second blood samples were conducted before breakfast and after breakfast. The final questionnaire and blood sample were taken 30 min after breakfast. ![]() , Standardised dinner;
, Standardised dinner; ![]() , appetite and thirst questionnaire;
, appetite and thirst questionnaire; ![]() , bedtime snack;
, bedtime snack; ![]() , Leeds Sleep Evaluation Questionnaire;
, Leeds Sleep Evaluation Questionnaire; ![]() , arrival at laboratory;
, arrival at laboratory; ![]() , indirect calorimetry;
, indirect calorimetry; ![]() , blood sample;
, blood sample; ![]() , ad libitum breakfast of cornflakes and semi-skimmed milk.
, ad libitum breakfast of cornflakes and semi-skimmed milk.
The next morning, participants woke up at 06.30 hours and immediately completed a questionnaire to assess sleep quality before attending the laboratory. Sleep quality (including sleep duration) was also assessed objectively using actigraphy (see the ‘Measurements of sleep quality’ section). Participants arrived at the laboratory fasted at 08.00 hours, having abstained from moderate-to-high intensity exercise, alcohol intake, and caffeine consumption for 24 h, and rested supine on a bed for 10 min. Subjective appetite and thirst were assessed at the end of the 10 min rest period. Metabolic measurements were then completed using indirect calorimetry for 30 min. Subsequently, subjective appetite and thirst were assessed again followed by collection of the first blood sample (08.45–08.50 hours) and the ad libitum breakfast. Subjective appetite and thirst also were assessed before and after breakfast and again 30 min after breakfast. Additional blood samples were collected immediately after the 15 min breakfast period (09.05–09.10 hours) and 30 min (09.35–09.40 hours) after the end of breakfast.
Bedtime beverage treatments
The study was randomised and cross-over in design. Treatments were double-blind except for the non-energetic placebo (BS0), which was water. A third party, not involved in other aspects of the study, prepared the beverages in advance and randomised the treatments in a counterbalanced order, with at least 4 d separating trials. Treatments were given to participants as pre-weighed Tesco© instant dried skimmed milk powder (Tesco Stores Ltd) in opaque plastic beverage bottles instead of fluid milk to ensure treatments were isovolumetric. Participants were instructed to add 400 ml of water to dissolve the skimmed milk powder thoroughly by shaking the bottle before ingestion at home. Macronutrient breakdown and energy content of treatments are described in Table 2. The treatment condition containing 10 g of protein (BS10) was chosen to mimic the approximate amount of protein in a typical glass of milk. The treatment with the highest amount of protein (BS30) was chosen to meet the 30 g protein threshold postulated to be required to suppress appetite( Reference Phillips, Chevalier and Leidy 18 ) and to match the protein dose administered in previous bedtime snack studies( Reference Kinsey, Eddy and Madzima 6 – Reference Ormsbee, Kinsey and Eddy 9 ). Participants were given an empty bottle for BS0 and filled it with 400 ml of tap water to be consumed at the time of the bedtime beverage.
Table 2 Energy and macronutrient content of bedtime snack treatments
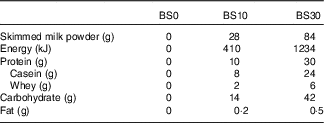
BS0, placebo; BS10, 10 g protein; BS30, 30 g protein.
Diet control
Participants completed a weighed food diary for three separate evening meals before beginning the study. Energy and macronutrient intakes were calculated using dietary analysis software (Nutritics Academic Edition version 4.267; Nutritics). The average energy intake of the three evening meals was used to determine the total energy content of the standardised evening meal. The standardised evening meal was designed to provide the same macronutrient breakdown of diets in UK adults according to the National Diet and Nutrition Survey 2008/2009–2011/2012 (carbohydrate: 50 %; fat: 32 %, protein 18 %)( 19 ). The standardised evening meal consisted of Tesco© Fusilli Pasta Twists, Tesco© Bolognese Pasta Sauce, Tesco© Beef Lean Steak Mince 5 % Fat (Tesco Stores Ltd) and olive oil. The ingredients were supplied to the participants and instructions were provided to prepare the meal at home. Compliance was verified verbally and by the return of empty food containers.
Participants also kept a 2-d food and activity diary 48 h before the first experimental trial and were asked to replicate the same food intake and activity in the 48 h before the subsequent trials. No other food or drink was permitted after consumption of the bedtime beverage the night before the morning trials. Participants were asked to consume 300 ml of water in the morning before visiting the laboratory.
Metabolic measurements
Oxygen consumption and carbon dioxide production were measured via indirect calorimetry (Oxycon Pro; Cardinal Health) using a ventilated metabolic hood placed over the participant’s head. Before starting the measurements, a calibration program within the software application accompanying the metabolic cart (LabManager, version 5.30.0) was used to determine ambient conditions (temperature, relative humidity and barometer pressure). Volume calibration was completed manually using a 3 litre calibration pump and gas analyzer calibration was completed using verified gases of known concentrations (16 % O2 and 5 % CO2). Measurements were completed with participants resting supine on a bed in a quiet and temperature-controlled room (20–24°C). Gas exchange was measured continuously for 30 min and data were captured every 30 s. The software application determined the RER and calculated the RMR using the formula derived by Weir( Reference Weir 20 ). Only the final 20 min of the data collection period was used for analysis to ensure participants were at a physiological steady state.
Subjective assessment of hunger, fullness, desire to eat and thirst
Hunger, fullness, desire to eat, and thirst were assessed subjectively using a validated visual analogue scale (VAS)( Reference Flint, Raben and Blundell 21 ). The questions accompanying the VAS were ‘How hungry do you feel?’, ‘How full do you feel?’, ‘How strong is your desire to eat now?’ and ‘How thirsty are you?’. The horizontal lines were anchored by the statements ‘Not at all hungry/full/thirsty’ and ‘As hungry/full/thirsty as I have ever felt’ at each end. For the desire to eat, the statements ‘Not at all’ and ‘Extremely’ were used at each end of the horizontal line. Participants placed a vertical mark on a 100-mm horizontal scale to rate how they felt regarding each sensation. Participants were instructed not to refer to previous scales when completing each new set of scales.
Ad libitum breakfast and 24 h post-trial energy intake
Participants were given 15 min to consume an ad libitum breakfast at a dining table in an isolated area of the research kitchen to minimise external distractions. Participants were provided a packet of Kellogg’s Corn Flakes® (Kellogg Company of Great Britain Ltd), a 500-ml jar of semi-skimmed milk, and instructed to eat as little or as much of each breakfast item as they wanted until they were satisfied. If participants finished eating before the allotted 15 min, they remained seated at the table. The packet of Kellogg’s Corn Flakes® (1582 kJ/100 g) was weighed before and after the ad libitum breakfast to determine the amount the participant consumed. The volume of semi-skimmed milk (Tesco© British semi skimmed milk, 209 kJ (50 kcal)/100 ml; Tesco Stores Ltd) remaining in the jar was measured in a graduated cylinder to determine volume consumed. All participants answered ‘yes’ to whether they would like cornflakes and milk for breakfast in the pre-study questionnaire. Participants were not informed that the energy intake of the cereal was being measured, as this information may have influenced their eating habits.
At the end of each trial, participants were instructed to keep a detailed food record of all food and beverages consumed in the 24-h post-trial period. The food records were analysed using dietary analysis software. Ten participants were included in the analysis of energy intake in the 24 h post-trial period as two participants were unable to provide complete food records.
Measurements of sleep quality
Given that sleep restriction has been associated with reduced next morning RMR( Reference Spaeth, Dinges and Goel 22 ), objective and subjective measurements of sleep were assessed to investigate the acute effect of bedtime milk ingestion on sleep. The MotionWatch 8© (CamNTech Ltd) tri-axial wrist-worn actigraphy device was used to obtain three objective measurements of sleep quality – actual sleep time, sleep latency, and fragmentation index. Actual sleep time was defined as total minutes categorised as sleep by the actigraphy device and the accompanying software (MotionWare, 1.125; CamNTech Ltd.). Sleep latency was defined as the time between ‘lights out’ and ‘fell asleep’ time points. Fragmentation index, expressed as the sum of total mobile time and immobile bouts not exceeding 1 min in duration, is a measure of disruption to sleep periods used as a marker of sleep quality, with a higher value indicating lower quality sleep.
Participants completed the Leeds Sleep Evaluation Questionnaire (LSEQ) immediately upon waking on the morning of the experimental trials for subjective measurements of sleep quality. The LSEQ was validated in individuals aged 18–49 years and consists of ten VAS questions that evaluate four domains of sleep: the ease of getting to sleep, the perceived quality of sleep, the ease of awakening from sleep, and behaviour following wakefulness( Reference Parrott and Hindmarch 23 ). Participants were asked to place a mark on the 100-mm line based on how they felt between two extremes, for example ‘less sleepy than usual’ and ‘more sleepy than usual’. The scores were averaged to give a score for each domain.
Blood sampling and analyses
A cannula (Becton, Dickinson & Company) was inserted into a forearm vein for blood sampling. At each timepoint, 10 ml of blood was dispensed evenly between lithium heparin or clot activator vacutainer tubes. Within 120 min, lithium heparin vacutainers were centrifuged at 3500 rpm at 4°C and plasma aliquots were dispensed into Eppendorf tubes. Clot activator vacutainers were allowed to clot for 60 min at room temperature before centrifugation and dispensing serum aliquots into Eppendorf tubes. Plasma and serum samples were stored at −80°C for future analysis of glucose and insulin concentrations, respectively. Plasma glucose concentrations were analysed with use of an automated analyser (ILab Aries; Instrumentation Laboratory) and serum insulin concentrations were analysed with use of a commercially available ELISA kit (Demeditec Diagnostics GmbH) according to manufacturer’s instructions. The HOMA2 Calculator version 2.2.3( 24 ) was used to determine the homoeostatic model assessment of insulin resistance (HOMA-IR) value. The averages of duplicate samples were used for data analysis. The intra-assay CV and inter-assay CV for insulin concentrations were 8·5 and 10·8 %, respectively. Two participants were unable to provide blood samples for all three trials; therefore ten participants were included in the final analysis of blood samples.
Data presentation and statistical analysis
Statistical analyses were conducted using IBM® SPSS® Statistics software package version 23 (IBM Corporation). AUC was calculated using the trapezoidal method with the baseline set as the value measured immediately after bedtime snack ingestion for the evening period and at 0 min for the next morning period (see Fig. 1). One-way repeated-measures ANOVA were conducted to examine differences in RMR, RER, estimated carbohydrate oxidation and fat oxidation rates, energy intake at ad libitum breakfast, 24 h post-trial energy intake, HOMA-IR, the AUC of subjective appetite and thirst assessments, actual sleep time, sleep latency, fragmentation index and the four domains of sleep in the LSEQ. Two-way repeated-measures ANOVA was conducted to test for treatment, time and treatment×time interaction effects on subjective assessments of hunger, fullness, desire to eat and thirst and also glucose and insulin concentrations. Where a significant treatment and/or interaction effect was detected, Bonferroni post hoc test was used to determine specific differences for both one-way and two-way repeated-measures ANOVA. Statistical significance was determined at an α level of P<0·05, and data were reported as mean with standard errors unless specified otherwise.
Results
Pre-trial dietary intake
Analysis of the pre-trial 2-d food diary revealed a daily mean energy intake of 26·3 (sem 3·4) kJ/kg/d and a macronutrient breakdown of 45·5 (sem 2·5) % carbohydrate, 19·2 (sem 1·2) % protein and 35·3 (sem 1·7) % fat.
Metabolic measurements
There was no significant effect of bedtime snack treatment on next morning RMR (P=0·19) (Fig. 2(a)) or RER (P=0·64) (Fig. 2(b)). Likewise, there was no significant effect of bedtime snack treatment on estimated carbohydrate (P=0·51) or fat (P=0·17) oxidation rates (Fig. 2(c)).
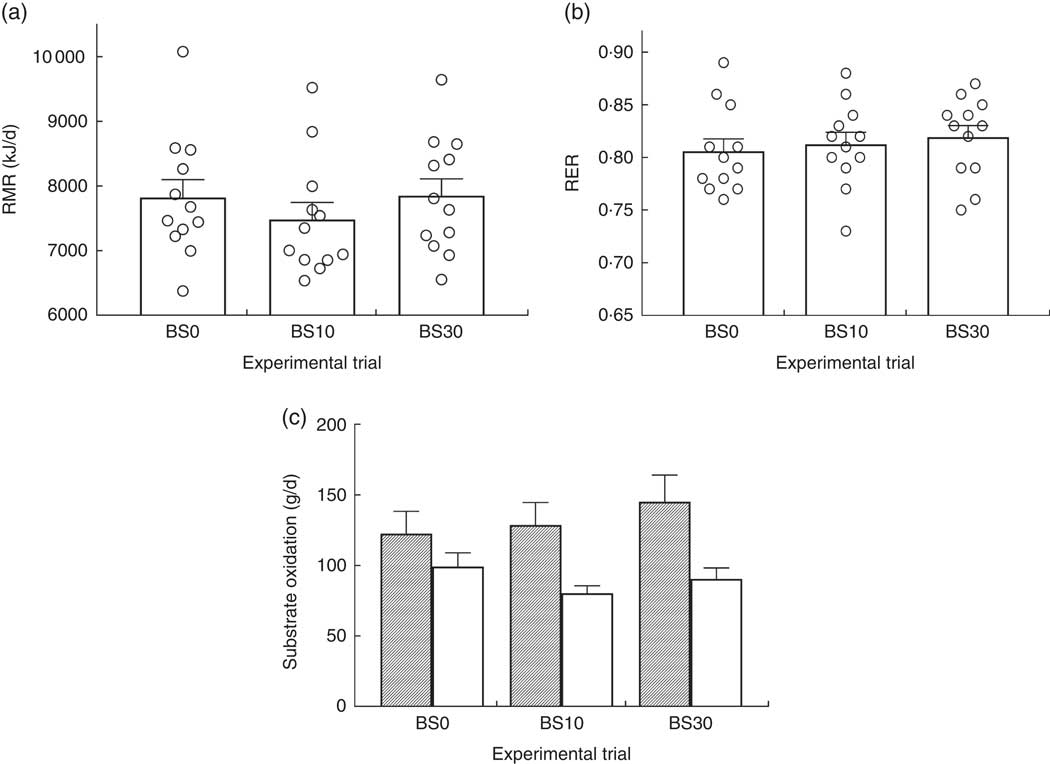
Fig. 2 Next morning (a) RMR, (b) respiratory exchange ratio (RER) and (c) carbohydrate (![]() ) and fat (
) and fat (![]() ) oxidation following bedtime milk ingestion. Values are means with their standard errors. No significant main effect of bedtime snack was observed for all measurements (P>0·05, one-way repeated-measures ANOVA). BS0, 0 g protein; BS10, 10 g protein; BS30, 30 g protein.
) oxidation following bedtime milk ingestion. Values are means with their standard errors. No significant main effect of bedtime snack was observed for all measurements (P>0·05, one-way repeated-measures ANOVA). BS0, 0 g protein; BS10, 10 g protein; BS30, 30 g protein.
Subjective assessment of hunger, fullness, desire to eat, and thirst
Subjective assessments of hunger, fullness, and desire to eat are represented in Fig. 3. A significant main effect of bedtime snack treatment was observed on subjective measurements of hunger (P=0·01) and fullness (P=0·04) during the evening period after bedtime milk ingestion. Hunger ratings for BS30 were significantly lower than BS0 during the evening at 5 (P=0·04) and 30 min (P=0·001) after bedtime milk ingestion, but were only significantly lower at 30 min for BS10 v. BS0 (P=0·01) (Fig. 3(a)). Evening fullness ratings for BS30 were significantly higher than BS0 at 30 min (P=0·007) after bedtime milk ingestion, whereas BS10 fullness ratings were higher than BS0 at 5 min (P=0·02) (Fig. 3(b)). There were no differences between BS30 and BS10 in subjective hunger or fullness during the evening after bedtime milk ingestion (P>0·05).
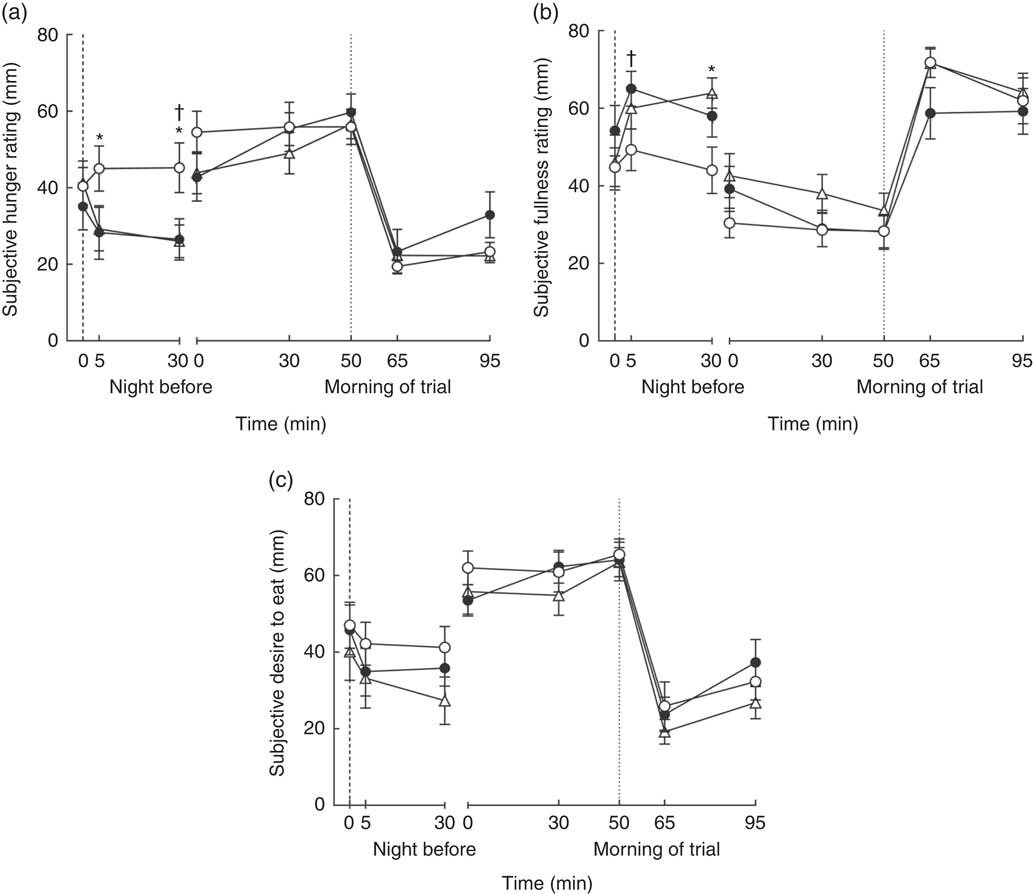
Fig. 3 Next morning subjective (a) hunger, (b) fullness and (c) desire to eat following bedtime milk ingestion. ![]() , the time point when bedtime milk was ingested. Values are means with their standard errors.
, the time point when bedtime milk was ingested. Values are means with their standard errors. ![]() , time when ad libitum breakfast was ingested;
, time when ad libitum breakfast was ingested; ![]() , 0 g protein (BS0);
, 0 g protein (BS0); ![]() , 10 g protein (BS10);
, 10 g protein (BS10); ![]() , 30 g protein (BS30). Data were analysed using a two-way (bedtime snack×time) repeated-measures ANOVA. Measurements from the night before and morning of trial were analysed separately. At night, there was a significant main effect of bedtime snack on hunger and fullness (P<0·05). The following morning, there was a trend towards a significant effect of bedtime snack on fullness (P=0·07), but no significant effect was observed for hunger and desire to eat (P>0·05). Bonferroni’s post hoc test was conducted to determine differences between means. * Mean value was significantly different between BS0 and BS30. † Mean value was significantly different between BS0 and BS10.
, 30 g protein (BS30). Data were analysed using a two-way (bedtime snack×time) repeated-measures ANOVA. Measurements from the night before and morning of trial were analysed separately. At night, there was a significant main effect of bedtime snack on hunger and fullness (P<0·05). The following morning, there was a trend towards a significant effect of bedtime snack on fullness (P=0·07), but no significant effect was observed for hunger and desire to eat (P>0·05). Bonferroni’s post hoc test was conducted to determine differences between means. * Mean value was significantly different between BS0 and BS30. † Mean value was significantly different between BS0 and BS10.
There was a trend for a significant effect of bedtime snack on the next morning rating of fullness (P=0·07), but not next morning hunger (P=0·60). No significant effect of bedtime snack was observed on the desire to eat or thirst both during the evening after ingestion (desire to eat: P=0·21; thirst: P=0·71) or the following morning (desire to eat: P=0·42; thirst: P=0·91).
Subjective appetite and thirst measurements also were expressed as AUC calculated over periods between bedtime snack ingestion and sleep, and from 0 to 95 min on the morning of the trials (Fig. 4). There was a significant effect of bedtime snack treatment on the AUC for hunger (P=0·006) and fullness (P=0·02) during the evening period. The bedtime snack treatment had no effect on AUC for hunger measured the following morning (P=0·62), but there was a trend for a significant effect on the AUC of fullness the following morning (P=0·07). No effect of bedtime snack treatment was observed for AUC of desire to eat and thirst calculated over the evening period (desire to eat: P=0·21; thirst: P=0·23) or the following morning (desire to eat: P=0·39; thirst: P=0·91) (data not shown for thirst).
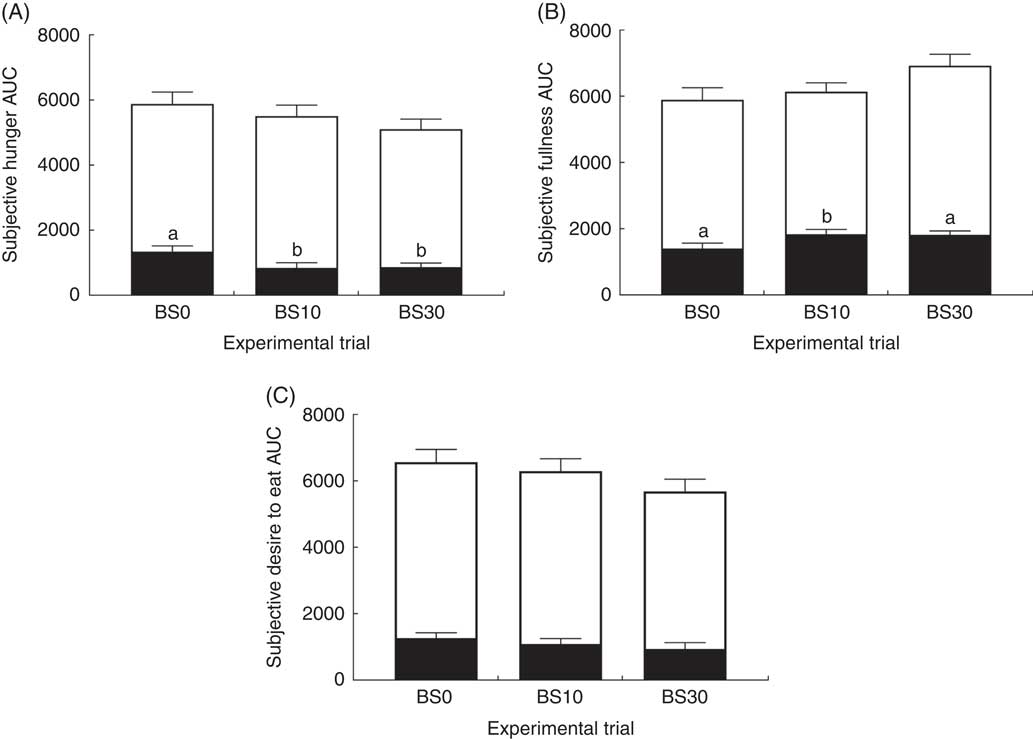
Fig. 4 AUC of subjective (A) hunger, (B) fullness and (C) desire to eat. Values are means with their standard errors. Data were analysed using a one-way repeated-measures ANOVA. Data from the night before and morning of the trial were analysed separately. There was a significant main effect of bedtime snack on hunger and fullness AUC at night (P<0·05), but not the next morning (P>0·05). No significant main effect of bedtime snack was found for the desire to eat AUC. Bonferroni’s post hoc test was conducted to determine differences between means. BS0, 0 g protein; BS10, 10 g protein; BS30, 30 g protein; ![]() , morning of trial;
, morning of trial; ![]() , night before trial. a,b Mean values with unlike letters were significantly different between conditions for the night before.
, night before trial. a,b Mean values with unlike letters were significantly different between conditions for the night before.
Ad libitum breakfast and 24 h post-trial energy intake
There was no significant effect of bedtime snack treatment on energy intake at the ad libitum breakfast (BS0: 2187 (sem 356) kJ, BS10: 2070 (sem 336) kJ, BS30: 2582 (sem 384) kJ, P=0·21). Likewise, bedtime snack did not have a significant effect on 24 h post-trial energy intake when expressed per kg body weight (BS0: 105 (sem 16) kJ/kg, BS10: 108 (sem 11) kJ/kg, BS30: 108 (sem 16) kJ/kg, P=0·95).
Blood glucose and insulin concentrations
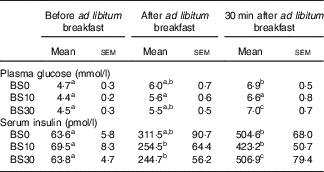
There was no significant bedtime snack and time interaction on next morning plasma glucose (P=0·60) or serum insulin (P=0·57) concentrations. Bedtime snack did not have a significant effect on next morning plasma glucose (P=0·61), serum insulin (P=0·56) or HOMA-IR (P=0·85) (Table 3). A main effect of time on plasma glucose and serum insulin concentrations (P<0·01) was observed.
Table 3 Plasma glucose and serum insulin concentrations (Mean values with their standard errors; n 10)
BS0, placebo; BS10, 10 g protein; BS30, 30 g protein.
a,b,c Mean values across a row with unlike superscript letters were significantly different from each other (P<0·05, repeated-measures two-way ANOVA, Bonferroni post hoc test).
Sleep measurements
As measured by the actigraphy devices, there was no significant effect of bedtime snack treatment on actual sleep time (BS0: 351 (sem 9) min, BS10: 366 (sem 12) min, BS30: 333 (sem 20) min, P=0·18). Likewise, no significant effect of bedtime snack treatment on sleep latency was observed (BS0: 20·3 (sem 7·0) min, BS10: 23·7 (sem 8·8) min, BS30: 30·3 (sem 11·6) min, P=0·76). There also was no significant effect of bedtime snack treatment on fragmentation index (BS0: 28·8 (sem 2·4), BS10: 29·2 (sem 4·9) and BS30: 35·9 (sem 5·5), P=0·41). Similarly, bedtime snack treatment had no significant effect on any of the four domains of subjective sleep in the LSEQ (data not shown): ‘getting to sleep’ (P=0·95), ‘quality of sleep’ (P=0·66), ‘awake following sleep’ (P=0·77) and ‘behaviour following awakening’ (P=0·86).
Discussion
The primary aim of the present study was to investigate the influence of bedtime skimmed milk ingestion on acute changes in whole-body metabolism and appetite the following morning in mildly overweight males. The main finding was that bedtime ingestion of a milk snack containing either 10 or 30 g of protein did not increase next morning RMR compared with a non-energetic placebo. In addition, next morning RER, as well as carbohydrate oxidation and fat oxidation rates, were similar between milk and non-energetic placebo conditions. Whereas the bedtime milk conditions tended (P=0·07) to increase subjective fullness the next morning, no differences in hunger and desire to eat were observed between milk and non-energetic placebo conditions. Accordingly, energy intake at an ad libitum breakfast the next morning and 24 h post-trial was similar between conditions. Hence, refuting our original hypothesis, bedtime milk ingestion failed to increase RMR and fat oxidation or reduce appetite the next morning compared with a non-energetic placebo in mildly overweight males. Consistent with our second hypothesis, next morning fasting insulin and glucose responses were similar between bedtime snack conditions in this cohort of healthy, mildly overweight males.
In the present study, we anticipated a dose-dependent increase in next morning RMR with bedtime milk intake due, at least in part, to differences in the protein and energy content of test drinks. The two primary factors known to influence diet-induced thermogenesis are protein and energy content, with protein estimated to contribute up to 30 % of diet-induced thermogenesis( Reference Westerterp 25 ). Hence, previous bedtime snack studies have proposed an energy-induced increase in thermogenesis to be a key mechanism behind the increase in next morning RMR following bedtime snack ingestion( Reference Kinsey, Eddy and Madzima 6 , Reference Madzima, Panton and Fretti 7 , Reference Ormsbee, Gorman and Miller 10 ). In the present study, the BS10 condition was chosen to mimic the 7–10 g of protein contained in a typical glass of milk and was similar to the 12 g of protein in the bedtime chocolate milk intervention administered previously by Ormsbee et al. ( Reference Ormsbee, Gorman and Miller 10 ). In addition to being higher in protein and energy content than BS10 and the previously described chocolate milk intervention( Reference Ormsbee, Gorman and Miller 10 ), the BS30 condition in the present study was protein matched to a similar bedtime snack study that found that 30 g of whey or casein increased next morning RMR( Reference Madzima, Panton and Fretti 7 ). Ormsbee et al. ( Reference Ormsbee, Gorman and Miller 10 ) reported a higher RMR with the bedtime ingestion of 355 ml of skimmed chocolate milk (12 g protein, 30 g carbohydrate, 0 g fat, 753 kJ) compared with a non-energetic placebo in young, trained, lean females. By contrast, in the present study of mildly overweight males, next morning RMR was similar between milk and non-energetic control conditions, irrespective of the dose of protein and energy content in the bedtime milk snack. Multiple factors may explain these discrepant findings, including differences in time elapsed between bedtime snack ingestion and metabolic measurements and differences in participant characteristics between studies. Sleep quality can be excluded because bedtime milk ingestion had no impact on sleep duration and quality in the present study.
One plausible explanation for the inconsistent findings regarding RMR between bedtime snack ingestion studies concerns time elapsed between bedtime snack ingestion and metabolic measurements the next morning. Utilising a respiratory chamber, previous studies have demonstrated that when an evening meal was consumed at 17.30 hours and then an evening snack at 19.30 hours, the increase in energy expenditure due to diet-induced thermogenesis returned to basal levels approximately 6 h after ingestion of the evening snack( Reference Westerterp 25 , Reference Verboeket-van de Venne, Westerterp and Hermans-Limpens 26 ). Conversely, data also exist demonstrating that diet-induced thermogenesis persists for longer than 6 h( Reference Reed and Hill 27 ). In the present study, we standardised the time between consumption of a bedtime milk snack (22.30 hours) and next morning measurements of indirect calorimetry (08.10 hours) at 9 h and 40 min and observed no increase in RMR with milk ingestion. Similarly, in a study of obese men, Kinsey et al. ( Reference Kinsey, Cappadona and Panton 8 ) reported no increase in next morning RMR measured about 8 h after bedtime ingestion of 30 g of casein protein compared with a non-energetic placebo. In contrast, Ormsbee et al. ( Reference Ormsbee, Gorman and Miller 10 ) demonstrated next morning RMR to be increased by about 5 % compared with a non-energetic placebo in lean, trained females when bedtime chocolate milk was consumed as little as 7 h before the measurement of RMR the following morning. As such, in the present study, we potentially missed the impact of diet-induced thermogenesis of bedtime milk ingestion on next morning RMR because we collected metabolic measurements 3 h and 40 min beyond the proposed approximately 6 h cut off point( Reference Westerterp 25 , Reference Verboeket-van de Venne, Westerterp and Hermans-Limpens 26 ). Taken together, these data suggest the time elapsed between bedtime snack ingestion and the next morning measurement of energy expenditure impacts, at least in part, the ability to detect an increase in next morning RMR through diet-induced thermogenesis.
In theory, the discrepant findings between past( Reference Kinsey, Eddy and Madzima 6 – Reference Ormsbee, Kinsey and Eddy 9 ) and present investigations of bedtime snack ingestion and next morning metabolism also may relate to the characteristics of recruited participants. Diet-induced thermogenesis has been reported to be greater in lean v. obese males( Reference Segal, Edaño and Tomas 28 ), which implies that bedtime snack ingestion confers a greater potential to increase next morning RMR in lean compared with obese males. Accordingly, a previous study in physically active men demonstrated an increase in RMR the following morning after bedtime ingestion of whey protein, casein protein, and carbohydrate( Reference Madzima, Panton and Fretti 7 ). In contrast, a study in obese men with a BMI of 36·1 kg/m2 observed no difference in next morning RMR following bedtime ingestion of casein protein compared with a non-energetic placebo( Reference Kinsey, Cappadona and Panton 8 ). Consistent with this finding, we observed no increase in RMR the following morning after bedtime skimmed milk ingestion in overweight men with a BMI of 27·4 kg/m2. Interestingly, although a previous study reported no difference in diet-induced thermogenesis between lean and obese females fed during the day( Reference Tentolouris, Pavlatos and Kokkinos 29 ), other studies have reported a higher next morning RMR after bedtime snack ingestion in lean, trained females( Reference Ormsbee, Gorman and Miller 10 ), but not in obese females( Reference Kinsey, Eddy and Madzima 6 , Reference Ormsbee, Kinsey and Eddy 9 ) when compared with no bedtime snack ingestion at baseline. Hence, future studies should compare sex-differences in next morning RMR following bedtime snack ingestion between lean and obese individuals.
The timing of next morning metabolic measurements and blood sampling also may explain why we failed to observe any modulation of substrate utilisation with bedtime milk ingestion. Milk consists of all macronutrients, of which protein composition constitutes 80 % casein and 20 % whey. The bedtime ingestion of casein protein has been shown to increase fat oxidation rates the next morning compared with whey protein and carbohydrate( Reference Madzima, Panton and Fretti 7 ). It was speculated that the lower insulin response to ingested casein compared with whey protein and carbohydrate resulted in reduced inhibition of fat oxidation the following morning( Reference Madzima, Panton and Fretti 7 ). Therefore, we anticipated that bedtime milk ingestion, which is rich in casein protein, would elicit an increase in fat oxidation the following morning. However, in the present study, morning fasting glucose and insulin concentrations in both milk conditions were similar to the non-energetic placebo condition, suggesting that, as perhaps could be expected, the glucose and insulin concentrations had returned to basal levels the next morning following bedtime milk ingestion. Accordingly, we observed no differences in substrate utilisation the following morning as estimated by RER between milk and placebo conditions. We also acknowledge that, in the present study, carbohydrate and fat oxidation rates may have been overestimated given that our calculations of substrate utilisation assumed negligible protein oxidation. Previous bedtime snack studies have made the same assumption with the bedtime provision of 30 g of protein( Reference Kinsey, Eddy and Madzima 6 – Reference Ormsbee, Kinsey and Eddy 9 ). Future studies are warranted that collect overnight gas exchange measurement using a respiratory chamber to determine the time course of change in overnight energy expenditure and substrate utilisation following bedtime snack ingestion.
Given that bedtime chocolate milk ingestion elicited a reduction in appetite the following morning compared with a non-energetic placebo in lean, trained females( Reference Ormsbee, Gorman and Miller 10 ), we anticipated that bedtime skimmed milk ingestion also would promote the suppression of appetite the following morning in mildly overweight males. However, in the present study, whereas evening hunger was suppressed and fullness increased immediately after bedtime consumption of milk compared with a non-energetic placebo, this effect was not maintained the following morning, even in the BS30 condition. Interestingly, other bedtime snack studies examining whey, casein, and carbohydrate ingestion reported inconsistent results relating to next morning appetite( Reference Kinsey, Eddy and Madzima 6 – Reference Ormsbee, Gorman and Miller 10 ). For example, the bedtime ingestion of 30 g of casein has been reported to be more satiating the next morning compared with whey or carbohydrate ingestion, but conversely, was found to increase the desire to eat the next morning compared with a non-energetic placebo at bedtime( Reference Kinsey, Cappadona and Panton 8 ). Future bedtime snack studies are required to clarify the differences in next morning appetite after intake of various mixed macronutrient food sources, for example milk, compared with single macronutrient snacks, both administered in solid and liquid form. Such studies should include measurements of candidate appetite regulating hormones (e.g. ghrelin) to provide mechanistic insight into the potential role of a bedtime snack in modulating next morning appetite.
The practical implication of modulating next morning RMR, substrate utilisation and appetite with bedtime snack ingestion relates to weight management. In theory, increasing next morning RMR and decreasing appetite may contribute to an overall negative energy balance. However, the hypothesis that there is a negative correlation between RMR (or energy expenditure) and body weight remains controversial. Two longitudinal studies in Native Americans( Reference Ravussin, Lillioja and Knowler 30 , Reference Piaggi, Thearle and Bogardus 31 ) and another in Caucasian adults( Reference Buscemi, Verga and Caimi 32 ) have demonstrated that lower values of energy expenditure and RMR were significant predictors of increases in body weight. Conversely, other studies observed no correlation between RMR and changes in body weight( Reference Seidell, Muller and Sorkin 33 – Reference Katzmarzyk, Pérusse and Tremblay 35 ). Therefore, in theory, increasing next morning RMR may contribute to an overall negative energy balance, but it is yet to be proven whether an association exists between RMR and changes in body weight.
In addition to obtaining subjective measurements of appetite, we also assessed subsequent energy intake the following morning using an ad libitum breakfast of cornflakes, as well as energy intake during the following 24 h. Given that subjective hunger and desire to eat were similar between conditions, and that there was only a trend (P=0·07) for an effect of bedtime snack on fullness the following morning, it follows that bedtime milk ingestion failed to modulate energy intake during the ad libitum breakfast. Interestingly, although not statistically significant (P=0·21), energy intake at breakfast for the BS30 condition was 18 and 25 % higher than BS0 and BS10 conditions, respectively, whereas no differences in energy intake over the 24 h post-trial period were observed between conditions. The bedtime milk snack interventions induced a similar pattern on next morning RMR (BS30>BS0>BS10). Taken together, these acute data are consistent with studies demonstrating that RMR is associated with ad libitum energy intake( Reference Caudwell, Finlayson and Gibbons 36 , Reference Piaggi, Thearle and Krakoff 37 ). It is possible that the lack of compensation in daytime energy intake when mildly overweight males consumed the most energy dense milk snack (BS30) at bedtime was related to the increase in energy intake as a result of the increased energy demands arising from the elevated post-prandial RMR. Future chronic bedtime snack intervention studies are warranted to confirm this seemingly adverse observation from a weight management perspective. Although not favourable in that respect, it is plausible that those with sarcopenia and aiming to retain lean mass, for example older adults( Reference Keller and Engelhardt 38 ), may benefit from chronic ingestion of a bedtime snack containing 30g protein and the resulting increase in energy intake the following morning. We acknowledge that participant preference for the sole breakfast option of cornflakes may have affected their overall energy intake as no alternative food choice to cornflakes was offered at breakfast. In addition, we cannot discount the possibility that participants may have under-reported or made changes to their usual food intake( Reference Ortega, Pérez-Rodrigo and López-Sobaler 39 ) as food records were the only method employed to assess 24 h post-trial energy intake. Moreover, in the present study, we did not assess any eating behaviour traits such as cognitive restraint disinhibition and susceptibility to hunger in our cohort of mildly overweight young men that may have influenced the energy intake between participants during the breakfast test meal. Hence, we acknowledge that highly restrained participants may have eaten less during breakfast than participants with low restraint. Nevertheless, based on our findings, it appears that bedtime milk ingestion does not impact energy intake the following day in mildly overweight men.
Although the bedtime milk snack did not impact appetite and subsequent energy at breakfast the following morning, perhaps unsurprisingly, appetite was reduced during the evening period immediately following milk ingestion compared with placebo. Hence, it may be argued that bedtime milk ingestion could play a role in reducing energy intake before bedtime. Evidence exists to suggest that individuals with weight management issues may benefit most from controlling appetite over the evening period( Reference de Castro 1 ). Night eating, defined as waking at night at least once a week to consume food and/or consuming 25 % or more of total daily energy intake after the last meal of the day, has been demonstrated to be 2·5 times more prevalent in obese compared with normal weight individuals( Reference Tholin, Lindroos and Tynelius 2 ). Furthermore, total daily energy intake appears to increase as energy intake increases at night between 18.00 and 02.00 hours( Reference de Castro 1 , Reference de Castro 40 ). In the present study, whilst milk ingestion suppressed appetite before bedtime, no differences in appetite were observed between BS10 and BS30 conditions. Therefore, ingesting a low energy and nutrient-rich snack such as a typical 200 ml glass of milk containing 7–10 g of protein (as in the BS10 condition in the present study) about 30 min before bedtime appears adequate to modulate appetite in the evening and may serve to displace intake of potentially energy dense foods that can contribute to higher total daily energy intake. This notion is supported by a study in which overweight or obese participants with self-reported night snacking behaviours were instructed to consume a fixed ready-to-eat cereal with milk daily 90 min after the evening meal( Reference Waller, Vander Wal and Klurfeld 41 ). After 4 weeks of the intervention, participants who complied with the daily evening snack protocol significantly reduced their post-evening meal energy intake, resulting in a trend towards greater body weight reduction compared with participants who continued on their normal diet( Reference Waller, Vander Wal and Klurfeld 41 ). Participants in the present study consumed each bedtime snack treatment on one occasion only. Hence future studies are warranted to investigate if the chronic ingestion of low energy and nutrient-dense bedtime snack can contribute to weight management, without long-term implications on cardiometabolic health.
To conclude, the bedtime ingestion of milk containing 10 or 30 g of protein does not make an impact on RMR, substrate utilisation and appetite the following morning (>9 h post-prandial) compared with a non-energetic placebo snack in mildly overweight males. Consequently, energy intake in the subsequent breakfast and 24 h post-trial period was similar between conditions. To date, findings from bedtime snack studies have been inconsistent, rendering the role of bedtime energy intake as a potential weight management strategy inconclusive. Future studies that include chronic bedtime energy intake of foods with different macronutrient composition and texture are warranted to characterise the long-term implications of a structured bedtime snack v. free living bedtime eating habits. The best time of day to consume a milk-based snack to suppress appetite and reduce subsequent energy intake also remains unknown.
Acknowledgements
The authors thank the study participants for their dedication in the present study. They also thank Thomas Di Virgilio, Jordan Philpott, Nidia Rodriguez, Connor Davidson, Kate McDougall and Amanda Nicol for their help with data collection.
No funding was received for the present study.
A. H. H. L., D. R. C., T. G. C., S. D. R. G., K. D. T. and O. C. W. conceptualised and designed the research. A. H. H. L., G. M. D. and O. C. W. conducted the research, while A. H. H. L. and G. M. D. analysed the data. A. H. H. L. and O. C. W. wrote the paper and had primary responsibility for the final content. All authors read, edited and approved the final manuscript.
The authors have no conflicts of interest to declare.









