Some folate-dependent in utero processes occur very early in gestation, making the periconceptional period especially sensitive to maternal folate status(Reference Naninck, Stijger and Brouwer-Brolsma1–Reference Greenberg, Bell and Guan4). Low prenatal folate concentration poses a public health problem, as it has been widely associated with poor pregnancy outcomes, including megaloblastic anaemia, pre-eclampsia, stillbirth, and pre-term delivery(Reference Molloy, Kirke and Brody5–Reference Green and Datta Mitra7). An inadequate maternal folate status may also have a harmful impact on offspring development, including neural tube defects(Reference Martinez, Weakland and Bailey8) but also neurodevelopmental disorders such as delayed cognitive abilities, hyperactivity and autism spectrum disorders(Reference Devilbiss, Gardner and Newschaffer9–Reference Steenweg-de Graaff, Roza and Steegers14).
One of the major advances to address this concern has been prenatal folic acid (FA) supplementation. The WHO currently recommends 400 µg/d of FA for childbearing-age women, especially those planning to get pregnant(15). Accordingly, supplementation should begin at periconceptional time, which is from 12 weeks before conception to the first month of gestation(15). Benefits of prenatal FA supplementation in prevention of congenital disorders are well known(Reference Imbard, Benoist and Blom3,Reference Greenberg, Bell and Guan4,Reference Martinez, Weakland and Bailey8) and, additionally, they have also been observed in cases ranging from neurostructural defects to neurobehavioural and cognitive disorders in the offspring(Reference Naninck, Stijger and Brouwer-Brolsma1,Reference Gao, Sheng and Xie10,Reference DeVilbiss, Magnusson and Gardner16,Reference Iglesias-Vázquez, Canals and Arija17) .
Despite growing awareness of the need for correct prenatal folate status, the limited data available indicate that folate deficiency and insufficiency are 0–5 %(Reference O’Malley, Cawley and Kennedy18–Reference Vandevijvere, Amsalkhir and Van Oyen22) and 40–50 %(Reference Herter–Aeberli, Wehrli and Bärlocher19,Reference Vandevijvere, Amsalkhir and Van Oyen22) , respectively, in developed countries. As for clinical significance and based on WHO recommendations, folate deficiency refers to depleted folate stores, while folate insufficiency indicates a high risk of neural tube defect despite having folate reserves(Reference Herter–Aeberli, Wehrli and Bärlocher19,Reference Vandevijvere, Amsalkhir and Van Oyen22–Reference de Benoist24) .
Furthermore, compliance of periconceptional FA supplementation remains low(Reference McNulty, Pentieva and Marshall25,Reference Ray, Singh and Burrows26) , while many women start it during the first months of pregnancy, when damage may already have occurred as a result of an inadequate folate level, if any(Reference Toriello27). Some sociodemographic and lifestyle characteristics have been identified as possible determinants of the prenatal use of FA and, in turn, of the maternal folate status in early pregnancy. In this sense, young maternal age, low educational level, immigrant status and unplanned pregnancy would be considered the main predictors of reduced use of FA supplements before becoming pregnant, according to previous studies(Reference Ray, Singh and Burrows26,Reference Nilsen, Leoncini and Gastaldi28–Reference Rowe, Garcia and Davidson33) . Furthermore, smoking, not going to the gynaecologist, and having previous children have also been associated with failure to adhere to recommended prenatal FA supplementation(Reference Navarrete-Muñoz, Monzó and De La Hera30).
Although the nutritional status of other macro and micronutrients is usually well studied in pregnant women, in Spain there are few data on the prevalence of inadequate folate status in early pregnancy. Moreover, adherence to prenatal FA supplementation by pregnant women in this country is not adequately understood either, despite its great importance for both mother and child. The objective of the present study was to describe the prevalence of folate deficiency and insufficiency at the end of the first trimester of gestation, the pattern of prenatal supplementation with FA, and their associated factors in a sample of healthy Spanish pregnant women.
Methods
Observational study nested in the ECLIPSES community randomised controlled trial, involving 791 pregnant women from Tarragona (Spain) recruited between 2013 and 2017. The aim of the ECLIPSES study was to evaluate the effectiveness of Fe supplements during pregnancy in different doses adjusted to the Hb levels of the first trimester. Participants were contacted in their primary care centres during the first routine visit with midwives before gestational week (GW) 12 and were allocated into three groups of Fe supplementation according to their Hb levels at GW12. Detailed information on ECLIPSES study was shown elsewhere(Reference Arija, Fargas and March34). The classification of the participants applied in the parent study did not interfere with the current analyses because we evaluated here folate status at GW12, while Fe supplementation started from that moment. Thus, the sample was considered as a whole for the present analyses.
Midwives were responsible for data collection from the clinical history and questionnaires, and for their introduction into an electronic database. This was monitored by an external service to ensure correct data entry and security. The following information of participants at GW12 was extracted from questionnaires and taken into consideration for this work: age, parity, pregnancy planning, BMI, smoking, ethnicity, use of supplements, educational level and occupational status. Educational level and occupational status were used to calculate the familiar socio-economic status (SES).
Dietary assessment at GW12 was performed using a FFQ and then the women’s degree of adherence to the Mediterranean diet was obtained, as it is considered a healthy food pattern with a large supply of foods rich in folate. Adherence to Mediterranean diet was calculated using an rMED score, a variation of the original Mediterranean diet score(Reference Trichopoulou, Costacou and Bamia35,Reference Trichopoulou, Kouris-Blazos and Wahlqvist36) based on the intake of nine components of this diet. Each rMED component (apart from alcohol) was expressed in grams per 1000 kcal/d and was divided by tertiles of dietary intake. Each tertile was assigned a value of 0, 1 and 2 points. Out of the nine components of the rMED, six scored positively (fruit, vegetables, legumes, cereals, fresh fish and seafood, and olive oil), and two scored negatively (total and processed meat and dairy products). Alcohol was scored as dichotomous variable: 0 when women consumed alcohol and 2 when women did not drink alcohol. The total rMED score ranged from 0 points (minimal adherence) to 18 points (maximum adherence) and pregnant women were classified into three categories accordingly: ‘“low adherence”’ from 0 to 6 points, ‘“medium adherence”’ from 7 to 10 points and ‘“high adherence”’ from 11 to 18 points. Detailed calculation can be found in Jardí et al. (Reference Jardí, Aparicio and Bedmar37).
Regarding supplementation with FA, women were asked whether or not they use FA supplements. If so, information on the daily dose they received and when they started taking it were self-reported. Regarding the dose of FA, women reported 400 or ≥1000 µg/d (including 5000 and 10 000 µg/d), so we classified the dose of FA in these two groups in the subsequent analyses. As for time of initiation, we divided our population into two groups to be able to observe the effect of FA supplementation including or not the periconceptional time: (1) women who took FA from 12 weeks before conception up to 4 weeks gestation and (2) women who started the FA supplementation from 4 weeks of gestation onwards. It has to be clarified that not all women in or out of the periconceptional period took supplements for one entire period or the other but started the FA use in that period. Combining these data, the pattern of FA supplementation was described based on whether women used the recommended dose of FA (400 µg/d) or an excessive amount (≥1000 µg/d) in the periconceptional time or after. The total amount of FA taken from the supplements in GW12 according to pattern was calculated by multiplying the number of days taking FA supplements by the daily dose of FA used by each woman.
The use of self-reported FA supplements was validated, considering both the dose and the length of time that the participants had been taking it, by the concentration of erythrocyte folate in GW12. Despite some limitations of erythrocyte folate measurement concerning the risk of haemolysis and other analytical variables, this biomarker reflects tissue stores more closely than serum folate so its concentration is considered the most reliable indicator of folate level(Reference McNeely, Pesce and Kaplan38–Reference West, Caudill and Bailey40). In addition, vitamin B12 concentration was also measured since its interplay with folate. Both folate and vitamin B12 concentrations were determined by using a chemiluminescence immunoassay (ADVIA Centaur, Siemens Healthcare Diagnostics Inc.). Then, erythrocyte folate concentration was calculated following this formula: (serum folate in haemolysed whole blood * dilution factor in haemolysis * 100)/haematocrit.
Folate status at GW12 was described as follows: folate deficiency or depleted folate stores when erythrocyte folate <340 nmol/l; folate insufficiency or at elevated risk of neural tube defect when erythrocyte folate <906 nmol/l(23,Reference de Benoist24,41) .
Statistical analyses
Student’s t test and ANOVA were used to describe continuous variables (mean and sd), while the χ 2 test was used to compare categorical variables (percentages). Multivariate regression models (multiple linear regressions and logistic regressions) were used to assess the association of possible determinant factors on maternal folate levels and folate status at GW12. Based on previous literature and the results of the bivariate analyses, the regression models were adjusted for the following variables: maternal age (<25 years, 25–34·99 years and ≥35 years), maternal initial BMI (underweight, BMI < 18·5 kg/m2; normal weight, BMI 18·5–24·99 kg/m2; overweight, BMI 25–29·99 kg/m2; and obesity, BMI ≥ 30 kg/m2), parity (yes and no), SES (low, middle and high), pregnancy planning (yes and no), maternal ethnicity (Caucasian or ethnic minorities), smoking (yes and no), adherence to Mediterranean diet and serum levels of ferritin and vitamin B12 at GW12.
Additional exploration on whether some sociodemographic characteristics (age, SES and ethnic origin) and health-related behaviours (smoking and adherence to Mediterranean diet) were associated with pregnancy planning was performed.
The analyses showed in this work are secondary analyses from a randomised controlled trial, from which sample size was calculated. Considering a two-sided significance level of 0·05 and a specified sample size of 791 subjects, the study would have 88·5 % and 94·5 % power to detect differences of 4 % in prevalence of folate insufficiency and folate deficiency, respectively.
All statistical analyses were performed using SPSS (version 25.0 for Windows; SPSS Inc.) and statistical significance was set at P < 0·05.
Ethical approval
The study was designed in agreement with the Declaration of Helsinki/Tokyo and was approved by Clinical Research Ethics Committee of the Jordi Gol University Institute for Primary Care Research (Institut d’Investigació en Atenció Primària; IDIAP), the Pere Virgili Health Research Institute (Institut d’Investigació Sanitària Pere Virgili; IISPV) and the Spanish Agency for Medicines and Medical Devices (Agencia Española del Medicamento y Productos Sanitarios; AEMPS). Signed, informed consent was obtained from all women participating in the study. The ECLIPSES study was registered at www.clinicaltrialsregister.eu as EudraCT number 2012-005480-28 and at www.clinicaltrials.gov with identification number NCT03196882.
Results
The overall prevalence of folate deficiency (erythrocyte folate <340 nmol/l) and folate insufficiency (erythrocyte folate <906 nmol/l) were 9·6 % and 86·5 %, respectively (Table 1). This table describes the patterns of prenatal FA supplementation performed by the women in the ECLIPSES study (n 791) and the prevalence according to them; we found that 32·5 % of women were not supplemented with FA, while 6·8 % of them took more than recommended dose. In addition, almost 60 % of study population started the preventive FA supplementation after the first month of gestation (GW4); 54·4 % did so using 400 µg/d and 3·8 % using more than 1000 µg/d. Consequently, only 6·3 % of the women were optimally compliant with the use of prenatal FA supplements, starting to take 400 µg/d in the recommended time (from 12 weeks before conception). Table 1 also shows that maternal folate level at GW12 was significantly higher in women taking daily 400 µg or ≥1000 µg of FA in the periconceptional period (609·69 and 745·74 nmol/l, respectively) than in those using any of the doses after the first month of pregnancy (545·02 and 599·17 nmol/l, respectively). As expected, maternal folate concentration was higher in any of these cases, compared with women who did not receive preventive FA supplements (528·21 nmol/l). Supplementation with high doses of FA (≥1000 µg/d) did not led to exceed the upper value of cut-off point for correct folate status (erythrocyte folate = 1020 nmol/l)(Reference LaGow42). Regarding the prevalence of folate deficiency (erythrocyte folate <340 nmol/l), it was nil for those women who reported FA supplementation around conception, while it increased to 10·5 % and 10 % in those using, respectively, daily 400 µg and ≥1000 µg out of the recommended period and up to 10·9 % in those not supplemented.
Table 1. Prevalence of inadequate folate status at GW12, in total sample and according to the pattern of prenatal FA supplementation
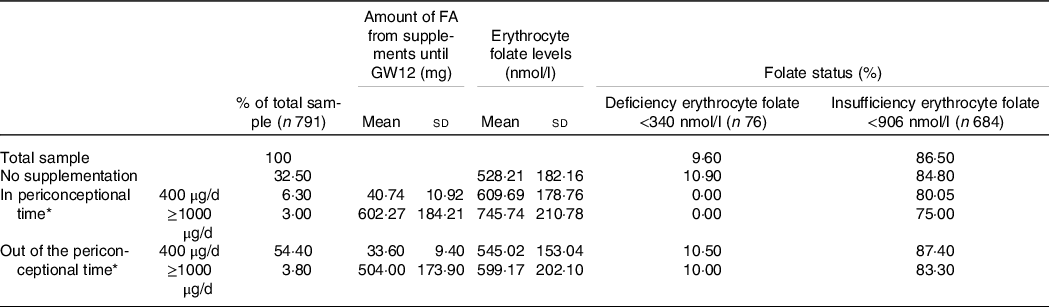
FA, folic acid; GW, gestational week.
* The periconceptional time is from 12 weeks before conception to GW4; out of the periconceptional time is from GW4 onwards.
Table 2 shows how different sociodemographic characteristics influenced the time of initiation of FA supplementation. Caucasian women, those aged between 25 and 34·99 years, with a medium SES level and pregnancy, tended to initiate FA supplementation in the periconceptional period to a greater extent than their counterparts. Likewise, significantly higher percentages of women under 25 years of age with low SES and ethnicity other than Caucasian were found among those who did not supplement or started the supplementation out of the periconceptional period than among those who met the recommendation. In addition, a significant greater percentage of high adherence to Mediterranean diet was found in women who met the recommendations compared with the others.
Table 2. Time of initiation of prenatal folic acid supplementation according to maternal characteristics

BMI, body mass index; SES, socio-economic status.
* Periconceptional time is from 12 weeks before conception to GW4; out of the periconceptional time is from GW4 onwards.
Data are expressed in %. P-values for comparisons between groups result from χ 2 test.
After measuring maternal folate levels, we found that Caucasian women, those aged over 35 years, non-smokers, with planned pregnancy, middle or high SES, and a high adherence to Mediterranean diet showed higher folate concentrations than their counterparts (Table 3). Here, coming out the observed association between adherence to the Mediterranean diet and erythrocyte folate levels, we verified the dietary intake according to the degree of adherence. As it is shown in Table 4, the greater the adherence to the Mediterranean diet, the greater the daily consumption of fruits, vegetables, legumes, nuts and fish. These results reinforce the representativeness of the Mediterranean diet as a whole as a good source of folate and its use in this study.
Table 3. Erythrocyte folate in early pregnancy, according to maternal characteristics and pattern of prenatal folic acid supplementation
(Mean values and standard deviations)
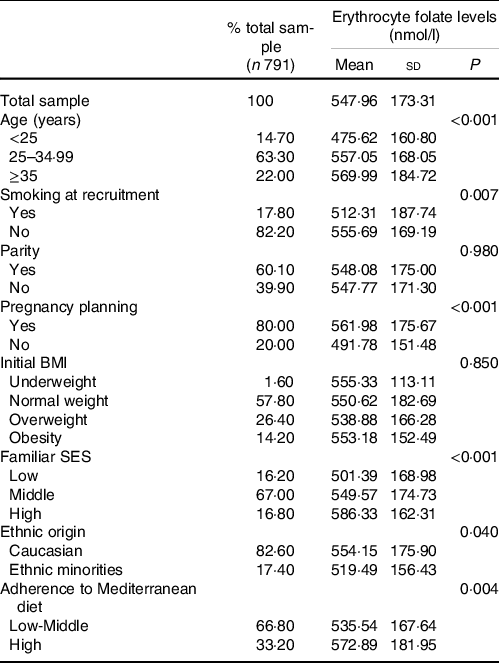
BMI, body mass index; SES, socio-economic status.
Table 4. Dietary intake (g/d) of folate-rich foods according to adherence to the Mediterranean diet
(Mean values and standard deviations)

Regarding the prevalence of folate deficiency, some of these factors were statistically significantly associated, the most notable being age, smoking, pregnancy planning and SES. Thus, the results showed that the prevalence of folate deficiency fell from 18·1 % in women under 25 years of age to 7·5 % in those over 35 years of age. Similar values were obtained for smokers and non-smokers, respectively. The prevalence was 8·4 % in women who had planned pregnancy, compared with 14·6 % in those who did not, and went from 16·4 % in those with a low SES to 5·4 % in those with high SES. Maternal characteristics with the most evident association with folate insufficiency were young age, the lack of pregnancy planning and non-Caucasian ethnicity (data not shown).
Multivariate adjusted analyses (Table 5) showed that, compared with the optimal pattern of FA supplementation which is daily 400 µg in a periconceptional period, both the lack of supplementation (β = −47·55, 95 % CI −98·51, 3·41) and the use of daily 400 µg after GW4 (β = −62·47, 95 % CI −111·68, −13·25) reduced in a great extent the concentration of maternal folate at GW12. On the contrary, the use of ≥1000 µg/d of FA increased maternal folate levels but only when it was taking during the recommended period (β = 137·92, 95 % CI 57·36, 218·49). As for the effect on folate status, compared with the use of daily 400 µg of FA in the periconceptional time, its use after GW4 increased the risk of folate insufficiency (adjusted OR = 3·32, 95 % CI 1·02, 15·36), while the use of ≥1000 µg in the recommended period seemed to reduce it by 75 % (adjusted OR = 0·23, 95 % CI 0·05, 1·13). On the contrary, no effect of the prenatal FA supplementation pattern was found on the risk of folate deficiency.
Table 5. Association between the pattern of prenatal folic acid supplementation and other characteristics on their erythrocyte folate levels and folate status at GW12†
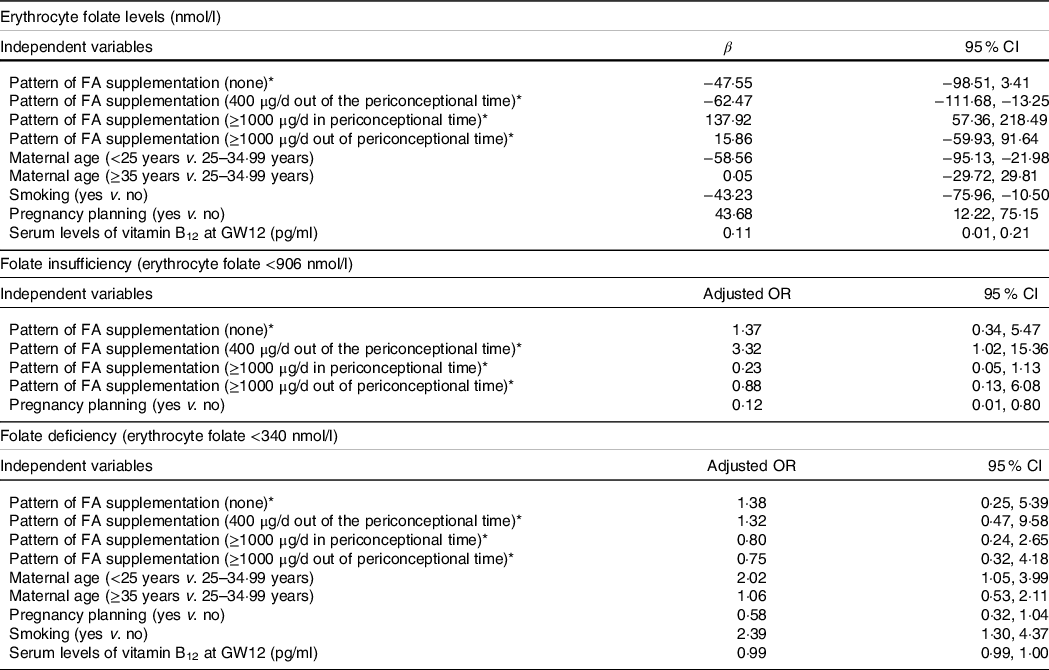
GW, gestational week; FA, folic acid.
* Reference category for pattern of FA supplementation: 400 µg/d in periconceptional time.
† Adjusted for: maternal age, smoking, parity, pregnancy planning, pattern of FA supplementation, hormonal contraception use, maternal initial BMI, socio-economic status, maternal ethnicity, adherence to Mediterranean diet, and serum levels of ferritin and vitamin B12 at GW12.
The most notable effects of sociodemographic factors on erythrocyte folate concentrations and folate status were as follows: age under 25 years, compared with ages between 25 and 34·99 years, greatly reduced maternal folate levels (β = −58·56, 95 % CI −95·13, −21·98) and doubled the risk of folate deficiency in GW12 (adjusted OR = 2·02, 95 % CI 1·05, 3·99); similarly, smoking reduced folate levels (β = −43·23, 95 % CI −75·96, −10·50) and increased the risk of folate deficiency more than twice (adjusted OR = 2·39, 95 % CI 1·30, 4·37). Multivariate analyses also confirmed that maternal folate levels in GW12 were positively associated with pregnancy planning increased, which protects against folate insufficiency (adjusted OR = 0·12, 95 % CI 0·01, 0·80). Additionally, increasing serum levels of vitamin B12 had a slight but statistically significant protective effect against inadequate folate status (Table 5).
We found that prevalence of smoking and low-middle rather than high adherence to Mediterranean diet were higher when there was no pregnancy planning. The results also showed that pregnancy planning was more common among Caucasian women, those between the ages of 25 and 34·99 years, and middle SES compared with their counterparts (online Supplementary Table 1).
Discussion
Given the scarcity of available data in Spain on the prevalence of inadequate folate status in early gestation as well as on the compliance of prenatal FA supplementation, the present study provides valuable information on these issues and associated factors in a sample of healthy Spanish pregnant women.
The prevalence of folate deficiency and insufficiency found in our study population (9·6 % and 86·5 %, respectively) was much higher than that in neighbouring countries, since available data indicate that they were around 0–5 % and 40–50 %, respectively(Reference O’Malley, Cawley and Kennedy18–Reference Vandevijvere, Amsalkhir and Van Oyen22).
Regarding compliance with FA supplementation at any time until the end of the first trimester, the percentage of women using FA in this study was similar to or higher than in other countries in Europe. Thus, participants reporting prenatal FA use represented the 55 % in a large European multicentre study evaluating about 23 000 women(Reference Bitzer, Von Stenglin and Bannemerschult44), 60·5 % in an Italian study with more than 2000 participants(Reference Nilsen, Leoncini and Gastaldi28) and 65·7 % in a Norwegian cohort including 811 healthy pregnant(Reference Kinnunen, Sletner and Sommer45).
Focusing on timing, current global evidence indicates suboptimal use of periconceptional FA supplements, with many countries reporting that fewer than 50 % of women started it before conception(Reference Ray, Singh and Burrows26). Recent studies in England(Reference Bestwick, Huttly and Morris46) and Norway(Reference Kinnunen, Sletner and Sommer45) found 30–31 % of women taking preconceptionally FA supplements, while lower percentages were found in Italy (23·5 %)(Reference Nilsen, Leoncini and Gastaldi28) and Spain (19·2 %)(Reference Navarrete-Muñoz, Monzó and De La Hera30). We believe that the much lower percentage of periconceptional use of FA found in our study (9·30 %) could be due to the relevant number of participants (∼20 %) from ethnic minorities in our population sample; as explained below, it could influence health care awareness(Reference Alderliesten, Vrijkotte and Van Der Wal32,Reference Rowe, Garcia and Davidson33,Reference Van Eijsden, Van Der Wal and Bonsel47,Reference Alomair, Alageel and Davies48) . An interesting finding that stand out the importance of using FA supplements during periconceptional time is in relation to the prevalence of folate deficiency. Our observation that the percentage of women with folate deficiency at GW12 was comparable between the group without supplementation and that of women supplemented from GW4 onwards, regardless the dose of FA, allows us to hypothesise that FA supplementation after the conceptional time is useless in improving maternal folate status in early pregnancy.
Based on our findings, and in agree with many studies, we highlight the central role of pregnancy planning in the optimal adherence to prenatal FA supplementation and the correct folate status(Reference Nilsen, Leoncini and Gastaldi28–Reference Stockley and Lund31,Reference Bixenstine, Cheng and Cheng49,Reference Navarrete-Muñoz, Valera-Gran and García de la Hera50) . We have found that it takes some time from the start of FA use until good folate stores built up, so pregnancy planning provides that time. We suggest, in addition, that pregnancy planning underlies the observed association between some other sociodemographic factors and the degree of compliance of FA supplementation in early pregnancy. Thus, younger women in our study have been found to be more likely to start FA supplementation after the first month of pregnancy which supports former findings(Reference Ray, Singh and Burrows26,Reference Nilsen, Leoncini and Gastaldi28,Reference Morin, De Wals and Noiseux29) and we hypothesise it is probably due to the lack of pregnancy planning in most cases. This make them more likely to have low prenatal folate concentrations and a greater risk of inadequate folate status(Reference Navarrete-Muñoz, Monzó and De La Hera30,Reference Stockley and Lund31,Reference Navarrete-Muñoz, Valera-Gran and García de la Hera50) . Similarly and supporting our findings, previous studies have repeatedly identified social and ethnic inequalities in regard to antenatal care and maternal folate status(Reference Alderliesten, Vrijkotte and Van Der Wal32,Reference Rowe, Garcia and Davidson33) . Women of ethnic minorities tend to neglect sexual and reproductive health more than Caucasian women, including prenatal use of FA supplements(Reference Stockley and Lund31,Reference Kinnunen, Sletner and Sommer45,Reference Timmermans, Jaddoe and Mackenbach51–Reference Baraka, Steurbaut and Leemans53) . Sometimes, they have to deal with low SES and educational level, which can lead to less healthy lifestyle, according to previous knowledge(Reference Stockley and Lund31,Reference Kinnunen, Sletner and Sommer45) . In addition, certain reluctance towards gynaecological and prenatal care has been identified in women from ethnic minorities or born in foreign countries, either for religious, cultural or linguistic reasons(Reference Alderliesten, Vrijkotte and Van Der Wal32,Reference Rowe, Garcia and Davidson33,Reference Van Eijsden, Van Der Wal and Bonsel47,Reference Alomair, Alageel and Davies48) . Such situations hinder pregnancy planning and correct compliance with FA supplementation(Reference Alderliesten, Vrijkotte and Van Der Wal32,Reference Rowe, Garcia and Davidson33,Reference Van Eijsden, Van Der Wal and Bonsel47,Reference Alomair, Alageel and Davies48) .
Blood folate concentration used to be lower in smokers(Reference Yila, Araki and Sasaki54–Reference Okumura and Tsukamoto56) and a recent systematic review confirmed the detrimental effects of tobacco exposure on folate levels specifically during pregnancy(Reference Tuenter, Bautista Nino and Vitezova57). One of the main postulated mechanisms by which smoking contributes to lowering folate concentrations and to increase the risk of inadequate folate status is the poor eating habits of smokers, who tend to consume less folate-rich foods such as fruits, vegetables and nuts(Reference Yila, Araki and Sasaki54–Reference Okumura and Tsukamoto56,Reference Segura, Javierre and Lizarraga58,Reference Ros59) . This suggests that a diet rich in plant-based foods is highly recommended even before pregnancy to achieve optimal folate levels(Reference Koebnick, Heins and Hoffmann60). Thereby, since nuts are not usually consumed so widely, it is especially interesting to highlight its beneficial role in relation to folate status and promote their incorporation into the prenatal diet.
The extensive data collection on sociodemographic characteristics, clinical and obstetrical information, and lifestyle strengthens the findings of this study. Available data on both the dose and the time of initiation of prenatal FA supplementation allowed us to know if women were following international recommendations. As erythrocyte folate reflected tissue stores during the previous 3–4 months, its measurement in GW12 accounts for maternal folate status at the periconceptional time. However, the risk of blood haemolysis and the high sensitivity of erythrocyte folate to some analytical variables could difficult the measurement procedure. In addition, some limitations have to be considered. Our findings have to be interpreted with caution given that the not-so-large sample size of the present study, despite being common in population-based studies, may limit the generalisability of the results. Furthermore, causal relationships could not be established due to the cross-sectional design.
In conclusion, study findings emphasise the importance of following the recommendation of starting FA supplementation at the periconceptional period to achieve optimal folate levels in early pregnancy. Although pregnancy planning is an accepted guideline, many women still do not adhere to it, so we continue to highlight its crucial role in routine obstetric visits for women who wish to become pregnant in order to strengthen public health strategies aimed to getting good pregnancy outcomes. These strategies should target young women, smokers, poorly adhere to Mediterranean diet which means less consumption of plant-based foods, and those especially vulnerable, such as those with low SES or those belonging to ethnic minorities.
Acknowledgements
We thank the Jordi Gol Research Institute in Primary Care (Institut d’Investigació en Atenció Primària; IDIAP) for their guidance regarding ethical matters. We thank the entities and participants in the ECLIPSES study: Research Group in Nutrition and Mental Health (NUTRISAM), Universitat Rovira i Virgili, Reus, Spain (Victoria Arija, Josepa Canals, Estefanía Aparicio, Núria Aranda, Cristina Bedmar, Carmen Hernández, Lucía Iglesias, Cristina Jardí, Núria Voltas and Nadine Khoury), Sexual and Reproductive Health Care Services (ASSIR) of Tarragona, Spain (Francesc Fargas, Francisca Ruiz, Gemma March and Susana Abajo) and the team of midwives recruited for the study (Irene Aguilar, Sònia Aguiles, Rosa Alzúria, Judit Bertrán, Carmen Burgos, Elisabet Bru, Montserrat Carreras, Beatriz Fernández, Carme Fonollosa, María Leiva, Demetria Patricio, Teresa Pinto, María Ramírez, Eusebia Romano and Inés Sombreo); the Research support Unit-Tarragona (Josep Basora, Meritxell Pallejà) and Central Unit-Barcelona (Rosa Morros) of the Institut d’Atenció Primària IDIAP Jordi Gol, Institut Català de la Salut, Laboratory of Institut Català de la Salut (ICS), University Hospital of Tarragona Joan XXIII, Tarragona, Spain (Núria Serrat).
The work was supported by the Instituto de Salud Carlos III, Fondo de Investigación Sanitaria, Ministerio de Sanidad y Consumo (grant number PI12/02777). The funding bodies play no part in the design of the study, collection and interpretation of data, or decision to publish.
Conception and planning, V. A.; biochemical measurement, N. S.; data curation, L. I. V., C. B. and M. P.; formal analysis, L. I. V.; investigation, L. I. V. and V. A.; writing – original draft, L. I. V.; writing – review and editing final version, L. I. V. and V. A.
There are no conflicts of interest.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0007114521004840








