There has been considerable public interest in the use of high-protein, low-carbohydrate (LC) weight-loss (WL) diets, as this type of diet can lead to modest fat loss, at least in the short term(Reference Johnstone, Horgan and Murison1, Reference Skov, Toubro and Ronn2). Despite widespread use, there are limited data on the possible consequences of this type of restrictive dietary regimen on indices of metabolic dysfunction and CVD risk, such as endothelial function, inflammatory markers and antioxidant status. Specifically, one major nutritional concern is the necessity of a limited intake of fruit and vegetables(Reference Mayor3) to achieve the LC requirement. This is counter-intuitive to current healthy eating advice, as inadequate intakes of fruits and vegetables are a risk factor for the development of CVD(Reference Nöthlings, Schulze and Weikert4) and certain cancers(Reference Nomura, Wilkens and Murphy5). This is associated, in part, with a dietary inadequacy of antioxidant micronutrients from plant-based foods. Moreover, it is known that LC intake has an impact on faecal bacterial populations, and this may have implications in the longer term for gut health(Reference Duncan, Belenguer and Holtrop6).
Although the efficacy and safety of high-protein, LC diets(Reference Bravata, Sanders and Huang7) has been questioned, most work has specifically examined the impact of the high protein component on health, for example kidney function(Reference Skov, Toubro and Bülow8) and bone health(Reference Skov, Haulrik and Toubro9). There are limited data that directly compare high-protein LC and high-protein, moderate carbohydrate (MC) WL diets on which to make recommendations. For example, it would be of interest to establish whether it is necessary to encourage antioxidant vitamin supplement(s) use while consuming a LC WL diet. Alternatively, recommended daily allowances of vitamins and micronutrients may be met by judicious inclusion of mixed fruits and vegetables, even when LC intake is met. The present study therefore focuses on comparing two isoenergetic high-protein diets, where the fat and carbohydrate contents have been altered. We hypothesised that the LC diet would have a negative effect on markers of antioxidant status, and the metabolic and cardiovascular profile in obese men undergoing WL, over a 4-week period.
Subjects and methods
A total of eighteen obese males with a BMI of >30 kg/m2 were recruited by newspaper advertisement to participate in a WL diet trial. Thus, the subjects were non-randomly selected individuals who were sufficiently motivated to actively respond to the request for volunteers. Because a subgroup of subjects underwent radioactive positron emission tomography scans (data reported separately), we were ethically constrained to recruit men >50 years, and the final recruitment profile reflects this older age grouping. Women of childbearing age were excluded from the study, on ethical advice, because of the radiation dose. During recruitment, the subjects underwent a medical examination, and their general practitioners were contacted to confirm medical suitability to participate in the study. The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the NHS North of Scotland Research Ethics Committee. Written informed consent was obtained from all subjects.
Study protocol
Participants were resident in the Human Nutrition Unit at the Rowett Institute of Nutrition and Health, Aberdeen, but were allowed to leave the Unit to attend their workplace. All food and drink were supplied by dietetic staff in the Unit. Subjects consumed a fixed intake maintenance (M) diet supplied at RMR (MJ/d) × 1·5 for 1 week. They then consumed two WL diets, each for a 4-week period, which provided approximately 70 % of energy maintenance requirements as high protein, LC and high protein, MC. The LC diet was designed to be ketogenic, and the MC diet was non-ketogenic. The order of treatments was randomised in a within-subject, cross-over design, whereby half of the subjects started on the LC diet and the other half on the MC diet.
Formulation and preparation of the diets
The composition of each meal, in terms of energy, fat, carbohydrate and protein, was calculated using McCance and Widdowson's The Composition of Foods (5th edition) and supplements(Reference Holland, Welch and Unwin10). All meals on the LC diet comprised 30 % protein, 4 % carbohydrate and 66 % fat by energy; while the MC meals contained 30 % protein, 35 % carbohydrate and 35 % fat. All meals within both diets had a fixed energy density of 5·75 MJ/kg. Lunch and dinner meals were fixed intake, with the LC meals containing 47·1 g (800 kJ) protein, 47·7 g (1765 kJ) fat, 6·63 g (106 kJ) carbohydrate and 2667 kJ energy. The MC lunch and dinner meals contained 47·2 g (802 kJ) protein, 25·3 g (936 kJ) fat, 58·5 g (936 kJ) carbohydrate and 2667 kJ energy. Subjects had a choice of seven breakfast meals, cooked on request, which varied from, per meal, 2·46 to 3·00 MJ and 2·31 to 2·92 MJ for the LC and the MC diets, respectively, with fixed macronutrient composition (%). See Appendices 1 and 2 for menus.
Presentation of the diets and measurement of food intake
While resident at the Human Nutrition Unit, each subject was allocated a refrigerator that was stocked daily with food. Kitchen research staff prepared and weighed all meals daily, with any leftovers weighed to the nearest gram. Breakfasts were eaten in the Unit. Subjects completed food diaries to record the time of eating. Subjects had free access to water and decaffeinated drinks. Energy and nutrient intakes were calculated using the Windiets program (Univation Limited; The Robert Gordon University, Aberdeen, Scotland).
Measurement of anthropometry, blood pressure and body composition
Measurements of body composition and metabolic rate were conducted under standardised conditions, with subjects instructed to fast overnight (10 h). Height was measured at the beginning of the study to the nearest 0·5 cm using a stadiometer (Holtain Limited, Crymych, Dyfed, Wales, UK). Subjects were weighed daily, after voiding, wearing only a pre-weighed dressing gown, to the nearest 50 g on a digital scale (DIGI DS-410; CMS Weighing Equipment Limited, London, UK). Abdominal and gluteal (hip) circumference was measured at the beginning and end of each dietary intervention period, as described previously(Reference Johnstone, Murison and Duncan11), following the guidelines of the International Standards for Anthropometric Assessment. Blood pressure was monitored at the beginning and end of each dietary intervention period, using an automated system (Omron M5-1; Omron Healthcare Incorporation, Bannockburn, IL, USA). Subjects were supine for 10 min before the measurement, and the average of three measures, taken 5 min apart, was recorded. Body composition was calculated by using a four-compartment model(Reference Fuller, Jebb and Laskey12) that involved dual-energy X-ray absorptiometry scanning (Norland XR-36, Mark II high-speed pencil beam scanner; Norland Corporation, Fort Atkinson, WI, USA, equipped with dynamic filtration, with version 2.5.2 of Norland software), body density by air displacement whole-body plethysmography (BodPod® Body Composition System; Life Measurement Instruments, Concord, CT, USA) and total body water by 2H dilution(Reference Pullicino, Coward and Stubbs13).
Blood sampling
Whole blood was sampled from a large antecubital vein in the morning after an overnight fast, before breakfast, using an 18G butterfly needle (Sarstedt, Nuernbrecht, Germany) and an adaptor and collected into separate EDTA and Li heparin tubes. The samples were immediately centrifuged (1000 g at 4°C for 10 min) and divided into aliquots before storage at − 80°C. Plasma for vitamin C analysis was diluted with an equal volume of chilled 10 % (v/v) metaphosphoric acid before freezing.
Carotenoids and antioxidant vitamins
Plasma vitamin C concentrations were determined by HPLC using an ion pairing technique, and reverse-phase HPLC was used to quantify vitamins A and E (α- and γ-tocopherols) and several carotenoids(Reference Duthie14). Analyses were conducted under the round-robin scheme of the National Institute of Standards Micronutrient Measurement Quality Assurance Programme and ISO9001 quality assurance status. Echinone was used as an internal standard, and intra- and inter-specific CV were 2 and 4 %, respectively. Analyses were conducted on weekly samples, so data are presented from weeks 1 to 4 for both WL (LC and MC) diets.
Hormone and metabolites
Analysis was conducted at the end of each dietary period, thus data are presented for the three diet periods (M, LC and MC). Insulin was measured on duplicate samples with an ELISA kit (Mercodia, Uppsala, Sweden), with a within-assay CV of 5 % and between-assay of 3 %. A Kone discrete automated clinical analyser (Kone Oyj, Espoo, Finland) was used for the analysis of urea, glucose, TAG, total cholesterol, LDL and HDL using commercial kits (Labmedics, Salford, Manchester, UK). The homeostasis model assessment of insulin resistance was applied using the fasting glucose and insulin values(Reference Matthews, Hosker and Rudenski15). The bead array Luminex system was employed for the determination of a range of inflammatory markers (IL-1β, IL-2, IL-6, IL-8, IL-10 and TNF-α), leptin, adhesion molecules (soluble intercellular adhesion molecule-1 and vascular cell adhesion molecule-1) and adiponectin (Millipore, Billerica, MA, USA).
Statistics
Data on energy intake, body weight and composition and blood metabolites for the two WL diets were analysed by ANOVA, with subject and period within subject as random effects, and diet and order as treatment terms (fixed effects). Data on plasma antioxidants were analysed by ANOVA, with subject, period within subject and the week within period as random effects, and diet, order, week and the week × diet and week × order interactions as fixed effects. Analyses of all three diets (i.e. including M) were also done, with order terms omitted (as diet M is always first), and antioxidant data for the WL diets averaged across weeks (as diet M was given 1 week only). All analyses were performed using Genstat 8.1 (Lawes Agricultural Trust, VSN International Limited, Hemel Hempstead, Herts, UK).
Results
The baseline characteristics of the sixteen participants are shown in Table 1; two subjects from the original eighteen withdrew for personal reasons.
Table 1 Baseline characteristics of the study participants
(Mean values, standard deviations and ranges, n 16)
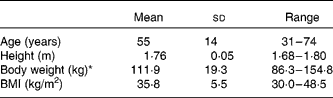
* Body weight at the end of maintenance with all subject data pooled, before randomisation to diet treatment.
Body weight and composition
During the M diet, subjects lost, on average, 1·0 kg body weight over the week, so may have been in slight negative energy balance. During the WL diets, on average, subjects lost 6·75 and 4·32 kg (6 and 4 %) body weight, on the LC and MC diets, respectively (P < 0·001, sed 0·350). This difference between the diets was explained, in part, by the larger total body water loss on the LC diet ( − 2·55 v. − 0·29 kg; P = 0·075; sed 1·175), probably linked to the mobilisation of hepatic glycogen stores. This was reflected in the change in fat-free mass stores with an apparent loss of − 2·72 and − 0·61 kg on the LC and MC diets, respectively (P = 0·042; sed 0·941). There was a gain in protein mass on the LC diet and a loss on the MC diet, but this did not reach significance. There were similar losses in fat mass between the diets with − 4·03 and − 3·71 kg on the LC and MC diets, respectively (P = 0·733; sed 0·919) (Table 2).
Table 2 Average measured change in body weight (kg) and composition (kg) on the study diets, where the low carbohydrate (LC) diet is a high-protein, low-carbohydrate diet and the moderate carbohydrate (MC) diet is a high-protein, medium-carbohydrate diet, for the 4-week intervention period*
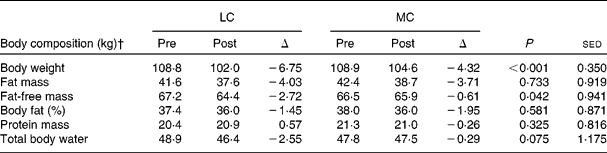
* The data are for the sixteen subjects analysed by ANOVA, where the P value refers to the analysis between the weight-loss diets (ΔLC and ΔMC).
† Measured with a four-compartment model(Reference Fuller, Jebb and Laskey12).
Energy and nutrient intake
On average, subjects consumed slightly less food and energy (0·3 MJ) on the LC diet compared with the MC diet (P < 0·001), when leftovers were accounted for. They consumed similar amounts of protein (30 % of energy) but varying amounts of fat (P < 0·001) and carbohydrate (P < 0·001). In terms of micronutrient intake, the LC diet contained twice as much retinol (P < 0·001) and vitamin E (P < 0·001), in comparison with the MC diet, possibly due to the presence of these lipid-soluble vitamins within the higher fat content of the diet. Interestingly, the LC diet also provided more vitamin C (P < 0·001) from the high citrus (LC) fruit intake and also vitamin D (P < 0·001). The MC diet provided more carotene, in the form of vegetables and tomatoes (P < 0·001). All the diets achieved the UK reference nutrient intake for vitamin E (4·0 mg/d), vitamin C (40 mg/d) and vitamin D (2·50 μg/d) but provided only 50–75 % of the reference nutrient intake for vitamin A (700 μg retinol equivalents for males)(16). Currently, there is no dietary recommendation for carotene intake (as a precursor of vitamin A), but the diets provided 3·7–4·9 mg carotenoids/d, within the range of 3–6 mg β-carotene/d suggested by the Food and Nutrition Board(17) as prudent (Table 3).
Table 3 Mean energy and micronutrient intake over the 4 weeks for the sixteen participants, with P value and sed for diet differences between the weight loss diets (low carbohydrate (LC) and moderate carbohydrate (MC))

M, maintenance; CHO, carbohydrate.
* M diet values provided for reference only.
Plasma vitamins and carotenoids
Data for values for selected plasma antioxidant vitamins and carotenoids (α-tocopherol, γ-tocopherol, xanthophyll, β-cryptoxanthin, lycopene, α-carotene, β-carotene, vitamin C and retinol) were compared across all three diets and for the two WL diets, with the effect of time (week on diet) also considered in Table 4. There were treatment effects of the WL diets compared with baseline (M) for all the antioxidant vitamins (P < 0·05). Values were lower on both the WL diets for α-tocopherol (P < 0·001), β-cryptoxanthin (P < 0·001) and retinol (P < 0·001). Relative to the M diet, γ-tocopherol (P < 0·001), xanthophyll (P < 0·001) and vitamin C (P < 0·001) were all greater on the LC diet (P < 0·05) but lower on the MC diet (P < 0·05). Relative to the M diet, lycopene (P < 0·001), α-carotene (P = 0·002) and β-carotene (P = 0·004) decreased on the LC diet and increased on the MC diet (P < 0·001).
Table 4 Mean values (μg/ml) for plasma antioxidants for maintenance (M), low-carbohydrate (LC) and moderate-carbohydrate (MC) weight-loss (WL) diets throughout the trial (weeks 1–4) for the sixteen participants

* Treatment effect relates to the P value from comparison of the M and LC and MC diets (average of weeks 1–4).
† WL diet effect relates to the P value from comparison of the LC and MC diets (average of weeks 1–4).
‡ WL diet × week effect relates to the interaction between the diet (LC and MC) and time course (weeks 1–4).
§ sed is for the WL diet effect.
There were differences between the two WL diets (LC and MC) for seven of the nine markers measured. For example, values were greater in the LC diet than in the MC diet for γ-tocopherol (P < 0·001), xanthophyll (P = 0·002) and vitamin C (P = 0·039). In contrast, concentrations were higher on the MC diet for lycopene (P < 0·001), α-carotene (P = 0·004), β-carotene (P < 0·001) and retinol (P < 0·001). When the effect of week within each diet was compared, there were WL diet × time interactions for seven of the nine markers. Only vitamin C and retinol did not show a change in concentration over the 4 weeks, on each WL diet. Notably, for α-tocopherol, γ-tocopherol, β-cryptoxanthin, lycopene and β-carotene, concentrations declined with time on the LC diet but remained constant or increased with the MC diet. In contrast, xanthophyll increased over the 4 weeks with the LC diet but decreased with the MC diet.
Plasma inflammatory markers
Of the cytokines measured (Table 5), only IL-10 (P = 0·049) and TNF-α (0·006) showed diet-related effects, with both reduced by WL therapy. The MC WL diet reduced IL-10 to a greater degree than the LC WL diet (P < 0·05), but the WL diet composition had no effect on TNF-α.
Table 5 Effect of the maintenance (M), low-carbohydrate (LC) and medium-carbohydrate (MC) diets on plasma inflammatory markers (pg/ml) in twelve volunteers
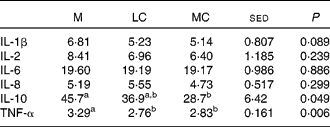
a,b Values within rows with unlike superscript letters were significantly different (P < 0·05).
Plasma cardiovascular risk markers
Mean values for the CVD risk markers adiponectin, soluble intercellular adhesion molecule-1 and soluble vascular cell adhesion molecule-1 in the plasma are presented in Table 6. There was no significant effect of WL or diet composition on any of these markers.
Table 6 Effect of the maintenance (M), low-carbohydrate (LC) and medium-carbohydrate (MC) diets on plasma cardiovascular risk markers (ng/ml) in twelve volunteers
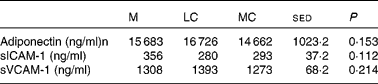
sICAM-1, soluble intercellular adhesion molecule-1; sVCAM-1, soluble vascular cell adhesion molecule-1.
Plasma metabolic health markers
Data on the homeostasis model assessment of insulin resistance, the plasma lipid profile, urea and β-hydroxybutyrate are presented in Table 7, and all parameters changed in response to WL (P < 0·05). Glucose was decreased by the WL diet (P < 0·001), and more so on the LC diet (P < 0·001). Homeostasis model assessment of insulin resistance was reduced during the WL diet, relative to the M diet (P < 0·001), but to a similar degree on both the WL diets. This was also reflected in insulin concentration, with a reduction relative to the M diet (P < 0·001), but there was no significant WL diet composition effect. As anticipated, the ketone body β-hydroxybutyrate was elevated in the LC (ketogenic) WL diet (P < 0·001) but remained similar between the M and MC WL diets. Urea concentration was elevated on both (high-protein) WL diets (P < 0·001) relative to the M diet, more so on the LC diet (P < 0·05). Lipid profiles were improved with the WL diet, with total cholesterol (P = 0·02) and LDL (P = 0·009) reduced, particularly with the MC diet (P < 0·05), whereas the improvement in HDL-cholesterol (P < 0·001) was better on the LC diet (P < 0·05). TAG levels were reduced to similar extents (P < 0·001) with both the WL diets. Leptin concentration was halved by WL treatment (P < 0·001), with both diets having a similar effect.
Table 7 Effect of the maintenance (M), low-carbohydrate (LC) and medium-carbohydrate (MC) diets on the homeostasis model assessment of insulin resistance (HOMA-IR) and plasma lipaemia* in sixteen volunteers
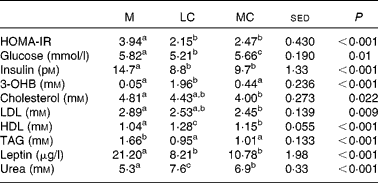
3-OHB, β-hydroxybutyrate.
a,b,c Values within rows with unlike superscript letters were significantly different (P < 0·05).
* HOMA-IR (from fasting insulin (pm) × fasting glucose (mm)/22·5).
Metabolic health – blood pressure and waist circumference (data not tabulated)
As anticipated, there was a significant effect of the WL diet on waist circumference (cm), relative to the baseline (M) diet (P < 0·001), with girth reduced from 117·7 to 111·1 and 114·2 cm at the end of the LC and MC WL diets, respectively. The LC diet promoted a greater reduction in girth than the MC diet (P = 0·011). As a group, the subjects were hypertensive, 144/90 mmHg at baseline (diastolic/systolic), which significantly dropped to a similar degree, (P < 0·001 for both) by the end of each WL diet regimen (127/79 and 128/79 mmHg) for the LC and MC diets, respectively.
Discussion
The present study compared the impacts of both a high-protein LC WL diet and a high-protein MC WL diet (in obese men) on plasma hormones (insulin), metabolites (glucose, antioxidant vitamins and lipid profile), inflammatory markers (cytokines) and endothelial markers (intercellular adhesion molecule, vascular cell adhesion molecule, adiponectin) as indicators of the metabolic and cardiovascular risk profile. It was hypothesised that the LC diet, which contained only 4 % carbohydrate, would have a deleterious impact on vitamin and antioxidant status, which would increase CVD risk. The present data suggest that the LC WL diet used in the present study does not impair plasma indices of cardiometabolic health, at least within a short time period (4 weeks) in otherwise healthy obese subjects. In general, improvements in metabolic health were similar between the LC and MC diets.
Body weight and composition changes
Although subjects consumed a similar amount of food energy, there was a 2·43 kg greater WL on the LC diet. This is similar to a previous study on subjects fed ad libitum (Reference Johnstone, Horgan and Murison1), though, in part, due to carbohydrate restriction depleting hepatic glycogen stores, with loss of associated water(Reference Astrup, Meinert Larsen and Harper18). In the four-compartment model of body composition used, glycogen is considered to be part of fat-free mass and is not directly measured. This could account for the greater apparent loss in fat-free mass on the LC diet. Volek et al. (Reference Volek, Sharman and Gómez19) concluded that LC diets favour the preservation of lean body mass, a finding supported by the present study.
Antioxidant vitamins and carotenoids
To our knowledge, this is the first dietary WL study to report plasma antioxidant values, with previous emphasis on the role of supplementation with the obesity drug Orlistat(Reference Melia, Koss-Twardy and Zhi20) and gastric surgery(Reference Gasteyger, Suter and Gaillard21). The present study found that plasma concentrations of vitamin E, vitamin C and carotenoids on the M diet were similar to that previously reported in a Scottish population(Reference Ross, Crosley and Brown22). The LC diet used in the present study used a wide range of easily obtained fruit and vegetable sources to achieve the 4 % carbohydrate (of energy) intake. This wide variety would help provide a number of the vitamin and antioxidant metabolites considered important for metabolic and cardiovascular health(Reference Moats and Rimm23). These diets were adequate in terms of recommended daily intake for vitamins C, D and E, but deficient in vitamin A. Indeed, plasma concentrations of retinol were lower for the LC diet than for either the MC or the M diet. Both the LC and MC diets produced a similar pattern of decline in plasma retinol over the 4 weeks of ingestion. This may have implications for longer-term WL on such diets. Interestingly, although vitamin E recommended daily intake was met on all diets, plasma concentrations of both α-tocopherol and γ-tocopherol declined over time on the LC diet. Deficiencies of α-tocopherol are implicated in several clinical conditions including heart disease, strokes and cancer(Reference Dietrich, Block and Norkus24). This decrease over a period of WL is in contrast to studies which indicate that obesity is associated with lower plasma vitamin E concentrations(Reference Gasteyger, Suter and Gaillard21, Reference Aasheim, Hofsø and Hjelmesaeth25). Smoking-related effects can be excluded, as all volunteers were non-smokers. Consequently, changes in plasma α-tocopherol concentrations probably reflect the tocopherol homologues present in the plant oil components of the WL diets. Nevertheless, concentrations were not indicative of an overt deficiency as classified by the UK guidelines of below 6·5 μg/ml(26). Whether such concentrations are attained with the prolonged use of WL diets is unclear, but supplementation with α-tocopherol may be warranted in the longer term. In contrast, vitamin C values were stable and greater on the LC diet compared with the MC and M diets. This was probably due to the use of unsweetened orange juice as part of the LC menu, but illustrates that dietary manipulation can be used to achieve a good nutritional status, even when food sources containing carbohydrate are severely restricted. Indeed, the LC diet contained twice as much fruit and vegetables (g) as the MC diet (Appendix 2) to match the weight of the meals. The duration of the study is relatively short, when considering the storage and turnover of fat-soluble vitamins v. water-soluble vitamins. For example, symptoms of scurvy (vitamin C deficiency) are apparent after a month on a deficient diet, but for vitamin E with tissue-specific stores, it could take up to 2 years for subjects to become deficient on the diets used. However, much of nutrition interest in this area is around defining ‘optimal levels for health’, and little is known about how diet and phenotype interact to determine the optimal nutrient profile at a population level or individual level during WL or weight maintenance.
Plasma cardiovascular risk markers
Markers of inflammation and endothelial dysfunction are elevated in obesity and type 2 diabetes mellitus and allegedly predict systemic atherogenesis risk(Reference Pontiroli, Pizzocri and Koprivec27). Interventions that lower the glycaemic response to foods, by lowering either the glycaemic index or the glycaemic load, have demonstrated improvement in risk markers for both type 2 diabetes and CHD(Reference Kelly, Frost and Whittaker28). WL by lifestyle modification also improves the cardiovascular risk profile, indicated by large-scale efficacy trials, as reviewed by Horton(Reference Horton29). Much work on these adhesion molecules have focused on surgical patients undergoing gastric procedures for weight control and known to improve metabolic health(Reference Pontiroli, Frigè and Paganelli30). Although the present study reports no statistical improvement in the markers with WL, larger studies have. The numerical values for intercellular adhesion molecules are similar to previous reports before (333 ng/ml) and after (274 ng/ml) WL, in patients undergoing laparascopic gastric banding(Reference Pontiroli, Pizzocri and Koprivec27). In terms of the role of diet composition in WL, Keogh et al. (Reference Keogh, Brinkworth and Clifton31, Reference Keogh, Brinkworth and Noakes32) compared the LC with MC WL diet in two groups of subjects and reported no specific diet effects on short-term (8-week) improvement in CVD risk factors, but observed a decrease in flow-mediated dilation in the LC group at 1 year(Reference Wycherley, Brinkworth and Keogh33).
Adiponectin is associated with markers of chronic inflammation, with a rise following WL in morbidly obese patients after gastroplasty(Reference Kopp, Krzyzanowska and Möhlig34) or modest WL(Reference Forsythe, Wallace and Livingstone35). The lack of change in the present study suggests that this marker to be less responsive to more modest WL. Indeed, Keogh et al. (Reference Keogh, Brinkworth and Noakes32) reported no change in adiponectin associated with short-term (8-week) WL on a LC or a MC diet, but there was an improvement after 1 year of maintenance following a LC diet(Reference Keogh, Brinkworth and Clifton31). Furthermore, while adiponectin is more responsive to modest WL of between 5 and 10 %(Reference Madsen, Rissanen and Bruun36), smaller losses (up to 5 % body weight) result in no change in obese women(Reference Varady, Tussing and Bhutani37).
Plasma inflammatory markers – cytokines
Plasma adiponectin concentrations are thought to be associated with the inhibition of vascular inflammation, being capable of lowering TNF-α levels in knockout mice(Reference Manigrasso, Ferroni and Santilli38). Obesity is associated with reduced insulin sensitivity linked to inflammatory status(Reference Eckel39). Many of the cytokines, including TNF-α, IL-1, IL-6 and IL-18, have shown elevated plasma concentration in the insulin-resistant state(Reference Eckel39) and an increase with obesity(Reference Ruan and Lodish40), but a decrease with WL(Reference Barinas-Mitchell, Kuller and Sutton-Tyrrell41). As with many other WL studies, the volunteers in the present study showed an improved (lowered) homeostasis model assessment of insulin resistance in response to WL(Reference Belobrajdic, Frystyk and Jeyaratnaganthan42, Reference Sheu, Chang and Lee43). This substantial improvement was not associated, however, with altered cytokine status, except for decreased TNF-α and IL-10. Responses in cytokines with WL have been inconsistent, however. For example, although IL-6 has been shown to decrease(Reference Bastard, Jardel and Bruckert44), in other studies, no change has been observed in others(Reference Shadid, Stehouwer and Jensen45). Similarly, TNF-α either decreases(Reference Bruun, Verdich and Toubro46) or is unaltered(Reference Bastard, Jardel and Bruckert44) by fat loss. Clearly, links between obesity, inflammatory status and response to WL need to be better understood.
Plasma metabolic markers
Despite the LC WL diet being high in fat (66 % energy), there was no detrimental effect on lipaemia. Indeed, the present study indicates a significant improvement in the lipid profile. This is in accordance with several reviews(Reference Westman, Mavropoulos and Yancy47–Reference Noble and Kushner49), reporting that LC diets, when accompanied with WL, positively improve the lipid profile. Nonetheless, greater improvements in total cholesterol and LDL were observed with the MC diet, which probably reflects the lower fat (35 % fat) content of this diet. Furthermore, it is well recognised that LC diets promote a reduction in fasting glucose and insulin(Reference Volek and Sharman48) and improve insulin sensitivity(Reference Bisschop, de Metz and Ackermans50), although in the present study, the LC diet did not offer any metabolic advantage over the MC diet. The measurement of metabolic markers was conducted during active WL, and it may be that the data reflect negative energy balance and not the lower body-weight status or diet composition, per se. It may be that these markers change accordingly once subjects return to energy balance status. We applied the Mensink & Katan(Reference Mensink and Katan51) predictive equation for estimating change in plasma cholesterol from diet composition. This regression equation assumes substituting carbohydrate with fatty acids for an isoenergetic diet. This predicted a decrease of 0·2 mmol/l from the MC to LC diets, reflecting the decrease in saturated fat consumption. There was a decrease of 0·43 mmol/l. There are a number of limitations of the equations, and in this scenario, they are being applied in negative energy balance, which may alter the predictive power. Although leptin concentrations are usually correlated with BMI and body fat, there is usually a rapid decrease associated with negative energy balance and WL(Reference Kerksick, Thomas and Campbell52), a finding confirmed in the present study where values halved from baseline, with a fat loss of only 3·7–4·0 kg or 1·5–2·0 % initial body fat.
Although the study has small subject numbers, it benefits from a within-subject design and complete control over dietary input, allowing precise metabolic measurements. The assumption that LC diets have a negative impact on vitamin and antioxidant intake is not supported. In conclusion, the present data would suggest that both high-protein LC and high-protein MC WL diets are safe within this relatively short period of time (4 weeks), as assessed by the reported biomarkers, and under medically supervised conditions, they could be used to achieve considerable WL in order to improve mortality and morbidity in obese patients. Antioxidant supplements may be warranted if LC WL diets are consumed for a prolonged period.
Acknowledgements
We are grateful for the assistance from Glenn Harden in processing the plasma samples, and Marion Scott, Jean Bryce, Nina Lamza and Kim Giles for the preparation of the study diets. We also thank Sylvia Hay and Linda Dewar for support in the Human Nutrition Unit. A. M. J., G. E. L. and G. G. D. were responsible for the study concept and design. A. M. J., D. M. B., P. C. M., C. L. F. and G. G. D. were responsible for the sample collection and analysis. A. M. J., G. E. L., G. G. D. and G. W. H. were responsible for the data analysis. A. M. J., G. G. D., G. W. H. and G. E. L. were responsible for the first draft and critical revision of the manuscript for important intellectual content. None of the authors had a personal or financial conflict of interest. The Rowett Institute and Biomathematics and Statistics Scotland are grateful for funding from the Scottish Government who provided funding for the present study.
Appendix 1 Menu of the maintenance meals provided
Appendix 2 Menu of high-protein weight-loss meals provided

LC, low carbohydrate; MC, moderate carbohydrate.











