Introduction
The geographical distribution and dynamics of a species are fundamental characteristics and essential tools for assessing its extinction risk (Gaston and Fuller Reference Gaston and Fuller2009; Pearson Reference Pearson2007; Syfert et al. Reference Syfert, Joppa, Smith, Coomes, Bachman and Brummit2014). The distribution of a species can be mapped by creating a polygon that connects the occurrence points that are farthest apart and quantifying the resulting area to determine the extent of occurrence (EOO) or by quantifying only the habitats actually occupied by the species within the EOO to determine the area of occupancy (AOO) (BirdLife International 2000; Gaston Reference Gaston1991; Gaston and Fuller Reference Gaston and Fuller2009; Jiménez-Alfaro et al. Reference Jiménez-Alfaro, Draper and Nogués-Bravo2012). IUCN Standards and Petitions Committee (2022) uses both types of geographical distribution as criteria to assess the conservation status of a species, because they express possible threats and vulnerabilities. The AOO is the most appropriate tool for analysing a wide variety of biological problems, but its assessment varies depending on the mapping scale and the level of biological knowledge about the species, with the most refined estimate possible being the sum of the area of the territories of a species (Gaston Reference Gaston1991). Difficulties of scale and dependence on in-depth knowledge to estimate geographical distributions as refined as possible were “resolved” by assigning 4 km² of AOO to any recording point that fell within a 2 km × 2 km grid (IUCN 2022). In addition to the cut-off line that limited more detailed assessments of AOO, there were methodological problems with (1) species with only one or a few linear records, thus preventing the delimitation of an EOO polygon, or (2) species of much reduced EOO, smaller than the minimum AOO of 4 km2 proposed (IUCN 2022). Both problems are in contrast with the concept that AOO represents smaller areas within the EOO, needing to align the two metrics (Bornschein et al. Reference Bornschein, Pie and Teixeira2019). The detailed measurement of the habitat has been proposed as a new metric called area of habitat (AOH), or extent of suitable habitat (ESH) (Brooks et al. Reference Brooks, Pimm, Akçakaya, Buchanan, Butchart and Foden2019; Lumbierres et al. Reference Lumbierres, Dahal, Soria, Marco, Butchart and Donald2022). Although this metric is not directly used to assess the extinction risk of species by International Union for Conservation of Nature (IUCN) based on geographical distribution (IUCN 2022), in addition to estimating important species distribution characteristics, when calculated using the same method as that used for EOO and AOO, minimum convex polygon (MCP) and 4 km² grid, respectively, it serves as an upper bound that represents the potential distribution of the species (Brooks et al. Reference Brooks, Pimm, Akçakaya, Buchanan, Butchart and Foden2019).
The Parana Antwren Formicivora acutirostris is a species endemic to the Atlantic Forest biome (Brooks et al. Reference Brooks, Tobias and Balmford1999) in southern Brazil, described in 1995 (Bornschein et al. Reference Bornschein, Reinert and Teixeira1995). This and its sister species, the Marsh Antwren F. paludicola, described in 2014, are the only marsh-dwelling thamnophilids worldwide (Zimmer and Isler Reference Zimmer, Isler, del Hoyo, Elliott and Christie2003; Buzzetti et al. Reference Buzzetti, Belmonte-Lopes, Reinert, Silveira and Bornschein2013). The geographical distribution of Parana Antwren was proposed to be divided into eight isolated populations from the central coast of Paraná to the north coast of Santa Catarina in southern Brazil, totalling an estimated AOH of 6,060 ha and an estimated population of 17,680 mature individuals (Reinert et al. Reference Reinert, Bornschein and Firkowski2007). This species has been assessed as being “Near Threatened” globally (BirdLife International 2019) and “Vulnerable” in Brazil (ordinance MMA no. 148/2022). Its conservation status is a constant concern because it occupies a coastal region that is under pressure from anthropogenic factors (Reinert et al. Reference Reinert, Bornschein and Firkowski2007). Hence, the geographical distribution of Parana Antwren assessed by Reinert et al. (Reference Reinert, Bornschein and Firkowski2007) has become outdated because it was based on aerial photographs taken over 40 years ago (in 1978 and 1980). Additionally, the species was recorded in Rio Grande do Sul (Bencke et al. Reference Bencke, Dias, Bugoni, Agne, Fontana and Maurício2010), 329 km south of the southernmost site of Reinert et al. (Reference Reinert, Bornschein and Firkowski2007), suggesting a better conservation scenario. In the present study, we assessed and updated the estimates of the EOO, AOO, AOH, population size, and conservation status of Parana Antwren. Herein, we also propose potential conservation measures.
Methods
Target species Parana Antwren
Parana Antwren displays sexual dimorphism (Reinert and Bornschein Reference Reinert and Bornschein1996) and has low flight capacity, typically not flying more than 25 m above vegetation (Reinert et al. Reference Reinert, Bornschein and Firkowski2007). It is a long-lived insectivorous species (Bornschein et al. Reference Bornschein, Pizo, Sobotka, Belmonte-Lopes, Golec and Machado-de-Souza2015), socially monogamous (Sobotka Reference Sobotka2011), that forms permanent pairs inhabiting territories that are defended over time (Reinert Reference Reinert2008). Most territories remain stable over time (Bornschein Reference Bornschein2013), despite turnover among their occupants (Sobotka Reference Sobotka2011). For example, the oldest individual of the species is a male named “Rosaldo” (Fi8), born on 6 October 2007, and still reproductively active at the age of 16 years and four months (last observed on 18 February 2024; M. R. Bornschein and G. Sandretti-Silva, unpublished data). Rosaldo has occupied the same territory, with nearly the same shape, since he was seven months old. The parents divide nest construction, incubation, and nestling care (Reinert Reference Reinert2008), but only females incubate the eggs at night (M. R. Bornschein and G. Sandretti-Silva, unpublished data). Parental care involves a brood division, with each parent attending to a single offspring (M. R. Bornschein and G. Sandretti-Silva, unpublished data).
Estimates of population density
We worked at four sites in Guaratuba Bay (Ramsar site Guaratuba), southern Brazil: (1) Jundiaquara Island (c. 25°52’25”S, 48°45’32”W; 11.3 ha); (2) the confluence of the Claro and São João Rivers (“Continente”; c. 25°52’28”S, 48°45’44”W; 8.5 ha); (3) Folharada Island (c. 25°51’58”S, 48°43’23”W; 16.3 ha); (4) Lagoa do Parado (c. 25°44’36”S, 48°42’53”W; 6.7 ha (Figure 1). We ringed all territorial individuals in the study sites with colour combinations to allow for individual identification. To capture individuals, we attracted them with playback and used 12-m wide ornithological nets with 28-mm meshes.

Figure 1. Study areas for Parana Antwren Formicivora acutirostris in Lagoa do Parado (1), Continente (2), Jundiaquara Island (3), and Folharada Island (4), Guaratuba Bay, Ramsar site Guaratuba in the municipality of Guaratuba, Paraná coast, southern Brazil. BR = Brazil; PR = Paraná. Background images from the National Water and Sanitation Agency (ANA), Brazilian Institute of Geography and Statistics (IBGE), Brazilian Annual Land Use and Land Cover Mapping Project (MapBiomas), Map data ©2015 Google, and Geomorphometric Database of Brazil (TOPODATA).
From January 2006 to mid-2008, we worked in the field every day during the reproductive season, from 1 September to 28 February, and 10 days per month outside it, from 1 March to 31 August. From mid-2008, we worked in the field for three to eight days per month every month. Daily, we worked from dawn until about 12h00 or 13h00 and for an additional 2–3.5 hours in the afternoon. Fieldwork was conducted by three to six people.
We marked the location of territorial individuals on each monthly trip or every three days when the fieldwork was uninterrupted. We determined the number of territorial pairs in the study areas and assessed the population densities in annual cycles: 15 cycles on Jundiaquara Island (2006–2021), 14 cycles at Continente (2007–2021), 11 cycles on Folharada Island (2010–2021), and one cycle at Lagoa do Parado (2012–2013).
Target environments
Jundiaquara Island, Folharada Island, and Continente are located in the Guaratuba Bay estuary, which is flooded twice daily by mixed-type tides (Lee and Chang Reference Lee and Chang2019). In Lagoa do Parado, where the influence of tides is indirect, poor water drainage (Reinert et al. Reference Reinert, Bornschein and Firkowski2007) leads to periodic flooding during the rainy season (c. October–March). The habitat types of the study sites are summarised in Supplementary material Table S1.
Estimation of the EOO
Since the Reinert et al. (Reference Reinert, Bornschein and Firkowski2007) study, we have found new sites of occurrence of the species in Paraná and Santa Catarina, where we conducted systematic searches. For each site, we recorded geographical coordinates and identified the species’ habitat, following Reinert et al. (Reference Reinert, Bornschein and Firkowski2007). Additionally, we reviewed the species’ geographical distribution by compiling records from the literature and the WikiAves website (http://www.wikiaves.com.br). We joined the extreme points of occurrence in an MCP to define the species’ EOO (IUCN 2012, 2022; see below).
Estimation of the AOO
We calculated this metric by adding 4 km2 to the AOO for each cell within a 2 km × 2 km grid containing species records (IUCN 2022). For species records, we compiled our data set obtained from 1996–2023, information from the literature and the WikiAves website.
Estimation of the AOH
We mapped the habitat types occupied by the species (Reinert et al. Reference Reinert, Bornschein and Firkowski2007) within the EOO to determine the AOH (Brooks et al. Reference Brooks, Pimm, Akçakaya, Buchanan, Butchart and Foden2019) on 2020 satellite images from Google Earth Pro 7.3.3.7786 (64-bit), noting the positions, textures, height impressions, and colour nuances of the vegetation. We conducted on-site verification in various regions of Paraná, Santa Catarina, and Rio Grande do Sul States. In situations when we could not identify the dominant trees in habitats that had an upper arboreal and a lower herbaceous stratum we treated them as “unidentified arboreal formations” (Table S1).
Based on the mapping of Reinert et al. (Reference Reinert, Bornschein and Firkowski2007), we conducted assessments of each habitat type using Google Earth satellite images from 2002 to 2020 (Table 1) as follows: (1) habitat loss according to different causes of disappearance; (2) whether the habitat had undergone progressive ecological succession, requiring reclassification as a different habitat type still occupied by the species; (3) whether the habitat had undergone progressive ecological succession to a vegetation type not occupied by the species; (4) whether a vegetation type not occupied by the species had undergone regressive succession and became an occupied habitat type (Sandretti-Silva et al. Reference Sandretti-Silva, Teixeira, Golec and Bornschein2023); (5) whether a habitat had been overlooked; (6) whether the habitat had been misclassified; (7) if new patches of habitat types occupied by the species appeared. Herbaceous formations that had been previously occupied by the species but were invaded by exotic grasses (i.e. Urochloa arrecta and U. mutica) were no longer considered as habitat (Reinert et al. Reference Reinert, Bornschein and Firkowski2007).
Table 1. Habitat gains and losses by populations of Parana Antwren Formicivora acutirostris compared with previous mapping (Reinert et al. Reference Reinert, Bornschein and Firkowski2007), based on aerial photographs from 1978 in Santa Catarina and from 1980 and 1995 in Paraná. The years or intervals of the years of the photographs or satellite images used for the analysis are indicated in parentheses

1 New secondary marshes formed in areas where they did not previously exist due to anthropogenic actions (see Reinert et al. Reference Reinert, Bornschein and Firkowski2007).
2 Formations where the species did not occur, modified through regressive ecological succession to habitats where it does occur.
3 Habitats where the species occurred, modified through progressive ecological succession to formations where it does not occur.
4 Tidal marshes that were almost completely submerged during the six-month period of high water levels in Lagoa do Parado and are not occupied by the species (M. R. Bornschein, unpublished data).
5 Loss mainly due to pasture formation, which may or may not include drainage, cutting of vegetation, and landfills for human occupation.
6 Biological invasions by exotic grasses, Urochloa mutica and, mainly, U. arrecta.
7 Patches of habitat smaller than the smallest territory for a pair of species observed in the same or similar environment as a reference (Table S2).
The new mapping approach included two key adjustments. We excluded the mapping of herbaceous formations from the central and southern coast of Santa Catarina to the northern coast of Rio Grande do Sul (south of 27°45’S) as the species’ habitat due to the absence of occurrences in those environments. Our mapping in this region focused exclusively on habitats characterised by an upper arboreal stratum and herbaceous stratum, in which we recorded the species. Next, we disregarded patches of habitat smaller than the smallest territory for a species pair observed in the same or in a similar reference habitat if they were isolated from other habitat patches by more than 6 m (Table S2). We even excluded different habitat patches if they were up to 6 m apart, but the combined ratio of each patch with regard to the smallest territory within the given habitat type or in habitat type similar to it did not meet the minimum area requirement (Table S2). We established a 7-m interruption in habitat as an insurmountable gap within a species’ territory, given that the largest gap in habitat continuity recorded in the species’ territories was 6 m (M. R. Bornschein, unpublished data). Finally, we added up all the areas of the mapped habitats by habitat type, following Reinert et al. (Reference Reinert, Bornschein and Firkowski2007) to assess the AOH at a fine scale. We also assessed the AOH by overlaying the mapped habitat with a 4-km² grid to make it comparable with the AOO as an upper bond that represented the potential of species occupancy (Brooks et al. Reference Brooks, Pimm, Akçakaya, Buchanan, Butchart and Foden2019). For this second method, we added up the area of each marked cell to obtain the AOH (IUCN 2012, 2022).
Delimitation of distinct populations
New sites of records were either assigned to one of the existing eight populations of the species or assigned to a new population if the habitat patches were close to each other and at least 10 km away from another set of habitat patches (Reinert et al. Reference Reinert, Bornschein and Firkowski2007). We confirmed the absence of suitable habitat within a distance of at least 10 km between mapped patches. In highly degraded regions where natural habitats have been suppressed, such as the central and southern coast of Santa Catarina, we inferred the absence of continuous habitats. Conversely, we regarded the presence of estuaries, meandering rivers flowing into estuaries or lagoons, and lagoons on plains as indicative of potential historical habitats for the species. These factors were taken into consideration when identifying habitat patches within a given population.
Estimation of population size
To estimate the population size of Parana Antwren, we multiplied the values of density of territorial individuals (Table 2) by the total area of each habitat type, following Reinert et al. (Reference Reinert, Bornschein and Firkowski2007). Some sites were studied for several years, so we averaged the population densities (Tables S3–S5). For habitats without available density data, we extrapolated densities obtained in habitats with comparable vegetation structures (Table 2; adapted from Reinert et al. [Reference Reinert, Bornschein and Firkowski2007]).
Conservation status and Green Status
We evaluated the conservation status of the species according to IUCN (2022). Additionally, we evaluated the Green Status according to the IUCN Species Conservation Success Task Force (2020) and IUCN (2021) to quantify the species’ recovery in different scenarios. Both assessments are related and complement each other to inform the conservation status of a species (IUCN 2021). For the Green Status, we obtained the Current, Counterfactual Current, Future-with-conservation, and Future-without-conservation scenarios to assess three of the proposed metrics: (1) Conservation Legacy, which quantifies the impact of past conservation actions; (2) Conservation Dependence, which quantifies the impact of ongoing conservation actions; (3) Conservation Gain, which quantifies the impact of ongoing and planned conservation action in a short-term future (IUCN 2021). We developed future scenarios by extrapolating the annual decline rates for habitats and individuals from populations and assessing threats in the areas.
Results
EOO, AOO, and AOH
The EOO of the Parana Antwren was estimated as 26,655 km². The MCP spanned 467 km from north to south, and up to 88 km from west to east (Figure 2A). The estimated AOO was 320 km² (Figure 2B). The AOH of the species was calculated to be 41 km² (Table 3) at fine scale and 980 km² using the 4 km² grid (Figure 2C and D).
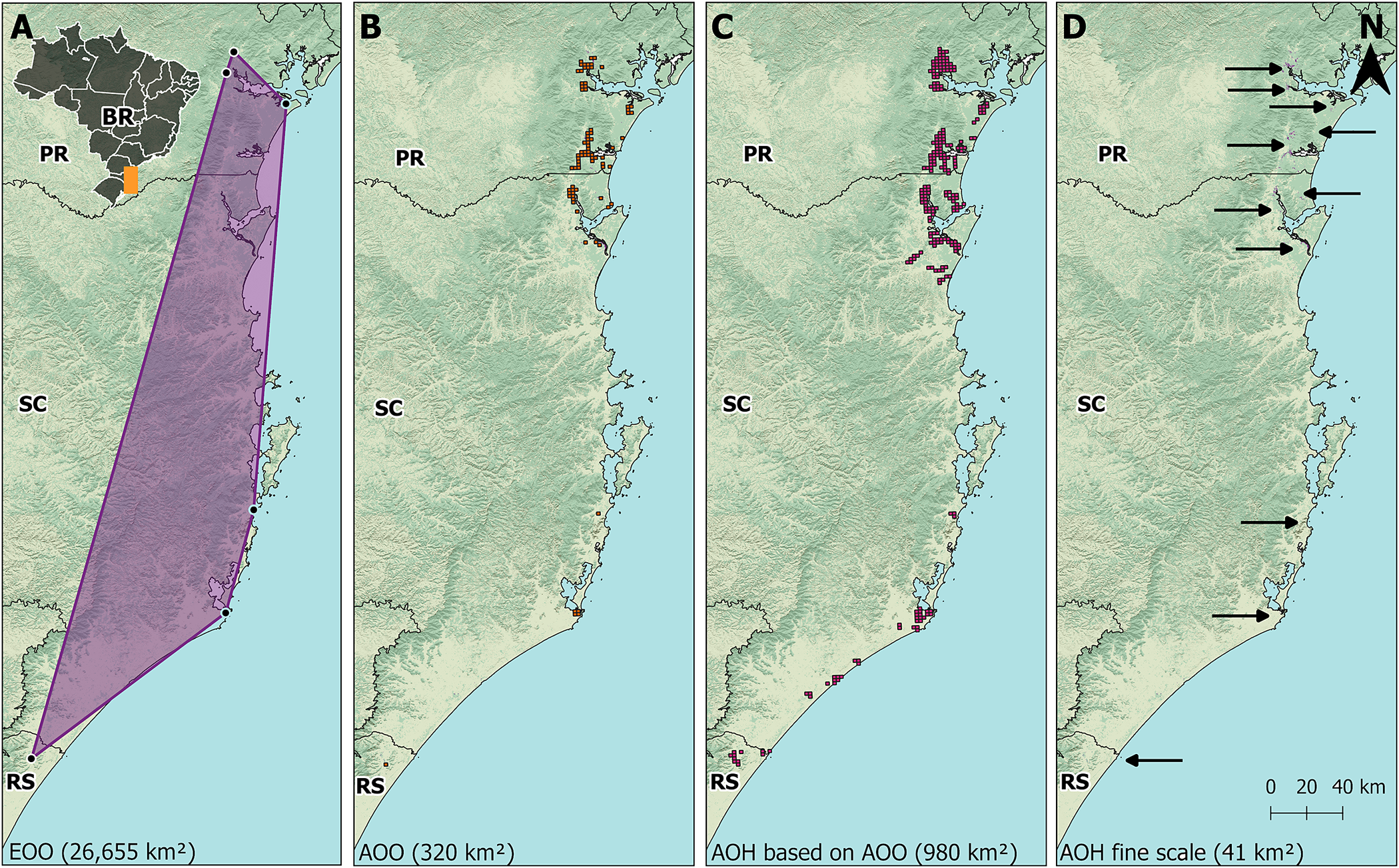
Figure 2. (A) Extent of occurrence (EOO) of Parana Antwren Formicivora acutirostris (purple polygon). (B) Area of occupancy (AOO) of the species calculated with a 4 km² grid according to IUCN (2022). (C) Area of habitat (AOH) of the species calculated with a 4 km² grid as an upper bond for the area of occupancy (AOO). (D) AOH of the species calculated in fine scale. PR = Paraná; RS = Rio Grande do Sul; SC = Santa Catarina. Background images from the National Water and Sanitation Agency (ANA), Brazilian Institute of Geography and Statistics (IBGE), Brazilian Annual Land Use and Land Cover Mapping Project (MapBiomas), and Geomorphometric Database of Brazil (TOPODATA).
Table 3. Estimate of area of habitat (AOH) (ha) and population size of mature territorial individuals of Parana Antwren Formicivora acutirostris by habitat type. PR = Paraná; RS = Rio Grande do Sul; SC = Santa Catarina.
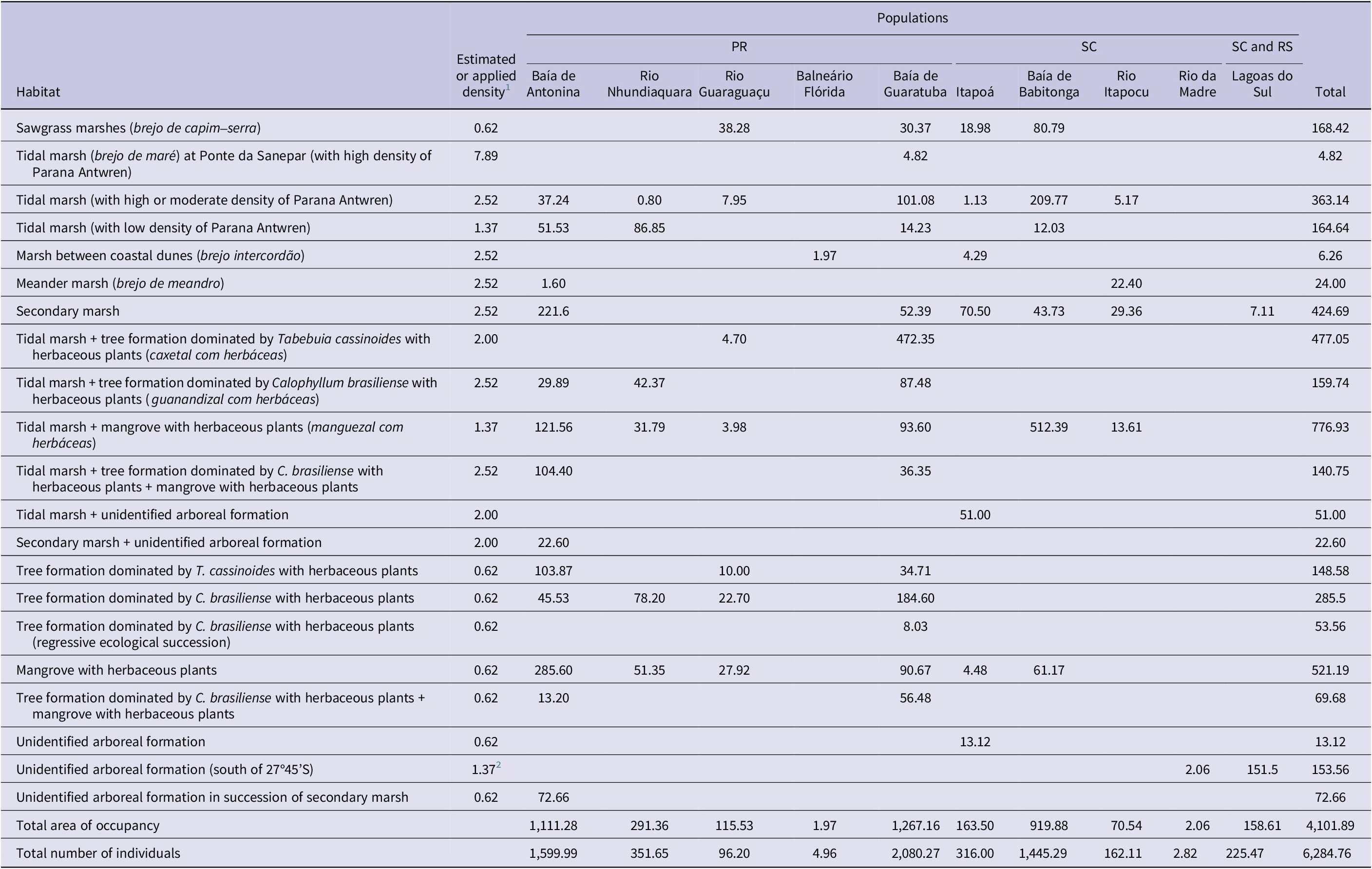
1 According to Table 2.
2 Field data for the Rio da Madre and Lagoas do Sul populations suggest a higher density than 0.62 individuals/ha, which is the population density value for similar habitats in other populations.
Estimation of population size
We estimated the population size of the species to be 6,285 mature territorial individuals (Table 3), 4,133 of which were present in Paraná. We estimated the largest population to have 2,080 mature territorial individuals (Baía de Guaratuba population, in Paraná), and the smallest population to have only two (Rio da Madre population, in Santa Catarina (Table 3).
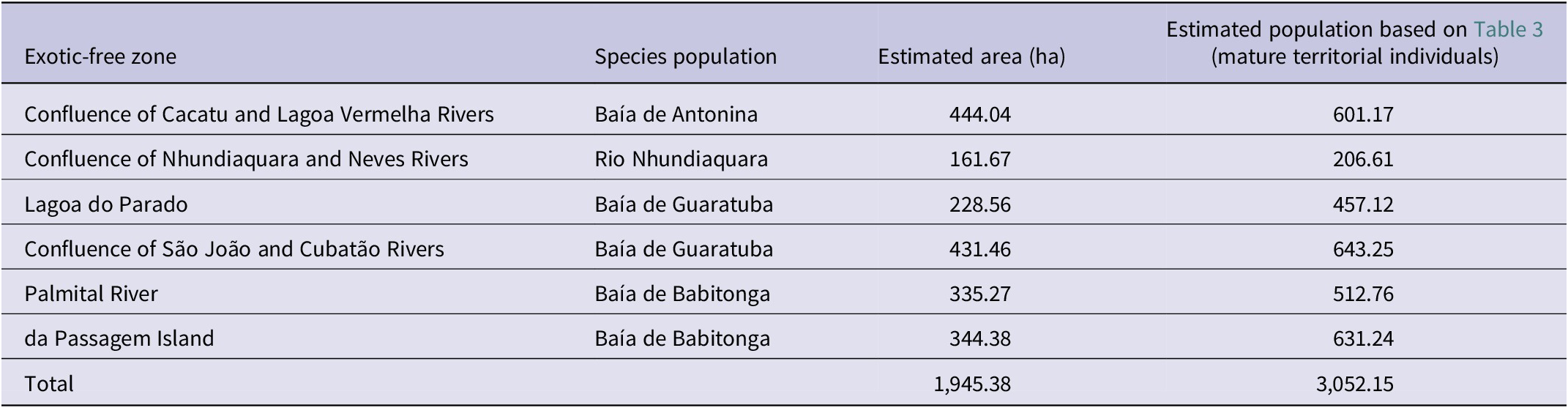
Table 4. The smallest area of occupancy of the Parana Antwren Formicivora acutirostris less impacted by the invasion of the exotic grasses Urochloa arrecta and U. mutica. They are proposed here as strategic units for management and conservation, called exotic-free zones
Species distribution and habitat
Parana Antwren was distributed across 10 populations (Figure 3), including two new populations named Rio da Madre and Lagoas do Sul (Table 3). We recorded the species at new sites, such as the Faisqueira River (25°21’58”S, 48°38’42”W), in Baía de Antonina, and at three other areas in Itapoá (26°07’53”S, 48°39’26”W; 26°10’51”S, 48°36’53”W; 26°09’23”S, 48°37’50”W). The Baía de Babitonga population in Santa Catarina has expanded due to the detection of habitat types used by the species that were overlooked in the previous mapping (Table 1). In the new Lagoas do Sul population, we recorded the species in Pedras Brancas (28°30’54”S, 48°47’29”W), municipality of Laguna, in the state of Santa Catarina, and in Lagoa do Forno (29°22’56”S, 49°53’12”W), municipality of Dom Pedro de Alcântara, in the state of Rio Grande do Sul.

Figure 3. Geographical distribution of Parana Antwren Formicivora acutirostris with approximate polygons (black lines) indicating the location of its 10 populations. BR = Brazil; PR = Paraná; RS = Rio Grande do Sul; SC = Santa Catarina. Background images from the National Water and Sanitation Agency (ANA), Brazilian Institute of Geography and Statistics (IBGE), Brazilian Annual Land Use and Land Cover Mapping Project (MapBiomas), and Geomorphometric Database of Brazil (TOPODATA).
We identified a 146-km distributional gap between the southernmost previously mapped population (Rio Itapocu) and the northernmost of the two new populations (Rio da Madre). In 1998–2012, we conducted extensive field surveys searching for the species in various areas within this distributional gap focusing on herbaceous formations dominated by California Bulrush Schoenoplectus californicus, Swamp Sawgrass Cladium mariscus, Southern Cattail Typha domingensis or the bulrush Scirpus giganteus, but we did not detect any individuals. However, following the first records of Parana Antwren in the Laguna municipality in early 2012, subsequent records by the authors, other researchers, and bird observers have indicated that the species’ habitat in the two southern populations was dominated by Mangrove Fern Acrostichum danaeifolium, together with Brazilian Peppertree Schinus terebinthifolius and Small-leaf Myrsine Myrsine parvifolia. Photographic evidence from WikiAves further confirmed this characteristic, since all images from the Rio da Madre and Lagoas do Sul populations showed the species exclusively in this habitat. However, our observations at Lagoa do Forno were in a flooded formation dominated by T. domingensis or by this plant alongside trees, and the photographs of Parana Antwren in Rio Grande do Sul on the WikiAves platform, likely taken at Lagoa do Forno, showed the species in a habitat characterised by the presence of the herbaceous plants T. domingensis, Schoenoplectus californicus, Umbrella Sedge Fuirena sp., and at least some Melastomataceae shrubs.
The habitat of Parana Antwren is prone to tidal flooding, except for the 10% fraction of its AOH (at fine scale), which consists of secondary marshes located along the borders of other habitats influenced by tides. The species occurred in habitat gradients that range from saline herbaceous vegetation to non-saline vegetation characterised by a lower stratum with marsh herbaceous plants and an upper stratum with trees commonly found in flood-prone areas (see Table S1). The eight first populations from north to south of the species’ geographical distribution occurred across the entire floristic gradient, whereas the two southern populations occurred only at the end of the gradient, although some observations in Rio Grande do Sul deviated from this pattern (see below).
Habitat changes
The habitat of the species changed between 1980 and 2020 due to eight factors (Table 1). Throughout this period, new habitat patches formed, but they were smaller than the minimum size for a territory in a similar habitat (Table S2), so this change is not accounted for in Table 1. Regressive ecological succession also created new habitat through tree mortality and the establishment of herbaceous species in the lower stratum (Table 1), probably due to extremely high tides and increased salinity. We detected an area of 0.22 km² with this phenomenon in the Baía de Guaratuba population (Table 1), always at higher positions within the tidal plain between herbaceous-dominated formations (sawgrass marshes) and forests. However, only 0.08 km² of these habitats formed through regressive succession met the minimum size (Tables 1 and S2).
There was a small habitat loss due to human activities between 1980 and 2020 (0.95 km²) (Table 1), contrary to our initial expectations. However, this assessment did not consider the Rio da Madre and Lagoas do Sul populations (Table 1), that potentially experienced significant historical losses before 1980. The primary habitat loss of Parana Antwren occurred through the natural and expected progressive ecological succession of habitats that had both arboreal and herbaceous strata with typical marsh plants in 1980 and was succeeded by environments without the typical marsh plants in the lower stratum (15.37 km²) (Table 1).
The most significant impact we detected on populations was the habitat loss due to invasion by the exotic grasses Urochloa arrecta and U. mutica, which were not observed before 1980. We observed the presence of one or both species of grasses associated in all populations, except in Rio da Madre. We observed areas where these grasses were dominant in seven populations (Table 1). The loss of habitat due to this factor was not accounted for the Balneário Flórida population as the invaded habitats were filled in 2009 (Table 1).
We detected six regions that had large, concentrated patches of habitat minimally invaded by Urochloa arrecta and U. mutica (Table 4, Figure 4), associated with four populations of Parana Antwren. We suggest designating these areas as “exotic-free zones” due to the limited resources available for exotic plant control projects and their associated high costs. These zones would encompass small geographical areas with a significant amount of non-invaded or minimally invaded environments, thus, having a favourable management cost–benefit ratio. This proposal is based on the premise that the protection of the species’ habitat could be maintained in the areas with the highest cost–benefit ratios, allowing the remaining areas to be considered irreversibly or functionally lost. The six proposed exotic-free zones account for a total of 19.45 km²; 47.4% of the total species’ AOH at fine scale (Table 4).

Figure 4. The total habitat (purple and orange) and the smallest habitat containing a significant range of area of habitat (AOH) that were less impacted by the invasion of the exotic grasses Urochloa arrecta and U. mutica (purple). We propose that these areas be considered “exotic-free zones” – a concept for landscape management that offers advantageous costs versus benefits. (A) I. Exotic-free zone confluence of the Cacatu and Lagoa Vermelha Rivers (Baía de Antonina population). (B) II. Exotic-free zone confluence of the Nhundiaquara and Neves Rivers (Rio Nhundiaquara population). (C) III. Exotic-free zone Lagoa do Parado (Baía de Guaratuba population); IV. Exotic-free zone confluence of the São João and Cubatão Rivers (Baía de Guaratuba population). (D) V. Palmital River (Baía de Babitonga population); VI. da Passagem Island (Baía de Babitonga population). PR = Paraná; SC = Santa Catarina. Background images from the National Water and Sanitation Agency (ANA), Brazilian Institute of Geography and Statistics (IBGE), Brazilian National Spatial Data Infrastructure (INDE), Brazilian Annual Land Use and Land Cover Mapping Project (MapBiomas), and Geomorphometric Database of Brazil (TOPODATA).
Conservation status
The estimation of how the losses and gains of habitat (Table 1) affected the losses and gains of individuals indicated an annual loss of 45 individuals (Table 5), adding up to a loss of 645 individuals over three generations (10.31% of the total population), using a generation length of 4.8 years (BirdLife International 2019). Since we estimated the population containing fewer than 10,000 mature individuals (Table 3), and considering the estimated loss of 10% over three generations, the species should be classified as “Vulnerable” (VU C1).
Table 5. Estimated gains or losses of individuals of Parana Antwren Formicivora acutirostris due to changes in the area of habitat (AOH) of the populations where both values could be calculated. The following values are presented: average annual change in area of occupancy; total changed area, intervals of years of this evaluation (in parentheses); estimated number of mature territorial individuals gained or lost annually (in brackets). To calculate the impact on the individuals, we multiplied the average annual change in area by the average population density in the respective habitats, as shown in Table 4. When the changes in area of occupancy involved ecological succession from one habitat to another, we multiplied the average annual change in area by the obtained value for the decreased population density of Parana Antwren in each of these habitats (according to Table 3)

1 Regressive ecological succession.
2 Phenomenon treated as tropicalisation (e.g. Bianchi and Morri Reference Bianchi and Morri2003; Encarnação et al. Reference Encarnação, Morais, Baptista, Cruz and Teodósio2019; Zarzyczny et al. Reference Zarzyczny, Rius, Williams and Fenberg2024).
3 Progressive ecological succession.
4 Impact considered across all types of habitats.
Green Status
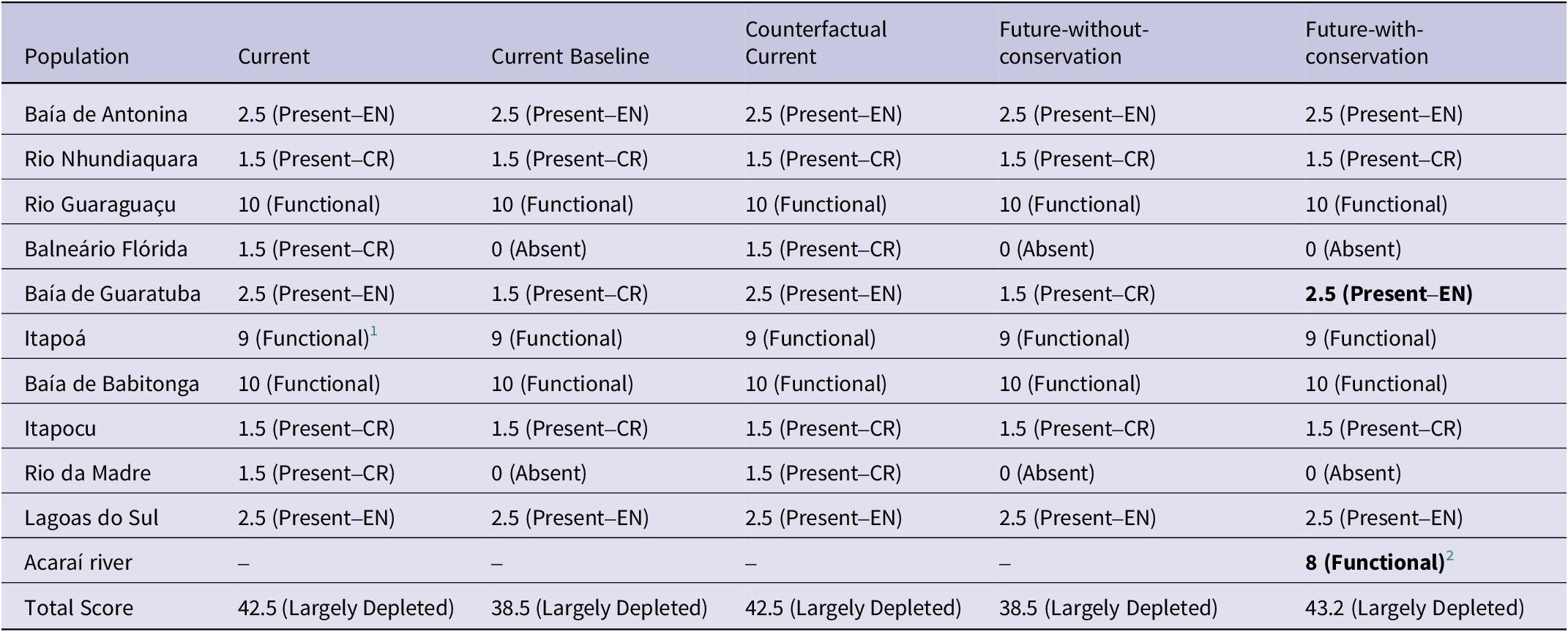
The current scenario showed that the Parana Antwren is Largely Depleted, with a Species Recovery Score of 42.5%. In this scenario, only three of the 10 populations were not facing a specific threat and could be deemed Functional despite their naturally small population sizes. Of the remaining populations, three larger populations were assessed as “Endangered” (EN), and four as “Critically Endangered” (CR) (Table 6). The Counterfactual Current scenario was similar to the Current scenario for all spatial units (Table 6), rendering a Zero (0%) Conservation Legacy of the actions implemented for the species. Among the considered actions were the creation of integral protection conservation units, an egg translocation between populations project, and programmes to eradicate the exotic grasses in the Baía de Guaratuba population (2012–2021) (Figure 5).
Table 6. Evaluation of the Green Status of Parana Antwren Formicivora acutirostris across its 10 populations and an eleventh (Acaraí River), if the suggested assisted colonisation occurs at this site, according to IUCN Species Conservation Success Task Force (2020) and IUCN (2021). The fine-resolution weights and categories for the spatial units and the resulting Green Score for the scenarios are presented. Bold values indicate the values that have changed compared with the Current Baseline scenario. CR = Critically Endangered; EN = Endangered
1 Indigenous range (historical and current distribution) of the population is larger than the current area of occupancy.
2 Expected additional range after the establishment of population is greater than the area of occupancy within 10 years.

Figure 5. Areas that were once habitats (AOH) for Parana Antwren Formicivora acutirostris under management for the eradication of the exotic grass Urochloa arrecta. (A) Areas invaded by exotic grasses prior to management. (B) The central region of the same area of (A) with management, with part of the biomass piled and stacked to prevent it from being carried by high tides. (C) and (D) Management by clear cutting of vegetation with brush cutters prior to piling and stacking. Photographed by Larissa Teixeira (A and B) and Gabriel Marchi (C and D).
In the Current Baseline and Future-without-conservation scenarios the species was assessed as Largely Depleted, with a Green Score of 38.5%. Therefore, the Conservation Dependency of the ongoing actions is Zero (0%). Despite an ongoing exotic grass eradication programme implemented in the Baía de Antonin population, its limited scale (6 ha) means it is unable to improve the Green Score for the Current Baseline. In both scenarios, the Balneário Flórida and Rio da Madre populations became extinct (Table 6). Additionally, the Baía de Guaratuba population could be classified as “Critically Endangered” due to a decline in habitat, estimated at 3.5 km² in 10 years (Table 5).
In the Future-with-conservation scenario the species was assessed as Largely Depleted, with a Green Score of 43.2%. Consequently, the Conservation Gain from planned conservation actions is low (4.7%), but high considering substantial recovery (12.2% of the Green Score of the Current Baseline scenario) with the potential to increase in a few years. In this scenario, the Balneário Flórida and Rio da Madre populations continue to face extinction without planned conservation action to maintain individuals in such small and degraded areas (see Table 3). The eradication of exotic grasses in the Baía de Guaratuba population at a rate of 8.5 ha/year could alter its status from Critically Endangered. Additionally, assisted colonisation would add a Functional spatial unit to 40% of its area (Table 6), but with the potential for full functionality following recruitment of juveniles in successive years (Figure S1).
Discussion
Species distribution, population, and habitat
Updating the geographical distribution and population size of Parana Antwren was extremely timely (Reinert and Bornschein Reference Reinert, Bornschein, Machado, Drummond and Paglia2008; Reinert et al. Reference Reinert, Bornschein, Sobotka, Vidolin, Tossulino and Britto2009) because the species occurs in coastal areas of Brazil that experience intense urban expansion (e.g. cities of Paranaguá, Guaratuba, and Joinville). In addition, most of the Reinert et al. (Reference Reinert, Bornschein and Firkowski2007) mapping was based on aerial photographs taken over 40 years ago. Reinert et al. (Reference Reinert, Bornschein and Firkowski2007) raised the possibility that the species could be recorded further south of the previously known southern limit, but its occurrence as far as 329 km south was unexpected (M. R. Bornschein, unpublished data). However, the species possibly occurs even further south than the currently mapped southern limit (29°23’04”S, 49°53’10”W, municipality of Dom Pedro de Alcântara in Rio Grande do Sul), for example, in the margins of lagoons with herbaceous and arboreal vegetation in the municipality of Osório in Rio Grande do Sul (29°51’43”S, 50°13’19”W).
The new AOH of the species is 32.3% smaller than the previous estimate, despite the discovery of two new populations, and the new estimate of population size is 64.5% smaller than the previous estimate (Reinert et al. Reference Reinert, Bornschein and Firkowski2007). This reduction is attributed to two main methodological factors that led to the overestimation of the population in the previous study. In the present study, population estimates were calculated using population density in study areas, which incorporated “leftover” areas between territories that might be too small or of insufficient low quality to support a species pair. In contrast, Reinert et al. (Reference Reinert, Bornschein and Firkowski2007) considered every AOH to be occupied by the species. In addition, the revised density in other habitats (Table 2) revealed that the population density of 7.89 individuals/ha (Reinert et al. Reference Reinert, Bornschein and Firkowski2007) was high and restricted to a small area in the Baía de Guaratuba population.
We propose categorising Parana Antwren as endemic to salt marshes, where it occurs in diverse compositions and floristic structures across altitude and salinity gradients in areas influenced by tides. It is also found in a few areas with sporadic tidal influence or even without such influence, possibly indicating secondary colonisation in these cases. Most populations of the species exhibit all floristic variation known across its occurrence, except for the two southernmost populations, where the bird is found in habitats with abundant trees. Notably, an exception was found in Lagoa do Forno, where it was also recorded in herbaceous-dominated habitats. The numerous anthropogenic impacts on this area, including landfills for roads, drainage canals, and trees harvesting (M. R. Bornschein, unpublished data), indicate that the habitat has become degraded, possibly facing regressive succession (Sandretti-Silva et al. Reference Sandretti-Silva, Teixeira, Golec and Bornschein2023). Therefore, the presence of Parana Antwren may indicate local adaptation or colonisation of nearby regions.
Habitat losses
The primary cause of habitat reduction in our study was ecological succession. All the habitats of Parana Antwren are pioneer formations that initiate vegetation colonisation on newly formed sediment banks and prepare these areas for ecological succession (IBGE 2012; Lin et al. Reference Lin, Huang, Hwang, Hsu, Chen and Hsieh2016; Veloso et al. Reference Veloso, Rangel-Filho and Lima1991). The regional climax community that encompasses the habitats of Parana Antwren is the rainforest, which implies that the current habitats may no longer be suitable for the bird when transitioning to an arboreal community without a lower stratum characteristic of typical marsh species in ecological succession. Continuous sediment accumulation and consequent gains in altitude reduce flooding levels in estuaries, enabling the establishment of plant species from more advanced ecological succession stages. Processes like sediment erosion or compaction may lead to cyclical phases of regressive ecological succession involving the mortality of different plant species, followed by the resumption of progressive ecological succession (M. R. Bornschein, unpublished data; see also Sandretti-Silva et al. Reference Sandretti-Silva, Teixeira, Golec and Bornschein2023).
The speed of ecological succession in Parana Antwren habitats is not well known, but Reinert et al. (Reference Reinert, Bornschein and Firkowski2007) reported cases of emerging herbaceous formations in two years (secondary marshes), four years (marshes between coastal dunes), and 10 years (tidal marshes); cases of emerging arboreal formations in five years (mangrove); cases of ecological succession from marshes between coastal dunes with tree formations dominated by Tabebuia cassinoides and herbaceous plants within a maximum of 27 years. In our study, we reported the succession from herbaceous formations to arboreal formations, with or without the presence of a lower stratum containing typical herbaceous marsh plants, within a maximum of 37–40 years, and from formations with an upper arboreal stratum and a lower stratum containing typical herbaceous marsh plants to formations without marsh species in the lower stratum within 32–40 years (Table 1). Additionally, we report cases of regressive ecological succession from tree formations without typical marsh herbaceous plants in the lower stratum (tree formation dominated by Calophyllum brasiliense) to the same tree formations with marsh herbaceous plants in the lower stratum (tree formation dominated by C. brasiliense with herbaceous plants) within a maximum of 37 years (Table 1). This phenomenon may be linked to increased tidal height, resulting in raised height and duration of flooding such that the increased salinity of the environment killed trees and increased light penetration into the lower stratum. It has occurred in other parts of the world, with formations adjacent to mangroves and other estuarine formations experiencing vegetation mortality and regressive succession (Butzeck et al. Reference Butzeck, Schroder, Nolte and Jensen2016).
The conversion of tidal marshes to mangroves with herbaceous plants is not an expected path for ecological succession (Table 1) (IBGE 2012; Reinert et al. Reference Reinert, Bornschein and Firkowski2007; Veloso et al. Reference Veloso, Rangel-Filho and Lima1991). Mangroves are floodplain formations found in tropical regions (Doody Reference Doody2001; D’Odorico et al. Reference D’Odorico, He, Collins, De Wekker, Engel and Fuentes2013), while salt marshes or subtropical salt marshes (Bornschein et al. Reference Bornschein, Reinert, Machado-de-Souza, Golec, Whitney and Favretto2017) in the case of study areas, are floodplain formations in colder regions (D’Odorico et al. Reference D’Odorico, He, Collins, De Wekker, Engel and Fuentes2013). The replacement of a community that is characteristic of colder regions (subtropical salt marsh) by another that is characteristic of warmer regions (mangrove) is an example of tropicalisation driven by climate change (e.g. Bianchi and Morri Reference Bianchi and Morri2003; Encarnação et al. Reference Encarnação, Morais, Baptista, Cruz and Teodósio2019; Zarzyczny et al. Reference Zarzyczny, Rius, Williams and Fenberg2024).
The current data support Reinert et al. (Reference Reinert, Bornschein and Firkowski2007), who stated that the greatest threat to the conservation of the Parana Antwren is invasion by exotic grasses, which must be actively controlled and managed (Reinert and Bornschein Reference Reinert, Bornschein, Machado, Drummond and Paglia2008; Reinert et al. Reference Reinert, Bornschein and Firkowski2007, Reference Reinert, Bornschein, Sobotka, Vidolin, Tossulino and Britto2009). These grasses are widespread invasive species that have a great impact on the herbaceous environments along the southern coast of Brazil, with no barriers to their dispersion except for salinity (see above and Reinert et al. Reference Reinert, Bornschein and Firkowski2007).
Conservation
The assessment of the Green Status of the species reveals that the Conservation Legacy of conservation actions taken thus far and the Conservation Dependence of the ongoing actions are null, due to the small scales of the projects. While these actions have contributed to the local maintenance and recovery of individuals, their impact has not been sufficient to improve the conservation status of the population based on the IUCN Red List criteria, which is used to assess the Green Status. However, the Conservation Gain shows the importance of eradicating exotic grasses.
The eradication of 1 ha of exotic grasses in estuaries where no seed bank is formed can be done swiftly, but is costly (Bornschein et al. Reference Bornschein, Teixeira, Guerra, de, Melchiori, Reinert and Sandretti-Silva2022). Invaded tidal marshes take 10 months to recover, from the start of management to the return of native vegetation (Bornschein et al. Reference Bornschein, Teixeira, Guerra, de, Melchiori, Reinert and Sandretti-Silva2022), although with a reduced number of species. The cost of managing 1 ha is US$13,404–29,356, depending mainly on the distance of the management areas from the workers’ homes (Bornschein et al. Reference Bornschein, Teixeira, Guerra, de, Melchiori, Reinert and Sandretti-Silva2022). This means that the cost of eradicating patchy AOH of Parana Antwren that have been invaded and dominated by exotic grasses is US$3,455,015.04–7,566,802.56, without considering the extent of the plant’s spread since the assessment years (2005–2022; Table 1), or its likely spread during the years of eradication activity.
The establishment of exotic-free zones could be vital for territorial management, particularly within conservation units, which might include the Roteiro Metodológico de Planejamento de Unidades de Conservação in Brazil (Methodological Guideline for the Planning of Conservation Units; D’Amico et al. Reference D’Amico, Mitozo, Abreu, Fritzen, Lorensi and Rivas2018; Galante et al. Reference Galante, Beserra and Menezes2002). The six proposed exotic-free zones represent 47.4% of the AOH of Parana Antwren and 48.6% of its population (Figure 4, Table 4). We consider them strategic areas for the conservation of the species where the management of Urochloa arrecta and U. mutica should be carried out (see details in Bornschein et al. Reference Bornschein, Teixeira, Guerra, de, Melchiori, Reinert and Sandretti-Silva2022). We emphasise the importance of matching the available resources with the size of managed patches to ensure the complete eradication of exotic species without depleting resources (Moody and Mack Reference Moody and Mack1988), and avoid plant regrowth and wasting resources.
A cheaper alternative to achieve the same environment management objectives would involve the ongoing selective cutting of trees, including those typical of forests and mangroves. This management approach would maintain vegetation communities at the same successional stage or even induce regressive succession to a more herbaceous stage, which could support a higher population of Parana Antwren (Table 2). However, this practice might lead to controversy in a country less accustomed to environmental management practices in the species’ natural habitats, potentially hindering the acquisition of approvals from competent authorities.
The primary conservation action leading to the increased Green Score in the Future-with-conservation scenario was the establishment of a new Functional population. Therefore, we propose the assisted colonisation of the species into coastal environments within the species’ EOO to enhance conservation, such as the Acaraí River (c. 26°14’53”S, 48°31’59”W), located at the Acaraí State Park, on São Francisco Island, municipality of São Francisco do Sul, on the northern coast of Santa Catarina, where there are 2.15 km² of similar habitats to those of the species’ occurrence with high conservation status (Figure S1). This place illustrates a potential area where the species may not have reached due to its low flight capacity, low habitat connectivity or insufficient time for colonisation (Reinert et al. Reference Reinert, Bornschein and Firkowski2007).
Conclusions
Parana Antwren is a threatened species due to its small population and the continuous loss of individuals primarily driven by human activities and ecological succession. The species occupies pioneer formations that quickly transition into unsuitable habitats (arboreal formations and forests). Recognising the dynamic and temporary nature of the species’ habitat is important for formulating plans for species’ conservation. Landscape transformation may limit the formation of new habitats and the ability of species to colonise them.
The invasion of exotic grasses is the most significant anthropogenic threat. It is important to continue eradicating these invasive exotic species and expanding managed areas. The costs are high, so our proposal for the establishment of exotic-free zones indicates strategic locations where meagre resources could be allocated to achieve the highest environmental returns. Assisted colonisation could mitigate the loss of habitats due to ecological succession. There are suitable areas where the species is currently absent that could support introduced individuals. The largest limiting factor, as with the management of exotic species, is fundraising.
Possibly due to more intense and frequent flooding, regressive succession is already evident. This phenomenon, which increases the species habitat, may be due to climate change, and may become more frequent and widespread over time, at least along the southern coast of Brazil. However, it is probably unlikely to compensate for more than a minimal fraction of habitat loss caused by progressive ecological succession.
The advance of mangroves across tidal marshes should not be considered progressive ecological succession, but rather an event related to climate change, known as tropicalisation (Zarzyczny et al. Reference Zarzyczny, Rius, Williams and Fenberg2024). In this study, we reported this phenomenon in Brazil for the first time.
Continuous monitoring of Parana Antwren and its habitats is strategically necessary to adjust management proposals and assess their effects. Gaining knowledge of this species can facilitate conservation efforts aimed at other birds facing similar challenges, both through replicating adopted management actions and through expeditiously obtaining environmental permits from competent authorities that have previously reviewed similar requests.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S095927092400008X.
Acknowledgements
Most of the projects that yielded results for this study were developed through Mater Natura – Instituto de Estudos Ambientais, with financial management support from Helena Zarantonieli. Alexandre Bianco and João A. B. Vitto provided valuable information regarding the localisation of Parana Antwren records in the municipality of Laguna. Claudia Golec, Ricardo Belmonte-Lopes, Felipe Shibuya, Larissa Teixeira, Maria Fernanda Ferreira Rivas, and Cecilia de Camargo Rocha assisted with many fieldwork activities. Carla S. Fontana and Márcio Repenning assisted with fieldwork in Rio Grande do Sul. Larissa Teixeira and Gabriel Marchi provided important photographs. Sérgio A. A. Morato, Mauro Pichorim, and Fernando de Camargo Passos reviewed a previous version of this work and made suggestions that improved its quality. The Associate Editor Judit Szabo and two anonymous reviewers contributed to the improvement of the study through their comments. This study was partially supported by Fundação Grupo Boticário de Proteção à Natureza (FGBPN; project numbers 0682/20052; 0740/20071, 0908_20112, BL0001_20111, 0004_2012, 1110_20172), Fundo Brasileiro para a Biodiversidade (FUNBIO), and 1º Vara Federal de Paranaguá (project number 50005063420184047008). Giovanna Sandretti-Silva received grants from Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, process numbers 2022/04847-7 and 2023/09718-3).














