Finland has the world's highest, and still increasing, incidence of type 1 diabetes, with the increase being particularly conspicuous among children aged below 5 years( Reference Harjutsalo, Sjoberg and Tuomilehto 1 ). Type 1 diabetes develops as the result of an immune-mediated inflammatory reaction that destroys β-cells in the pancreatic islets( Reference Vaarala, Atkinson and Neu 2 ). Available data suggest that type 1 diabetes is caused by a complex interplay between intestinal microbiota, gut permeability and mucosal immunity( Reference Vaarala, Atkinson and Neu 2 ). Prenatal modulation of the gut is dependent on which factors the fetus encounters via the placenta( Reference Blumer, Pfefferle and Renz 3 ). Accordingly, maternal nutrition during pregnancy may modulate the development of the gut immune system in the offspring. Maternal fatty acid status during pregnancy influences the fatty acid status of the fetus and newborn infant( Reference Prescott and Dunstan 4 ). The fatty acids may influence immune programming in many ways, e.g. by altering the lipid composition of fetal cell membranes, cellular metabolism, eicosanoid synthesis and gene expression regulation( Reference Prescott and Dunstan 4 ).
Epidemiological studies have suggested that the consumption of n-3 fatty acid-rich cod-liver oil during pregnancy or infancy might help in the prevention of the development of type 1 diabetes( Reference Stene and Joner 5 , Reference Stene, Ulriksen and Magnus 6 ). In contrast, in a US cohort, maternal n-3 and n-6 fatty acid intake has been shown to be not associated with the appearance of islet autoimmunity in the offspring( Reference Fronczak, Baron and Chase 7 ), and in a Norwegian cohort study, maternal serum concentrations of n-3 fatty acids during pregnancy have been reported to be not associated with the risk of clinical type 1 diabetes in the offspring( Reference Sorensen, Joner and Jenum 8 ). By contrast, in a US cohort, the intake of n-3 fatty acids and their content in erythrocyte membranes in children have been shown to be inversely associated with β-cell autoimmunity( Reference Norris, Yin and Lamb 9 ). Animal studies have also indicated a protective effect of n-3 fatty acids( Reference Kagohashi, Abiru and Kobayashi 10 , Reference Wei, Li and Shen 11 ).
There are only a few studies that have considered the effect of various fatty acids on the development of type 1 diabetes. In our previous nested case–control analysis in children, serum fatty acid biomarkers of milk consumption have been shown to be positively associated, and linoleic acid inversely associated, with the risk of advanced β-cell autoimmunity at or close to the time of seroconversion( Reference Virtanen, Niinisto and Nevalainen 12 ). In our recent study in children, we have found that the intake of fat from all milk products is associated with a higher risk of preclinical type 1 diabetes( Reference Virtanen, Nevalainen and Kronberg-Kippila 13 ). Lipid-induced impairment of β-cell function (lipotoxicity) has been well established both in vitro and in vivo in animal models in the pathogenesis of type 2 diabetes( Reference Giacca, Xiao and Oprescu 14 ). This raises the question of whether lipotoxicity is involved in the pathogenesis of type 1 diabetes as well. By contrast, MUFA have been shown to protect β-cells in some in vitro studies( Reference Morgan and Dhayal 15 ).
In the present study, we evaluated the intake of fatty acids and foods important in relation to fatty acid intake among pregnant Finnish women and assessed associations with the development of preclinical and clinical type 1 diabetes in their offspring.
Methods
Participants
The present investigation is part of the Finnish Type 1 Diabetes Prediction and Prevention (DIPP) Study, which is a large prospective, population-based birth cohort study of Finnish children at an increased genetic risk of type 1 diabetes. Increased human leucocyte antigen (HLA)-conferred disease susceptibility is defined by a high-risk genotype (HLA-DQB1*02/*0302) or a moderate-risk genotype (HLA-DQB1*0302/x, where x= other than *02, *0301 or *0602). Details of the genetic screening methods and enrolment criteria have been published previously( Reference Kupila, Muona and Simell 16 ). The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the Ethical Committees of the University Hospitals of Oulu and Tampere, Finland. Written informed consent was obtained from all the subjects.
The study population comprised 5605 children with HLA-conferred susceptibility to type 1 diabetes. They were born in Tampere or Oulu University Hospital between October 1997 and beginning of September 2004 (72 % of those invited). FFQ were completed by the mothers of 4887 children during pregnancy (87 %).
Assessment of maternal nutrition
The participants completed a validated 181-item semi-quantitative FFQ concerning their habitual diet during 1 month. The mothers were asked about their food consumption during the eighth month of pregnancy (the month preceding maternity leave in Finland). If more than ten of the 181 food frequency questions were not answered, the FFQ was excluded (n 53). The FFQ contained questions about the frequency (number of times per d, week or month) and amount of foods consumed, in units of common serving sizes. In addition, the FFQ contained questions related to what type of fat was used in cooking and baking and in salad dressings as well as questions related to the extent of home baking. The individual type and quantity of fat were taken into account when calculating food and nutrient intakes. FFQ content, validity and data processing have been described earlier( Reference Erkkola, Karppinen and Javanainen 17 ). Information on the intake of fatty acids from all kinds of supplements throughout pregnancy was requested. Sociodemographic and other factors, including maternal education and diabetes in close relatives, were recorded using a structured questionnaire completed by the parents after delivery.
Autoantibody assays and definition of outcome
The DIPP children were monitored for the appearance of signs of β-cell autoimmunity by analysing primarily islet cell antibodies (ICA). If a child tested positive for ICA, all his or her samples obtained from birth were analysed for insulin autoantibodies, autoantibodies to 65 kDa isoform of glutamic acid decarboxylase and antibodies to tyrosine phosphatase-related islet antigen 2. Autoantibody samples were obtained during each of the study centre visits, which were scheduled to take place at the age of 3, 6, 12, 18 and 24 months and subsequently at an interval of 12 months. If the child became positive for ICA, the interval between visits was shortened to 3 months. The autoantibodies were analysed in the Research Laboratory, Department of Pediatrics, University of Oulu. ICA, insulin autoantibodies, autoantibodies to 65 kDa isoform of glutamic acid decarboxylase and autoantibodies to tyrosine phosphatase-related islet antigen 2 levels were quantified as described previously( Reference Kukko, Kimpimaki and Korhonen 18 ).
We defined preclinical type 1 diabetes as being repeatedly positive for ICA and one or more of the three other autoantibodies analysed. Altogether, 283 of the 5605 (5·0 %) children seroconverted to repeated ICA positivity and positivity to one of the three other autoantibodies during this period at a mean age of 3·6 (range 0·5–10·0) years. Of the children, 145 (2·6 %) had progressed to clinical type 1 diabetes at a mean age of 4·6 (range 0·5–11·5) years. Among the children who progressed to clinical type 1 diabetes, 103 had been repeatedly positive for ICA and at least one other autoantibody. However, fifteen of the remaining forty-two children who progressed to type 1 diabetes had or had had one or more autoantibodies before or at the time of diagnosis. Moreover, ten children who developed type 1 diabetes had been persistently seronegative, with their last blood sample being drawn at a mean age of 4·0 (range 0·3–7·7) years before diagnosis. A total of seventeen children were not subjected to any autoantibody analysis before the diagnosis of diabetes. Thus, preclinical type 1 diabetes endpoint was defined as the first occurrence of either (1) repeated positivity for ICA in combination with positivity for one or more of the autoantibodies analysed or (2) clinical type 1 diabetes. Due to missing maternal FFQ data, statistical analyses were carried out with 240 preclinical and 112 clinical type 1 diabetes endpoints.
Statistical analyses
The endpoint of preclinical type 1 diabetes is interval censored and possibly dependent on siblings. To accommodate this structure, a piecewise linear log-hazard survival model was used to analyse the associations of fatty acid intake and food consumption with the risk of preclinical type 1 diabetes, assuming linear log-hazards in the intervals 0–1·99, 2–3·99 and >4 years. The results were not sensitive to the particular choice of intervals used. Observation intervals beyond positivity did not contribute to the analyses. The models were fitted using maximum likelihood in SAS PROC NLMIXED, with standard errors of the estimates derived from the observed information matrix. Random effects for family were introduced to accommodate familial dependence, and these were assumed to follow a normal distribution. The proportionality of the hazards was tested by adding linear interaction terms of the exposure variables with time to the models. To illustrate such time-varying effects, the endpoint analyses were carried out in two settings. First, all the data were used in the analyses. Second, data that included endpoint information only up to 3 years of age were used. There was no interval censoring in the diagnosis of clinical type 1 diabetes. Therefore, the associations of fatty acid intake and food consumption with the risk of clinical disease were analysed by Cox proportional-hazards regression.
Fatty acid variables were adjusted for energy intake by the residual method( Reference Willett 19 ) after logarithmic transformation. Food variables were adjusted for energy intake by adding energy intake as a covariate to the survival model. Fatty acid and food variables were used as both continuous and categorical (the latter defined by falling into intervals given by the first and the third sample quartile) explanatory variables in the analyses. Some food variables were dichotomised because of a high proportion of non-users. If there is no indication of a non-linear association between dietary variables and endpoint, only results for continuous covariates are presented. Otherwise, results for categorical variables are presented. In addition to energy-adjusted fatty acid variables, analyses were carried out for the relative proportions of fatty acids (the percentages of total fatty acid intake). We also separately analysed the intakes of fat and protein from milk products.
The possible confounding by background characteristics (hospital of birth, familial diabetes and maternal vocational education) was controlled by adding background variables as covariates to the survival model; SAS version 9.2 (SAS Institute) was used in the analyses. Statistical significance was taken as less than 5 %.
Results
The characteristics of the study children are given in Table 1. The maternal average daily intake of fatty acids from foods and mean consumption of foods during pregnancy are given in Tables 2 and 3, respectively. The number of users of supplements containing fatty acids was small (n 81, 1·7 % of all women). Thus, intakes of fatty acids only from food sources are presented and used in the aetiological analysis. The maternal mean daily intake of total fatty acids was 32·8 % of energy intake (E%), SFA 14·4 E%, MUFA 11·7 E%, PUFA 4·7 E%, n-3 PUFA 1·0 E% and n-6 PUFA 3·5 E%. Fresh and sour milk, cheese, butter and butter-oil spreads were the most important sources of SFA, the most important of which were myristic, pentadecanoic and palmitic acids (Table 2). Red meat and meat products were the main sources of palmitoleic acid (both n-9 and n-7), stearic acid, oleic acid, cis-vaccenic acid and arachidonic acid. Linoleic acid and α-linolenic acid were derived mainly from oils and EPA and DHA from fish.
Table 1 Characteristics of the study participants (Number of participants and percentages)
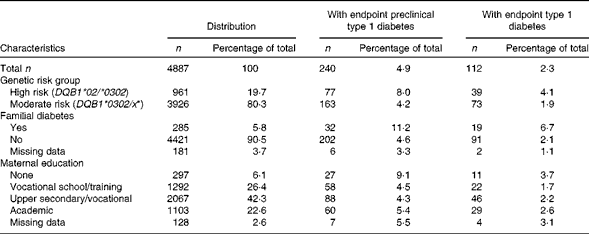
*x not equal to *02, *0301 and *0602.
Table 2 Maternal mean daily intake of fatty acids from food sources among pregnant Finnish women (n 4887) during the years 1997–2004

* Eggs, snacks, sweets, chocolates, seeds and nuts, industrial and animal fats, bread and cereals, and other less important food groups in relation to fatty acid intake.
Table 3 Associations of maternal food consumption during pregnancy with the risk of preclinical and clinical type 1 diabetes in the offspring* (Mean values and standard deviations; hazard ratios (HR) and 95 % confidence intervals)

* For continuous food variables, HR describes the change in risk, when the food variable is changed by an amount corresponding to its standard deviation. For categorical food variables, reference category is the intermediate half, and for dichotomised variables, reference category is the non-users.
† Model was adjusted for maternal energy intake.
‡ Likelihood ratio test was used to test whether the model with and without the food variables differed.
§ Model was adjusted for maternal energy intake, genetic risk, hospital of birth, familial diabetes and maternal vocational education.
∥ Salmon, rainbow trout, Baltic herring, mackerel, lavaret, trout, European cisco, pike-perch, flounder, bream, rosefish, herring, anchovy and tuna.
¶ Fish fingers, frozen fish, perch, northern pike, burbot, shrimps and shell.
The maternal intake of saturated palmitic acid (HR 0·82, 95 % CI 0·67, 0·99; P= 0·039) was weakly associated with a decreased risk of clinical type 1 diabetes (Table 4). For the endpoint clinical type 1 diabetes, interactions between fatty acids and time were significant for the following fatty acids: palmitoleic acid isomers 16 : 1n-7 (P= 0·019) and 16 : 1n-9 (P= 0·014); EPA (P= 0·037); dihomo-γ-linolenic acid (P= 0·013). To illustrate these apparently time-varying effects, analyses were carried out separately for children aged below 3 years. When the follow-up time was less than 3 years, monounsaturated palmitoleic acid (16 : 1n-7) (HR 0·54, 95 % CI 0·36, 0·86; P= 0·014) was associated with a decreased risk of clinical type 1 diabetes, but not during the overall follow-up period.
Table 4 Associations of maternal fatty acid intake during pregnancy with the risk of preclinical and clinical type 1 diabetes in the offspring* (Hazard ratios (HR) and 95 % confidence intervals)

* Fatty acid variables used in statistical analyses were energy adjusted using Willett's residual method. HR describes change in risk, when fatty acid variable is changed by an amount corresponding to its standard deviation. These results are not adjusted for any potential confounders.
† Likelihood ratio test was used to test whether the model with and without the fatty acid variables differed.
‡ Model was adjusted for genetic risk, hospital of birth, familial diabetes and maternal vocational education.
High consumption of cheese was associated with a decreased risk of clinical type 1 diabetes (highest quarter v. intermediate half HR 0·52 (95 % CI 0·31, 0·87)) (Table 3). The consumption of sour milk was associated with an increased risk of preclinical type 1 diabetes (HR 1·14, 95 % CI 1·02, 1·28), and the consumption of low-fat margarines was associated with a decreased risk (HR 0·67, 95 % CI 0·49, 0·92). No significant interactions with time were detected for any of the foods, and results are thus presented for only the whole period. The adjustment for the putative confounding variables (genetic risk, hospital of birth, familial diabetes and maternal vocational education) modified the results slightly (Tables 3, 4 and 5).
Table 5 Associations of maternal intake of fat and protein from dairy products during pregnancy with the risk of preclinical and clinical type 1 diabetes in the offspring* (Hazard ratios (HR) and 95 % confidence intervals)

* For continuous fat and protein variables, HR describes change in risk, when the food variable is changed by an amount corresponding to its standard deviation. For categorical fat and protein variables, reference category is the intermediate half.
† Model was adjusted for energy intake.
‡ Likelihood ratio test was used to test whether the model with and without the food variables differed.
§ Model was adjusted for maternal energy intake, genetic risk, hospital of birth, familial diabetes and maternal vocational education.
To determine which fraction of milk products accounts for the observed associations with the development of preclinical or clinical type 1 diabetes, we separately examined the intakes of fat and protein from milk products. High intakes of fat from fresh milk (HR 1·43, 95 % CI 1·04, 1·96) and protein from sour milk (HR 1·15, 95 % CI 1·02, 1·29) were associated with an increased risk of preclinical type 1 diabetes (Table 5).
Discussion
In the present prospective cohort study with genetically susceptible offspring, it was found that maternal diet during pregnancy was weakly associated with the development of preclinical and clinical type 1 diabetes. The maternal intake of palmitic acid and consumption of cheese during pregnancy were inversely associated with the development of clinical type 1 diabetes. The consumption of sour milk products, intake of protein from sour milk and intake of fat from fresh milk products were associated with an increased risk of preclinical type 1 diabetes, and the intake of low-fat margarines was associated with a decreased risk.
The strength of the present study is the population-based prospective cohort setting as well as the fact that the endpoints used comprised both preclinical type 1 diabetes and, separately, clinical type 1 diabetes. The endpoint ‘preclinical type 1 diabetes’ is strongly associated with clinical disease( Reference Siljander, Simell and Hekkala 20 ). The FFQ used in the present study was validated against two 5 d food records, and it estimated the consumption of food groups relatively well( Reference Erkkola, Karppinen and Javanainen 17 ). The consumption of milk products had very good validity (Pearson's correlation coefficients for different milk products varied between 0·58 and 0·86)( Reference Erkkola, Karppinen and Javanainen 17 ).
The major limitation of the study is the high number of fatty acids and food groups analysed. Altogether, we tested over seventy associations. None of the associations was highly significant and may be false positive and occur due to chance. Therefore, the results must be interpreted with caution. Another limitation is that the children's diet during infancy was not taken into account, because the food records of all the children were not available. In the validation study, for some important food sources of fat, the correlations measured by FFQ and food records were low, as Pearson's correlation coefficients for the consumption of oils and low-fat spreads were 0·22 and 0·25, respectively( Reference Erkkola, Karppinen and Javanainen 17 ).
The present observation is the first epidemiological one to indicate that the maternal intake of palmitic acid, which reflects the consumption of certain milk products, and consumption of cheese are inversely associated with clinical type 1 diabetes in the offspring. The results indicate that maternal exposure to certain milk products during pregnancy might induce tolerance to cows' milk in genetically susceptible offspring. A previous study has shown that the maternal intake of protein from cheese during pregnancy and maternal intake of protein from fresh milk products and cheese during lactation are marginally inversely associated with the humoral immune responses to cows' milk proteins in offspring with a high risk of type 1 diabetes( Reference Erkkola, Kronberg-Kippila and Savilahti 21 ). An enhanced humoral immune response to various cows' milk proteins has been observed in infancy in children who later were diagnosed with type 1 diabetes( Reference Luopajarvi, Savilahti and Virtanen 22 ).
In contrast to the inverse associations between the maternal intake of milk-related fatty acids and the risk of type 1 diabetes in the offspring in the present study, our previous case–control analysis in 315 children has shown that the fatty acid biomarkers of milk consumption are directly associated with the risk of islet autoimmunity at or before the time of seroconversion( Reference Virtanen, Niinisto and Nevalainen 12 ). Our newest findings in children were that the consumption of fresh milk products and cows' milk-based infant formulas and the intake of fat from all milk products and intake of protein from fresh milk products are weakly associated with a higher risk of advanced preclinical type 1 diabetes( Reference Virtanen, Nevalainen and Kronberg-Kippila 13 ). These findings support the hypothesis that the direct exposure of children to cows' milk leads to immunisation or the results could be partly explained by possible lipotoxicity. Several other case–control and cohort studies as well as randomised trial findings also support the hypothesis that cows' milk is associated with the development of type 1 diabetes, although there are conflicting results also( Reference Knip, Virtanen and Akerblom 23 , Reference Knip, Virtanen and Seppa 24 ). Savilahti & Saarinen( Reference Savilahti and Saarinen 25 ) observed that very early exposure to cows' milk in the maternity hospital may decrease the risk of type 1 diabetes in the offspring by the age of 8 years.
In the present study, the maternal intake of palmitoleic acid (16 : 1n-7) during pregnancy was inversely associated with the risk of clinical type 1 diabetes when the children were observed up to the age of 3 years. An inverse association of palmitoleic acid intake may reflect the maternal consumption of meat or dairy products. The observation could also reflect that the effect of palmitoleic acid may be protective in accordance with some in vitro studies( Reference Morgan and Dhayal 15 ).
Type 1 diabetes has been shown to be associated with increased inflammation, and n-3 fatty acids have been implicated as anti-inflammatory agents, while n-6 acids fatty acids have been shown to be pro-inflammatory agents( Reference Galli and Calder 26 ). In line with other cohort findings, the maternal intake of n-3 or n-6 fatty acids during pregnancy was not associated with the risk of preclinical or clinical type 1 diabetes in the present study( Reference Fronczak, Baron and Chase 7 , Reference Sorensen, Joner and Jenum 8 ).
In accordance with our previous analyses using a smaller dataset, the maternal intake of low-fat margarines during pregnancy was found to be associated with a decreased risk of preclinical type 1 diabetes( Reference Virtanen, Uusitalo and Kenward 27 ). In contrast to our earlier results( Reference Virtanen, Uusitalo and Kenward 27 ), butter was not associated with the risk of prediabetes and sour milk was associated with an increased risk of prediabetes in the present study. However, in the present study, the maternal intake of protein from sour milk was also associated with an increased risk of prediabetes.
The present findings that the maternal intake of fat from fresh milk products is weakly associated with an increased risk of advanced preclinical type 1 diabetes may indicate lipotoxicity to be responsible for the pathogenesis of type 1 diabetes. However, palmitic acid intake exhibited a protective association with the clinical type 1 diabetes endpoint.
In other analyses carried out in the cohort of the present study, interesting findings emerged for fatty acids and other immune-mediated endpoints, and these suggest that maternal diet and fatty acid intake during pregnancy may influence the development of the immune system in the offspring. High maternal consumption of butter and a high ratio of n-6:n-3 fatty acids during pregnancy have been reported to be associated with an increased risk of allergic rhinitis in the offspring( Reference Nwaru, Erkkola and Lumia 28 ). The maternal intakes of α-linoleic acid (n-3), total n-3 PUFA, SFA and palmitic acid during pregnancy have been reported to be associated with a decreased risk of asthma and that of arachidonic acid (n-6) with an increased risk( Reference Lumia, Luukkainen and Tapanainen 29 ). These and present findings support the importance of fatty acids when studying relationships between nutrition and immune-mediated diseases.
Acknowledgements
The authors express their gratitude to the children and parents who participated in the present study. They thank the DIPP research nurses, doctors, nutritionists and laboratory staff for their excellent collaboration over the years. They are also grateful to Heli Tapanainen for her skilful assistance. An abstract of the present study has been published at the 46th Annual meeting of the European Diabetes Epidemiology Group, 15–18 May 2011.
The present study was supported by the Doctoral Program in Public Health, the Academy of Finland (grants 63672, 79685, 79686, 80846, 201988 and 210632), the Finnish Diabetes Association, the Finnish Diabetes Research Foundation, the Finnish Pediatric Research Foundation, the Häme Foundation of the Finnish Culture Fund, the Juho Vainio Foundation, the Yrjö Jahnsson Foundation, Medical Research Funds, Turku, Oulu and Tampere University Hospitals' Juvenile Diabetes Research Foundation (grants 197032, 4-1998-274, 4-1999-731 and 4-2001-435), the Novo Nordisk Foundation and the EU Biomed 2 Program (BMH4-CT98-3314). None of the funders had any role in the design and analysis of the study or in the writing of this article.
The authors' contributions were as follows: S. N. shared responsibility for the writing of the manuscript with S. M. V., H.-M. T., L. U., M. K., J. N. and M. L.; S. M. V., L. U., S. N., H.-M. T. and J. N. were responsible for the study concept and design; J. R. updated the fatty acid values of their national database; J. I., M. K. and O. S. designed the DIPP Study; S. M. V. designed the nutrition study within the DIPP Study; H.-M. T. carried out the statistical analyses under the supervision of J. N. and M. G. K.; R. V. had responsibility for the clinical work in Oulu. All the authors contributed to the critical revision of the manuscript.
The authors declare that there is no conflict of interest associated with this paper.







