Implications
Feed digestion represents a critical process of the utilization of nutrients for productive purposes such as meat or milk production. In high-producing ruminants, starch represents the major component of dietary energy, whereas protein represents a high cost and dietary, environmental concern; thus, it is critical that the utilization of both be optimal. Evolution has dictated that ruminants use protein derived from microbial fermentation, and this tends to be a critical driver for nutrient assimilation. In contrast, starch does not result in signalling to increase starch assimilation, and evolutionary constraints may exist for maximal use in the small intestine of ruminants.
Introduction
In ruminants, the composition of digesta flowing to the small intestine differs substantially from what is consumed in the diet because ruminants have a complex stomach with four compartments allowing for pregastric fermentation (Merchen, Reference Merchen and Church1988; Swanson, Reference Swanson, Smithers and Knoerzer2019). This differing digesta composition is because of fermentation in portions of the stomach (rumen, reticulum and omasum) resulting in the production of volatile fatty acids (VFAs) and microbial biomass. The VFA provides a large proportion (approximately 50% to 85%) of the total metabolizable energy to the animal. A portion of the feed carbohydrates and proteins are degraded in the forestomachs, and a portion escapes fermentation in the forestomachs and flows to the small intestine along with microbial biomass containing microbial protein and is utilized by the animal. Microbial protein supplies a substantial portion (approximately 50% or more) of the protein digested and utilized by the animal.
The small intestine is the primary site of digestion and absorption of the macronutrients, escape starch, protein (microbial and escape) and lipids, aside from VFA absorption in the forestomachs. Because of the microbial influence on the nutrient profile, a complete description of these processes remains elusive. The objectives of this review are to describe: (1) the processes and potential limitations of small intestinal starch and protein assimilation and (2) the mechanisms regulating the nutritional modulation of digestive function in the small intestine in ruminants.
Small intestinal starch digestion
The feeding of large amounts of grain to ruminants is still a relatively new practice encompassing approximately the past 70 years. The continued availability of comparatively inexpensive cereal grains has insured that the practice will continue for the foreseeable future as modern production practices continually expand in scale with ever-decreasing profit margins. Research into these practices is also relatively recent with the earliest work characterizing the adaptive responses in ruminants fed high-starch ingredients (Clary et al., Reference Clary, Mitchell, Little and Bradley1969).
In forage-based diets, fibre and microbial polysaccharides are the primary carbohydrates flowing to the small intestine (Swanson, Reference Swanson, Smithers and Knoerzer2019). Limited fibre is digested in the small intestine because there are no fibre-digesting enzymes produced by the animal and there is a much smaller population of microbes in the small intestine than in the forestomach. When forage-based diets are fed, limited amounts of soluble carbohydrates such as starches flow to the small intestine as the small amounts in forages are fermented by the microbes in the forestomach, and any α-glucosides present may arise from microbial sources (Branco et al., Reference Branco, Harmon, Bohnert, Larson and Bauer1999). However, from 4% to 60% of dietary starch passes to the small intestine, depending on grain source and processing methods, when high-concentrate diets based on cereal grains are fed (Theurer, Reference Theurer1986). A recent summary for dairy cows (Moharrery et al., Reference Moharrery, Larsen and Weisbjerg2014) reported that ruminal digestion averaged 68% with a range of 22% to 94%.
Pancreatic α-amylase
Most species readily adapt their complement of digestive enzymes to match their diet (Brannon, Reference Brannon1990), ensuring maximum digestion of major dietary components. Early work suggested that ruminants fed high-grain diets had increased pancreatic concentrations of α-amylase (Clary et al., Reference Clary, Mitchell, Little and Bradley1969; Russell et al., Reference Russell, Young and Jorgensen1981; Janes et al., Reference Janes, Weekes and Armstrong1985). Kreikemeier et al. (Reference Kreikemeier, Harmon, Peters, Gross, Armendariz and Krehbiel1990) were the first to demonstrate that pancreatic α-amylase was linked to dietary energy intake and that earlier studies suggesting that pancreatic α-amylase was up-regulated with increased starch intake were confounded by dietary energy. This study (Kreikemeier et al., Reference Kreikemeier, Harmon, Peters, Gross, Armendariz and Krehbiel1990) demonstrated that cattle do respond to increased dietary energy, whether it is from forage or concentrate, by increasing pancreatic α-amylase content. When starch intake was increased while controlling energy, the content of pancreatic α-amylase decreased.
This adaptive response is unique and unexpected. Follow-up experiments using steers with pancreatic cannula confirmed that starch infused directly into the abomasum would decrease pancreatic α-amylase secretion compared with water or starch infused into the rumen (Walker and Harmon, Reference Walker and Harmon1995).
The nature of ruminant digestion insures that increased dietary energy increases the intestinal supply of microbial protein. Thus, the interpretation of experiments reporting that increased dietary energy intake increases pancreatic α-amylase is inherently confounded with energy and protein. Experiments have shown that increasing the small intestinal protein supply by infusing casein abomasally increases small intestinal starch disappearance (Richards et al., Reference Richards, Branco, Bohnert, Huntington, Macari and Harmon2002) and abomasal casein infusion increases pancreatic α-amylase secretion (Richards et al., Reference Richards, Swanson, Paton, Harmon and Huntington2003). These results could indicate that the increased pancreatic α-amylase responses to increased dietary energy resulted from the increased supply of small intestinal protein.
To directly examine the relationship between the small intestinal supply of protein and energy, calves were infused abomasally for 10 days with casein and starch in a 2 × 2 factorial arrangement (Swanson et al., Reference Swanson, Matthews, Woods and Harmon2002a). Compared with control (water infusion), calves receiving starch had reduced pancreatic α-amylase, whereas calves receiving casein had increased pancreatic α-amylase. However, calves receiving both starch and casein had reduced pancreatic α-amylase, similar to starch alone. These results suggest that the positive effects of casein to increase pancreatic α-amylase are suppressed by increased small intestinal starch.
The regulation of pancreatic α-amylase is obviously complex and involves translational events. In the casein and starch infusion study (Swanson et al., Reference Swanson, Matthews, Woods and Harmon2002a), casein infusion increased both pancreatic α-amylase mRNA expression and α-amylase protein, whereas starch + casein decreased both.
The ability of starch, or a partially hydrolysed starch solution (Walker and Harmon, Reference Walker and Harmon1995; Swanson et al., Reference Swanson, Matthews, Woods and Harmon2002a), to down-regulate pancreatic α-amylase questions the capacity of the ruminant to hydrolyse starch and how starch influences the regulation of pancreatic α-amylase. However, a comparison of glucose infused abomasally compared with starch demonstrated that glucose also down-regulates pancreatic α-amylase (Swanson et al., Reference Swanson, Richards and Harmon2002b) indicating that the hydrolysis of α-glucosides is not limiting the adaptive response.
The downregulation of pancreatic α-amylase in cattle remains an intriguing and unexplained adaptive response. The concept has been studied and repeated across multiple experiments and experimental models. Several experiments have sought to characterize factors that affect pancreatic α-amylase, particularly factors that appear to stimulate increases in pancreatic α-amylase. The most notable of these is increasing casein supply to the small intestine. Research infusing starch and casein either ruminally or abomasally into steers (Taniguchi et al., Reference Taniguchi, Huntington and Glenn1995) reported that starch and casein infused abomasally increased the net portal and total splanchnic fluxes of glucose suggesting greater small intestinal starch hydrolysis and glucose absorption. Casein infused into the abomasum of lambs has been reported to increased glucose transporter activity in the small intestine (Mabjeesh et al., Reference Mabjeesh, Guy and Sklan2003).
The observation that casein could enhance small intestinal starch assimilation was followed by experiments showing that small intestinal starch disappearance (Richards et al., Reference Richards, Branco, Bohnert, Huntington, Macari and Harmon2002; Brake et al., Reference Brake, Titgemeyer, Bailey and Anderson2014b) and pancreatic α-amylase secretion (Richards et al., Reference Richards, Swanson, Paton, Harmon and Huntington2003) increased with casein infusion. Subsequent work comparing the feeding of intact and acid-hydrolysed casein demonstrated that intact casein stimulated pancreatic α-amylase secretion in steers as well as increasing cholecystokinin (CCK) secretion (Lee et al., Reference Lee, Choi, Jin, Wang, Lee, Ku, Hwang, Kim, Vega and Lee2013).
The exact mechanism for stimulation of pancreatic α-amylase and increasing starch digestion remains unclear but attempts to refine the response to individual amino acids have been made. Brake et al. (Reference Brake, Titgemeyer and Anderson2014a) infused duodenally and ileally cannulated steers with starch and compared additions of casein, crystalline amino acids similar to casein and essential or non-essential amino acids similar to casein. The small intestinal starch digestion was highest for the casein and crystalline amino acids similar to casein treatments. These authors then followed with a second experiment comparing casein, glutamate equivalent to casein, phenylalanine plus tryptophan plus methionine equivalent to casein or both. Small intestinal starch digestibility was highest in the casein and glutamate treatments, and based on differences in the ileal flows of small-chain α-glycosides the authors suggested that casein and non-essential amino acids may increase starch digestion by different mechanisms with casein favouring increased pancreatic α-amylase. The differential response suggesting a greater pancreatic α-amylase response was not present in a follow-up study where glutamate infusion from 30 to 120 g/day linearly increased small intestinal starch digestibility (Blom et al., Reference Blom, Anderson and Brake2016).
Numerous mechanisms may contribute to what is measured as increased small intestinal disappearance. Research on milk-fed calves reported that 89% of starch intake might have been fermented prior to the terminal ileum (Gilbert et al., Reference Gilbert, Pantophlet, Berends, Pluschke, van den Borne, Hendriks, Schols and Gerrits2015) suggesting that microbial activity may contribute substantially to the small intestinal carbohydrate disappearance. The infusion of casein into the small intestine causes dramatic increases in large intestinal digestion suggesting that stimulation of microbial activity in the small intestine is undoubtedly a contributor to the increased disappearances observed with casein and may explain some of the differential responses attributed to casein and amino acids (Brake et al., Reference Brake, Titgemeyer and Anderson2014a and Reference Brake, Titgemeyer, Bailey and Anderson2014b; Blom et al., Reference Blom, Anderson and Brake2016), albeit 89% disappearance from fermentation may be unique to the milk-fed calf. However, the increases in intestinal starch disappearance observed with individual amino acids are less attributable to increased microbial activity and have been associated with increased pancreatic α-amylase.
Other amino acids have been evaluated for their effect on small intestinal starch digestion. Goats were infused duodenally with phenylalanine at 0, 2, 4 and 8 g/day for 2 weeks and pancreatic secretion was measured (Yu et al., Reference Yu, Xu, Yao, Liu, Li, Liu, Wang, Sun and Liu2013). Pancreatic α-amylase secretion responded quadratically with a small increase at 2 g/day. A follow-up experiment using short-term, 10 h infusions showed that pancreatic α-amylase secretion responded cubically with increases in secretion at 2 and 10 g/day of phenylalanine. A similar experiment using goats reported that both short- and long-term infusions of leucine at 0, 3, 6 or 9 g/day increased pancreatic α-amylase secretion (Yu et al., Reference Yu, Xu, Liu, Yao, Yu and Wang2014a).
The inconsistent responses may result from the difficulty in assessing pancreatic α-amylase secretion with few animals and because of the pulsatile and variable secretion from the pancreas. However, these studies (Yu et al., Reference Yu, Xu, Yao, Liu, Li, Liu, Wang, Sun and Liu2013 and Reference Yu, Xu, Liu, Yao, Yu and Wang2014a) indicate that phenylalanine and leucine have the ability to up-regulate pancreatic α-amylase secretion, whereas the combination of phenylalanine plus tryptophan plus methionine did not increase small intestinal starch digestion in steers (Brake et al., Reference Brake, Titgemeyer and Anderson2014a). These observations were confirmed in goats receiving duodenal infusions of leucine (3 and 9 g/day) and phenylalanine (2 g/day) that were slaughtered and enzyme activity in the small intestine measured (Yu et al., Reference Yu, Xu, Wang, Liu, Yao, Wu, Qin and Sun2014b). The infusion of leucine and phenylalanine caused large increases in pancreatic α-amylase activity in the proximal small intestine and tended to increase small intestinal starch digestibilty.
The effects of leucine on pancreatic secretion have also been studied in cattle. Heifers fitted with pancreatic cannula also received duodenal infusions of 10, 20 and 30 g/day of leucine (Liu et al., Reference Liu, Liu, Liu, Xu, Yu, Wang, Cao and Yao2015). They reported that leucine infused at 10 g/day increased pancreatic α-amylase secretion. However, supplementing additional phenylalanine and leucine in milk-fed calves did not increase pancreatic α-amylase (Cao et al., Reference Cao, Yang, Guo, Zheng, Wang, Cai, Liu and Yao2018).
The milk-fed calf may have differing mechanisms of regulation compared with the previous studies in mature ruminants. However, leucine has been shown to up-regulate pancreatic α-amylase in pancreatic acinar cells isolated from new-born calves and maintained in culture (Guo et al., Reference Guo, Liang, Zheng, Liu, Yin, Cao and Yao2018a). They demonstrated an upregulation of the m-TOR signalling pathway that may have resulted in increased α-amylase synthesis. This response contrasted with the influence of phenylalanine that was studied using pancreatic acinar cells and tissue segments isolated from 2-month-old calves (Guo et al., Reference Guo, Tian, Shen, Zheng, Liu, Cao, Cai and Yao2018b). Phenylalanine stimulates α-amylase secretion and mRNA expression as well as the phosphorylation of S6K1 and 4EBP1 indicating that phenylalanine could regulate the synthesis of α-amylase through the mRNA translation initiation factors, S6K1 and 4EBP1. Thus, these studies report that phenylalanine and leucine both stimulate pancreatic enzyme synthesis but through different mechanisms.
Mucosal carbohydrases
Research on the deficiencies of mucosal carbohydrases in infants has dramatically increased our understanding of the processes involved in mucosal carbohydrase function (Nichols et al., Reference Nichols, Baker and Quezada-Calvillo2018). The major starch hydrolysis activities within the small intestine function through two proteins that contribute four hydrolytic activities, sucrase-isomaltase and maltase-glucoamylase (Galand, Reference Galand1989). These proteins have a high degree of homology (Figure 1), and sucrase-isomaltase is generally present in much higher quantities. These four activities are better described as α-glucosidases because they digest multiple linear starch oligosaccharides to glucose, not just maltose. An excellent chronology of the study of intestinal disaccharidases is available (Lentze, Reference Lentze2018).

Figure 1 Maltase-glucoamylase and sucrose-isomaltase protein structures. Percentages represent sequence identity. Size differences represent greater relative protein abundances for sucrose-isomaltase. Adapted with permission from Lee et al. (Reference Lee, Rose, Lin, Quezada-Calvillo, Nichols and Hamaker2016) Copyright ©2016 American Chemical Society.
The process of starch assimilation in humans has been described in detail by numerous authors. The process involves six carbohydrase activities: salivary and pancreatic α-amylase, n-terminal and c-terminal activities of sucrase-isomaltase and maltase-glucoamylase (Lin et al., Reference Lin, Nichols, Quezada-Calvillo, Avery, Sim, Rose, Naim and Hamaker2012b). Ruminants lack salivary α-amylase, and they possess no sucrase activity (Huber et al., Reference Huber, Jacobson and Allen1961). Thus, of the six required enzyme activities ruminants possess perhaps four, pancreatic α-amylase, mucosal isomaltase activity and mucosal maltase(s) activities (Coombe and Siddons, Reference Coombe and Siddons1973). The enzyme profile of ruminants resembles humans exhibiting congenital sucrase-isomaltase deficiency where patients have genetic mutations resulting in the absense of one or both subunits of sucrase-isomaltase resulting in limitations in carbohydrate digestion.
A complete understanding of starch assimilation may also be limited by terminology. The textbook description has been that pancreatic α-amylase α-1,4 endoglucosidase hydrolysis in the intestinal lumen produces maltose and a collection of limit dextrins, so named because of the presence of α-1,6-bonds ‘limits’ the activity of α-amylase in these regions. These products of pancreatic α-amylase are then exposed to mucosal carbohydrases that hydrolyse this collection of starch fragments at the brush border membrane prior to glucose transport. While this description is not inaccurate, it is simplistic. For example, studies characterizing the substrate preferences of the n- and c-terminal subunits of recombinant mammalian maltase-glucoamylase and sucrase-isomaltase reported that the c-terminal subunit of maltase-glucoamylase provided rapid and high digestion of cooked starch, nearly 80%, while other subunits showed 20% to 30% digestion (Lin et al., Reference Lin, Nichols, Quezada-Calvillo, Avery, Sim, Rose, Naim and Hamaker2012b). Thus, multiple proteins may contribute to the hydrolysis of starch molecules.
Referring to maltase as a specific enzyme is also misleading, but rather there are multiple proteins possessing maltase activity, or more specifically, each subunit possessing carbohydrase activity has activity on multiple substrates (Lin et al., Reference Lin, Hamaker and Nichols2012a). Characterization of the substrate preferences of the intestinal carbohydrases for various α-linked substrates demonstrated that c-terminal and n-terminal maltase-glucoamylase and c-terminal and n-terminal sucrase-isomaltase all possessed some hydrolytic capacity for isomaltose, whereas both c-terminal sucrase-isomaltase and c-terminal maltase-glucoamylase hydrolysed sucrose (Table 1). This would suggest that since ruminants possess no measurable sucrase activity there are differences in the structure and function of the mucosal carbohydrases.
Table 1 Hydrolysis of different substrates by c-terminal (ct) and n-terminal (nt) mouse recombinant α-glucosidases

One unit of enzyme activity was arbitrarily defined as the amount of enzyme that released 1 μg of glucose from 1% maltose per 10 min at 37°C.; Mean value ± SD of measurement of experiments performed in triplicate. Adapted from Lee et al. (Reference Lee, Rose, Lin, Quezada-Calvillo, Nichols and Hamaker2016).
The process of multiple entities acting on multiple substrates increases the complexity of carbohydrate assimilation exponentially. However, strides have been made in understanding this process. The roles of maltase-glucoamylase and sucrase-isomaltase have been characterized using a maltodextrin substrate chosen to emulate a pancreatic α-amylase end-product (Quezada-Calvillo et al., Reference Quezada-Calvillo, Robayo-Torres, Ao, Hamaker, Quaroni, Brayer, Sterchi, Baker and Nichols2007). They reported that at low-substrate concentrations maltase-glucoamylase was more active than sucrase-isomaltase; however, at higher substrate concentrations, maltase-glucoamylase was inhibited, whereas sucrase-isomaltase was not. Thus, maltase-glucoamylase contributed only 20% of the hydrolytic activity, and pancreatic α-amylase was stimulatory to both the hydrolytic activities of sucrase-isomaltase and maltase-glucoamylase. This inhibitory activity was later localized to the C-terminal ‘glucoamylase’ subunit (Quezada-Calvillo et al., Reference Quezada-Calvillo, Sim, Ao, Hamaker, Quaroni, Brayer, Sterchi, Robayo-Torres, Rose and Nichols2008). It has been proposed that maltase-glucoamylase is responsible for the rapid hydrolysis at low-starch intakes, whereas sucrase-isomaltase provides sustained hydrolysis at high-starch intakes (Diaz-Sotomayor et al., Reference Diaz-Sotomayor, Quezada-Calvillo, Avery, Chacko, Yan, Lin, Ao, Hamaker and Nichols2013). This greater overall activity of sucrase-isomaltase is consistent with the relative abundances of the proteins in that sucrase-isomaltase is approximately 3-fold greater than maltase-glucoamylase (Amiri and Naim, Reference Amiri and Naim2017).
While our knowledge of the brush border carbohydrases has increased dramatically for non-ruminants, much less is known in regard to their function in ruminants. The expression of sucrase-isomaltase and maltase-glucoamylase has been shown to be highly responsive to diet changes in mice, increasing in response to increased digestible starch and regressing when fed resistant starch (Goda and Honma, Reference Goda and Honma2018). This response is thought to be elicited by available hexose as corresponding increases in glucose transporter (SGLT1) accompany increases in sucrase-isomaltase with both glucose and fructose feeding, with the reponse being more significant for fructose (Kishi et al., Reference Kishi, Takase and Goda1999).
The structural similarities of sucrase-isomaltase and maltase-glucoamylase (59% homologous) suggest a common route for post-translational processing in that both are type II membrane glycoproteins (Nichols et al., Reference Nichols, Eldering, Avery, Hahn, Quaroni and Sterchi1998; Amiri and Naim, Reference Amiri and Naim2017). A similar path of post-translational processing could result in a common alteration in c-terminal processing affecting both proteins. However, differences in processing do exist (Amiri and Naim, Reference Amiri and Naim2018). Particularly, sucrase-isomaltase is cleaved at the luminal membrane by trypsin into the two subunits (Naim et al., Reference Naim, Sterchi and Lentze1988) whereas maltase-glucoamylase is not.
Generally, ruminant mucosal carbohydrase activities are non-responsive to changes in diet (Siddons, Reference Siddons1968; Russell et al., Reference Russell, Young and Jorgensen1981; Janes et al., Reference Janes, Weekes and Armstrong1985; Kreikemeier et al., Reference Kreikemeier, Harmon, Peters, Gross, Armendariz and Krehbiel1990; Bauer et al., Reference Bauer, Harmon, McLeod and Huntington1995; Gorka et al., Reference Gorka, Schurmann, Walpole, Blonska, Li, Plaizier, Kowalski and Penner2017). Ruminants possess measurable activities for maltase (Siddons, Reference Siddons1968), isomaltase (Coombe and Siddons, Reference Coombe and Siddons1973), trehalase (Coombe and Siddons, Reference Coombe and Siddons1973; Kreikemeier et al., Reference Kreikemeier, Harmon, Peters, Gross, Armendariz and Krehbiel1990) and lactase (Siddons, Reference Siddons1968).
Heat inactivation suggested that activities of trehalase, isomaltase and lactase were single entities, whereas maltase represented multiple activities (Coombe and Siddons, Reference Coombe and Siddons1973). Based on the Lee et al. (Reference Lee, Rose, Lin, Quezada-Calvillo, Nichols and Hamaker2016) study (Table 1), the n-terminal activities of both proteins would represent multiple enzymes with no sucrase activity. Whether both proteins differ in ruminants remains to be determined.
The apparent absence of changes in mucosal carbohydrase activities in ruminants suggests that ruminants do not adapt to increased intake of carbohydrate. However, increased intestinal length (Kreikemeier et al., Reference Kreikemeier, Harmon, Peters, Gross, Armendariz and Krehbiel1990) and increased mucosal mass (Kreikemeier et al., Reference Kreikemeier, Harmon, Peters, Gross, Armendariz and Krehbiel1990; Gorka et al., Reference Gorka, Schurmann, Walpole, Blonska, Li, Plaizier, Kowalski and Penner2017) led to increases in total hydrolytic capacity of the intestine and in the jejunum with increases in energy intake (Kreikemeier et al., Reference Kreikemeier, Harmon, Peters, Gross, Armendariz and Krehbiel1990; Gorka et al., Reference Gorka, Schurmann, Walpole, Blonska, Li, Plaizier, Kowalski and Penner2017).
These studies suggest that, as ruminants consume increased amounts of high-concentrate diets, there is a greater ability to assimilate the starch in the small intestine. However, that capacity when compared with the ability of the non-ruminant to adapt, and perhaps with a more efficient complement of enzymes, may explain the inefficiencies of ruminant small intestinal digestion. We have calculated that starch digestibility in the small intestine must be maintained to at least 70% (Huntington et al., Reference Huntington, Harmon and Richards2006) to maintain the energetic efficiency advantages of small intestinal digestion. These limitations may explain some of the challenges of meeting that requirement.
Glucose transport
Early research suggested limited amounts of glucose are absorbed into the portal blood of functioning ruminants (Schambye, Reference Schambye1951). However, studies directly evaluating glucose transport by measuring disappearance of sugars from isolated loops of the small intestine filled with sugar solutions determined that absorptive capacity decreased along the length of the small intestine as measurements proceeded distally and that the capacity decreased following weaning (White et al., Reference White, Williams and Morris1971). These workers also suggested that the capacity for glucose absorption was less than the rat, mainly as a function of intestinal length per kg BW.
The presence of active transport of sugars was reported (Scharrer, Reference Scharrer1976) and a decrease in transport capacity associated with weaning was described (Scharrer et al., Reference Scharrer, Peter and Raab1979). These authors (Scharrer et al., Reference Scharrer, Peter and Raab1979) also demonstrated that the declining transport of glucose associated with weaning could be delayed by prolonged milk feeding.
These early studies, which contributed significantly to our understanding of sugar transport in ruminants, were all conducted using anaesthetized sheep, with measurements made using intestinal perfusions and measurement of glucose disappearance. These observations were later confirmed using brush border membrane vesicles prepared from sheep small intestine (Shirazi-Beechey et al., Reference Shirazi-Beechey, Kemp, Dyer and Beechey1989). These authors reported that Na+-dependent glucose transport (SGLT1) was present throughout the small intestine of pre-ruminant lambs but absent in ruminants. These observations were later extended (Shirazi-Beechey et al., Reference Shirazi-Beechey, Hirayama, Wang, Scott, Smith and Wright1991a) to show that SGLT1 was maximum 2 weeks following birth then declined to negligible amounts following weaning and that increased transport activity could be maintained by maintaining lambs on milk replacer. This study was also the first to report SGLT1 could be induced in the small intestine of 2- to 3-year-old sheep infused for 4 days with 30 mM glucose or α-methyl-D-glucopyranoside (a non-metabolizable analogue).
Subsequent work (Lescale-Matys et al., Reference Lescale-Matys, Dyer, Scott, Freeman, Wright and Shirazi-Beechey1993) showed that maintaining lambs on milk maintained tissue SGLT1 mRNA levels, whereas infusion of glucose into functional ruminant sheep increased mRNA only 2-fold compared with a 60- to 90-fold increase in transporter activity. Changes in SGLT1 activity in sheep were associated with changes in SGLT1 protein abundance (Shirazi-Beechey et al., Reference Shirazi-Beechey, Dyer, Allison and Wood1996) whereas the regulation of SGLT1 synthesis was thought to occur post-translationally.
The presence of SGLT1 in cattle jejunum has been established (Kaunitz and Wright, Reference Kaunitz and Wright1984) and one of the first to address SGLT1 expression throughout the gastrointestinal tract was conducted in lactating cows (Zhao et al., Reference Zhao, Okine, Cheeseman, Shirazi-Beechey and Kennelly1998). They reported that SGLT1 was expressed throughout the gastrointestinal tract of cattle and that SGLT1 was active in the small intestine, being greater in the proximal small intestine. Bauer et al. (Reference Bauer, Harmon, McLeod and Huntington2001b) infused both cattle and sheep abomasally or ruminally with a partially hydrolysed starch solution for 7 days before slaughtering and measuring transport activity in small intestinal tissues. They reported that SGLT1 increased 2.1-fold in the proximal jejunum of animals receiving the abomasal compared with the ruminal infusion. However, a subsequent study (Bauer et al., Reference Bauer, Harmon, Bohnert, Branco and Huntington2001a) was unable to demonstrate changes in SGLT1 activity throughout the small intestine in response to abomasal v. ruminal infusion of partially hydrolysed starch. Obviously, a limitation of this model could be the conversion of starch hydrolysate to glucose or that mechanisms other than SGLT1 contribute to small intestinal glucose disappearance in cattle.
To determine if increased glucose in the small intestine upregulates glucose transport, glucose was abomassaly infused into steers and compared with steers receiving either ruminal or abomasal partially hydrolysed starch (Rodriguez et al., Reference Rodriguez, Guimaraes, Matthews, McLeod, Baldwin and Harmon2004). Sodium-dependent glucose uptake was not affected by treatment, but uptake decreased distally along the intestine. This work is supported by results from dairy cows (Lohrenz et al., Reference Lohrenz, Duske, Schönhusen, Losand, Seyfert, Metges and Hammon2011) fed high- (24%) and low-starch diets (12%). These workers reported no differences in expression of SGLT1 or GLUT2 mRNA or protein in brush border membrane vesicles prepared from mid-duodenum and mid-jejunum. Thus, it appears SGLT1 is functional in cattle, activities are highest in the proximal intestine, but activity does not appear to respond to higher intakes of starch-based diets.
The contribution of diffusion was assessed in cattle (Krehbiel et al., Reference Krehbiel, Britton, Harmon, Peters, Stock and Grotjan1996) by infusing glucose along with 2-deoxyglucose, a non-metabolizable, non-SGLT1 transportable analogue, into the proximal and mid-intestine of steers. They reported that glucose disappearance was much higher in the proximal small intestine and that passive diffusion was a minor contributor to portal glucose appearance. These results would suggest that SGLT1 is the major pathway for glucose transport from the intestinal lumen.
Dyer et al. (Reference Dyer, Vayro, King and Shirazi-Beechey2003) using glucose molecules bound to polyethylene glycol to make them non-absorbable showed that glucose stimulates increased SGLT1 protein by interacting luminally with a glucose sensor. An alternative mechanism for enhancing luminal sugar removal was proposed using mice (Gouyon et al., Reference Gouyon, Caillaud, Carriere, Klein, Dalet, Citadelle, Kellett, Thorens, Leturque and Brot-Laroche2003) where the presence of sugars stimulated the recruitment of basolateral GLUT2 into the brush border membrane and the presence of this facilitated transporter contributed to the upregulation of glucose removal. This mechanism, however, remains controversial (Daniel and Zietek, Reference Daniel and Zietek2015) or may be species dependent (Moran et al., Reference Moran, Al-Rammahi, Arora, Batchelor, Coulter, Ionescu, Bravo and Shirazi-Beechey2010). The latter work using piglets (Moran et al., Reference Moran, Al-Rammahi, Arora, Batchelor, Coulter, Ionescu, Bravo and Shirazi-Beechey2010) demonstrated that GLUT2 was expressed only in the basolateral membrane and that there was no uptake of substrate specific for SGLT1. Similarly, work using SGLT1 and GLUT2 knockout mice (Roder et al., Reference Roder, Geillinger, Zietek, Thorens, Koepsell and Daniel2014) reported that SGLT1 was the major intestinal apical glucose transporter. At this writing, there is no information on whether GLUT2 plays a role in apical glucose transport in ruminants.
Sheep v. cattle
Evidence would suggest that perhaps sheep are more able to adapt to increasing small intestinal starch. When starch is infused into the abomasum, pancreatic α-amylase secretion decreases as it does in cattle (Wang and Taniguchi, Reference Wang and Taniguchi1998); however, when casein is infused with the starch pancreatic α-amylase secretion is restored, unlike cattle (Swanson et al., Reference Swanson, Matthews, Woods and Harmon2002a). While the early work was confounded by dietary energy (Janes et al., Reference Janes, Weekes and Armstrong1985) there were increases in carbohydrases with increased intake and when dietary energy was balanced using moderate starch diets (Swanson et al., Reference Swanson, Matthews, Matthews, Howell, Richards and Harmon2000), pancreatic α-amylase protein and activity tended to increase despite the trend for reduced pancreatic α-amylase mRNA.
Adaptive responses in glucose transport to carbohydrate in the small intestine have been demonstrated in sheep (Shirazi-Beechey et al., Reference Shirazi-Beechey, Kemp, Dyer and Beechey1989; Shirazi-Beechey et al., Reference Shirazi-Beechey, Hirayama, Wang, Scott, Smith and Wright1991a and Reference Shirazi-Beechey, Smith, Wang and James1991b; Mabjeesh et al., Reference Mabjeesh, Guy and Sklan2003) but changes have been more difficult to demonstrate in cattle (Bauer et al., Reference Bauer, Harmon, Bohnert, Branco and Huntington2001a; Klinger et al., Reference Klinger, Zurich, Schröder and Breves2013). Collectively, these data suggest that sheep may be better able to adapt to high-starch diets, but at present, there is no definitive comparison of starch utilization in sheep and cattle.
Limitations to post-ruminal starch digestion
Many researchers have suggested that the ruminant small intestine has a limited capacity for starch digestion (Orskov, Reference Orskov1986; Owens et al., Reference Owens, Zinn and Kim1986; Swanson and Harmon, Reference Swanson, Harmon, Zabielski, Gregory and Westrom2002; Swanson, Reference Swanson, Smithers and Knoerzer2019). Owens et al. (Reference Owens, Zinn and Kim1986) summarized several studies and reported that only 55% of starch entering the small intestine disappears in the small intestine of cattle fed high-concentrate diets. A recent summary for dairy cows reported that small intestinal disappearance ranged from 11% to 90% with a mean of 60% (Moharrery et al., Reference Moharrery, Larsen and Weisbjerg2014). Similar conclusions have been drawn from studies with dairy cattle (Nocek and Tamminga, Reference Nocek and Tamminga1991) and studies with both beef and dairy cattle (Harmon et al., Reference Harmon, Yamka and Elam2004). Aside from inefficiencies of undigested starch exiting the small intestine, large quantities of starch flowing to the large intestine can result in excess fermentation which can result in diarrhoea and acidosis.
Specific factors limiting starch digestion, proposed by Owens et al. (Reference Owens, Zinn and Kim1986), include limited carbohydrase activity, insufficient time for complete starch hydrolysis, inadequate access of enzymes to starch granules and limited glucose absorption.
Pancreatic α-amylase has been suggested as a possibility by numerous authors; however, attempts to increase small intestinal α-amylase have not enhanced starch assimilation (Remillard et al., Reference Remillard, Johnson, Lewis and Nockels1990; Westreicher-Kristen et al., Reference Westreicher-Kristen, Robbers, Blank, Troescher, Dickhoefer, Wolffram and Susenbeth2018).
The downregulation of pancreatic α-amylase by starch has been overcome by casein infusion (Richards et al., Reference Richards, Swanson, Paton, Harmon and Huntington2003; Brake et al., Reference Brake, Titgemeyer and Anderson2014a) and this has been associated with increased small intestinal starch disappearance (Richards et al., Reference Richards, Branco, Bohnert, Huntington, Macari and Harmon2002) and similar responses have been shown with amino acids mimicking casein (Brake et al., Reference Brake, Titgemeyer and Anderson2014a; Blom et al., Reference Blom, Anderson and Brake2016). Whether these treatments achieve this increased intestinal disappearance through increased pancreatic α-amylase or some other means is unknown. It has been suggested that luminal pH in the proximal small intestine limits pancreatic α-amylase resulting in a shift in carbohydrate hydrolysis to the distal intestine where glucose transport is more limiting (Mills et al., Reference Mills, France, Ellis, Crompton, Bannink, Hanigan and Dijkstra2017); however, attempts to increase intestinal pH have not been shown to increase starch assimilation (Remillard et al., Reference Remillard, Johnson, Lewis and Nockels1990).
The apparently striking differences in mucosal carbohydrases in ruminants may pose another limitation. Knockout mice without maltase-glucoamylase had a 40% reduction in their ability to generate blood glucose from starch (Nichols et al., Reference Nichols, Quezada-Calvillo, Robayo-Torres, Ao, Hamaker, Butte, Marini, Jahoor and Sterchi2009). This essential role plus the recent demonstration of the role of mucosal enzymes in hydrolysing starch (Quezada-Calvillo et al., Reference Quezada-Calvillo, Robayo-Torres, Ao, Hamaker, Quaroni, Brayer, Sterchi, Baker and Nichols2007) suggests that the ruminants evolutionary limits to starch hydrolysis may be greater than previously thought.
While glucose transport has been shown to be inducible in the small intestine of ruminants (Moran et al., Reference Moran, Al-Rammahi, Zhang, Bravo, Calsamiglia and Shirazi-Beechey2014) benefits of this increase for increased small intestinal starch assimilation are lacking.
Combined data suggest that ruminants are limited users of small intestinal starch and that the low digestibilities in the small intestinal are likely the outcome of multiple factors that are only overcome by supplying small amounts of highly digestible substrate.
Limitations in post-ruminal protein assimilation
Compared with carbohydrates, research on the processes of protein assimilation has received little attention. Excellent reviews covering many of the processes are available (Beck, Reference Beck1973; Snook, Reference Snook1973; Hooton et al., Reference Hooton, Lentle, Monro, Wickham, Simpson, Nilius, Gudermann, Jahn, Lill, Petersen and DeTombe2015). However, scant information is available describing the processes in ruminants. The fact that ruminant digestion produces a relatively continual flow of microbial protein to the small intestine places a high priority on protein assimilation suggesting a highly efficient system is in place. Estimates used for small intestinal assimilation of protein are usually 80% (NASEM, 2016) based on N measurements; however, this is an apparent measure and values for true digestibility in the small intestine may be substantially higher. Estimates of small intestinal digestibility of feedstuffs made using the mobile nylon bag technique (Hvelplund et al., Reference Hvelplund, Weisbjerg and Andersen1992) suggest that a single value for small intestinal digestion is inadequate or more importantly, the digestibility of protein sources does vary in the small intestine. Previous research in ruminats showing that protein assimilation has not been exceeded when protein was infused at very high levels suggests that the small intestine has a high digestive and absorptive capacity for protein (Owens et al., Reference Owens, Zinn and Kim1986). Apparent small intestinal digestion of N compounds in ruminants has been reported to be between 65% and 75% of duodenal N flow (Santos et al., Reference Santos, Satter and Stern1984).
Pancreatic proteases
The major endopeptidases, trypsin, chymotrypsin and elastase are all present in the ruminant pancreas, and bovine sources have been well characterized (Walsh et al., Reference Walsh, Kauffman, Kumar and Neurath1964). The exopeptidases, carboxypeptidases A and B are also present, but there are limited data on their nutritional characterization.
The adaptation of protease activity in the small intestine of rats has been known for many years (Snook, Reference Snook1965 and Reference Snook1973). These adaptations to changes in diet involved increases in the synthesis and content of proteases in the pancreas and increased secretion of enzymes (Brannon, Reference Brannon1990). The complexity of ruminant digestion, that is, the pregastric fermentation necessitates that the majority of these studies infused proteins or amino acids into the abomasum or small intestine. Most of these studies reported steady amounts of trypsin or chymotrypsin activities in concert with the steady flows of microbial protein in the ruminant small intestine. However, adaptation did occur with changes in activity up to 1.5-fold common with the highest being 2.0- (Swanson et al., Reference Swanson, Benson, Matthews and Harmon2004) to 2.85-fold increases (Yu et al., Reference Yu, Xu, Wang, Liu, Yao, Wu, Qin and Sun2014b). Contrast this with changes up to 6-fold reported in rats (Brannon, Reference Brannon1990) and it appears ruminant pancreatic protease activity is less responsive to changes in diet. Some of the apparent differences in relative changes may occur because much lower activities occur in the fasting and protein deficient non-ruminant models making relative changes much greater. In the fed ruminant, fermentation produces a nearly continuous flow of microbial protein to the small intestine, and changes in diet produce much more subtle changes in protein flow and changes in pancreatic proteases may be more subtle as well. However, adaptation or stimulation of synthesis and secretion does occur.
Mucosal peptidases
A complete accounting of mucosal peptidases in ruminants is not available at the present time. A summary of current information is available (Hooton et al., Reference Hooton, Lentle, Monro, Wickham, Simpson, Nilius, Gudermann, Jahn, Lill, Petersen and DeTombe2015) and a partial summary of the most common brush border peptidases is in Table 2. The majority have been identified in bovine tissues (Uniprot, 2017) and characterized. Nomenclature for many peptidases has changed, and the identity of all present in any species remains elusive.
Table 2 Common peptidases in the mammalian small intestine brush border

Peptidases are ubiquitous and multifunctional in that the same peptidase may be anchored in the brush border membrane, present in the cytosol of the enterocytes and present in the intestinal lumen. Peptidases have broad specificities allowing them to act on a variety of substrates generated from the hydrolysis of proteins by pancreatic enzymes. In general, peptidases are complementary to pancreatic enzymes in that multiple peptidases target proline containing residues where pancreatic enzymes have little or no activity (Erickson and Kim, Reference Erickson and Kim1990), others may act on intact proteins producing small peptides and amino acids eliminating the need for pancreatic proteases (Guan et al., Reference Guan, Yoshioka, Erickson, Heizer and Kim1988). Brush border peptidases are highest in the proximal to the mid-gut region (Yoshioka et al., Reference Yoshioka, Erickson and Kim1988) in concert with their role in protein assimilation.
Peptide and amino acid transporters
The process of moving the products of digestion from the intestinal lumen across the brush border membrane is multi-faceted involving numerous amino acid and peptide transporters. A thorough description of these processes is beyond the scope of this paper. However, numerous excellent reviews are available describing both amino acid (Bröer, Reference Bröer2008) and peptide transporters (Daniel, Reference Daniel2004; Gilbert et al., Reference Gilbert, Wong and Webb2008; Daniel and Zietek, Reference Daniel and Zietek2015).
Multiple systems have been described for both amino acid (Matthews et al., Reference Matthews, Wong, Bender and Webb1996b; Knapp, Reference Knapp2004; Liao et al., Reference Liao, Vanzant, Harmon, McLeod, Boling and Matthews2009) and peptide (Matthews and Webb, Reference Matthews and Webb1995; Matthews et al., Reference Matthews, Wong, Bender, Bloomquist and Webb1996a) transport in ruminants similar to other species. To date, aspects of amino acid or peptide transport in ruminants have not been reported limiting to protein assimilation.
Mechanisms regulating the nutritional modulation of digestive function in the small intestine
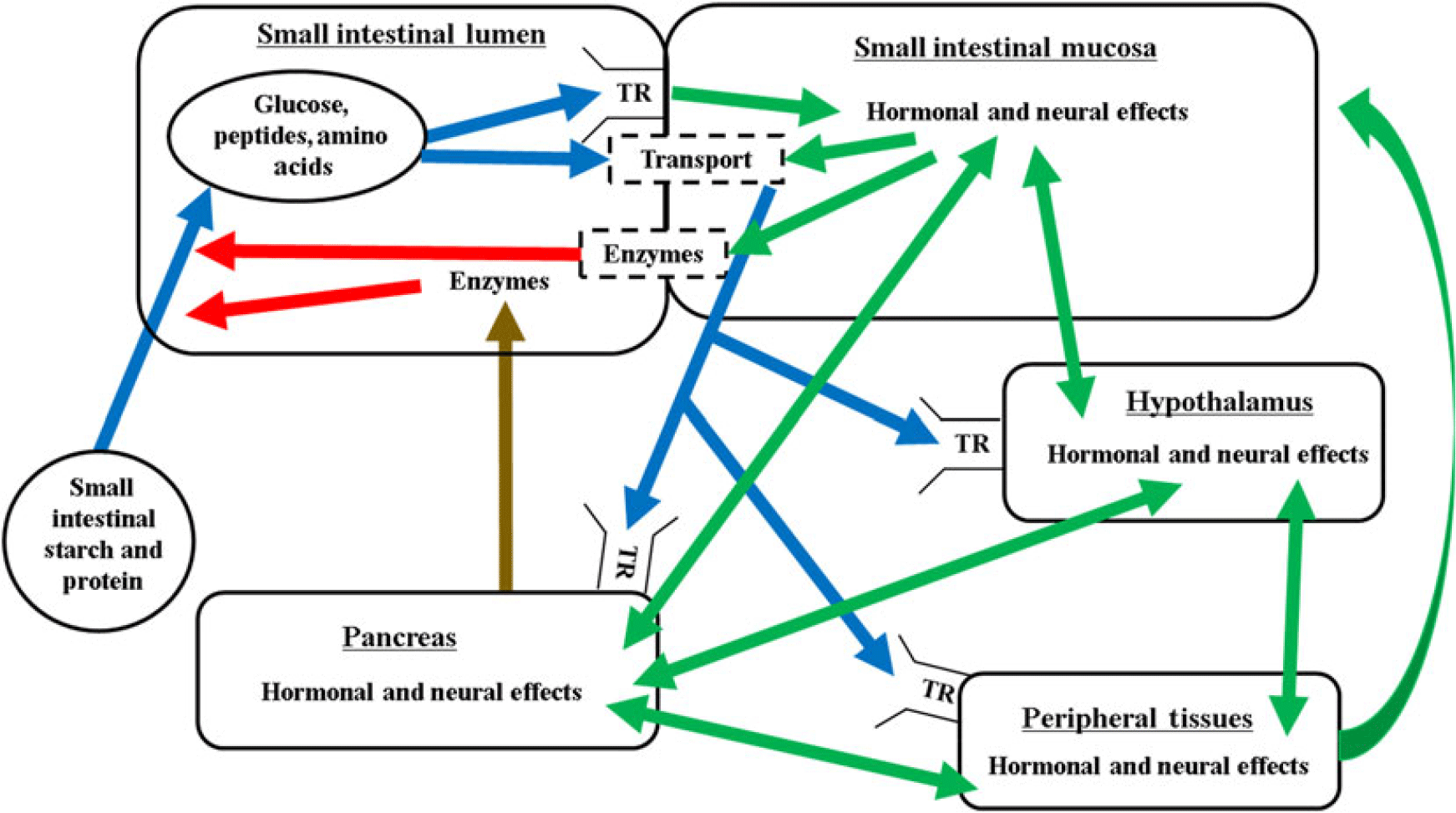
The mechanisms regulating the nutritional modulation of digestive function in the small intestine are complex and coordinated via the substrate, neural and hormonal effects in the small intestine, pancreas, peripheral tissues and the pituitary–hypothalamic axis (Figure 2). The overall regulation is also closely linked with factors regulating feed intake, glucose and amino acid metabolism, and energy balance.

Figure 2 Proposed interrelationships of factors controlling digestion and absorption in ruminants. Blue lines represent nutrient flow, green lines represent hormonal and neural signalling, brown line represents secretion through the pancreatic duct, red lines represent digestive enzyme activity, dashed boxes indicate brush border. Enzymes = pancreatic and brush border carbohydrases and proteases; TR = taste receptor; transport = glucose or amino acid/peptide transporter.
The small intestine plays a vital role in the sensing, digestion and absorption of nutrients. Nutrients are an essential signal for the release of gut peptides (Bauer et al., Reference Bauer, Hamr and Duca2016) within the small intestine and absorbed nutrients can have intestinal, pancreatic or other peripheral effects in mediating dietary effects on pancreatic exocrine function (Call et al., Reference Call, Mitchell and Little1975; Blouet and Schwartz, Reference Blouet and Schwartz2010).
The importance of taste receptors on nutrient sensing in the digestive tract and other tissues in animals and humans is becoming more apparent (Moran et al., Reference Moran, Al-Rammahi, Zhang, Bravo, Calsamiglia and Shirazi-Beechey2014; Lushchak et al., Reference Lushchak, Strilbytska, Yurkevych, Vaiserman and Storey2019). Taste receptors for sweet and umami (T1R), bitter (T2R) and salty (ENaC) have been described in vertebrate animals (Bachmanov et al., Reference Bachmanov, Bosak, Lin, Matsumoto, Ohmoto, Reed and Nelson2014) with much of the research conducted using laboratory animals. Initially, the taste receptors were identified not only in the oral cavity but also in many metabolically active tissues in the body including the small intestine (Kochem, Reference Kochem2017). In the small intestine, the taste receptors are primarily concentrated in the enteroendocrine cells (Herzig et al., Reference Herzig, Louie and Owyang1994; Lee and Owyang, Reference Lee and Owyang2017). The gastrointestinal hormones that are secreted from the neuroendocrine cells containing taste receptors in response to stimulation of the taste receptors include secretin (S-cells), CCK (I-cells), ghrelin (X/A-like cells), GIP (K-cells), and peptide YY, glucagon peptide 1 and glucagon peptide 2 (GLP-2; L-cells) (Calvo and Egan, Reference Calvo and Egan2015). There is also a recent evidence suggesting that multiple gastrointestinal regulatory proteins can be co-localized within the enteroendocrine cells of the small intestine (Fothergill and Furness, Reference Fothergill and Furness2018). The primary functions of the gastrointestinal hormones are to regulate feed intake, feed digestion and whole animal metabolism (Gribble and Reimann, Reference Gribble and Reimann2017; Fothergill and Furness, Reference Fothergill and Furness2018).
Cholecystokinin and secretin have long thought to be key regulators of pancreatic exocrine function (Miyasaka and Funakoshi, Reference Miyasaka and Funakoshi1998; Chey and Chang, Reference Chey and Chang2014) with CCK thought to have primary effects on enzyme secretion and secretin on the buffer and fluid secretion. These effects may be mediated by stimulating neural effects in the small intestine that regulate pancreatic exocrine function or directly on the pancreas via CCK receptors (Bourassa et al., Reference Bourassa, Laine, Kruse, Gagnon, Calvo and Morisset1999). In pigs, this effect is likely mediated at the intestinal level via CCK receptors located in the duodenum which activate neural signals to increase pancreatic enzyme secretion (Evilevitch et al., Reference Evilevitch, Westrom and Pierzynowski2004). Less is known in ruminants. However, incubation of pancreatic tissue explants with caerulein, a CCK mimic, was shown to increase α-amylase release in bovine pancreas from steers previously abomasally infused with casein or when incubated with amino acids (Swanson et al., Reference Swanson, Matthews, Woods and Harmon2003). The role of other gut peptides on pancreatic function is less well defined, especially in ruminants.
The primary nutrients that activate taste receptors in the small intestine are likely amino acids (Bachmanov et al., Reference Bachmanov, Bosak, Glendinning, Inoue, Li, Manita, McCaughey, Murata, Reed, Tordoff and Beauchamp2016) and monosaccharides (Moran et al., Reference Moran, Al-Rammahi, Zhang, Bravo, Calsamiglia and Shirazi-Beechey2014). For example, recent research suggests that the positive effect of increased luminal glucose has on SGLT1 expression is mediated through neuroendocrine cells producing GLP-2 (Moran et al., Reference Moran, Al-Rammahi, Batchelor, Bravo and Shirazi-Beechey2018). Similarly, amino acids have been shown to elicit CCK secretion via taste receptor activation in mice (Daly et al., Reference Daly, Al-Rammahi, Moran, Marcello, Ninomiya and Shirazi-Beechey2013). The physiological effects of taste receptors in the gastrointestinal tract are less well understood in ruminants. However, it has been shown that ruminants do express taste receptors in the small intestine and the artificial sweetener, Sucram, increases SGLT1 mRNA abundance, Na+-dependent glucose uptake, maltase activity, and villus height and crypt depth in the small intestine in lambs and calves (Moran et al., Reference Moran, Al-Rammahi, Zhang, Bravo, Calsamiglia and Shirazi-Beechey2014). Although it seems that the small intestine in ruminants responds to increased glucose supply by increasing mass and carbohydrase activity, in past research from our laboratory, post-ruminal infusion of glucose decreased α-amylase secretion in steers (Swanson et al., Reference Swanson, Richards and Harmon2002b) suggesting a complex and perhaps uncoordinated regulation between intestinal and pancreatic responses related to the adaptation of post-ruminal starch digestive function.
Insulin also has long been implicated as an important regulator of pancreatic exocrine function (Brannon, Reference Brannon1990). Diabetic sheep have decreased α-amylase and lipase secretion (Pierzynowski and Barej, Reference Pierzynowski and Barej1984) suggesting a role for insulin in regulating exocrine pancreatic function in ruminants. Also, an insulin-dependent element has been identified in the α-amylase gene in mice (Keller et al., Reference Keller, Rosenberg, Johnson, Howard and Meisler1990) suggesting a direct role for insulin in regulating pancreatic exocrine function.
The hypothalamus is critical in sensing whole body signals (substrate, hormonal and neural) related to nutrient and energy balance and coordinating whole body responses to stimuli (Blouet and Schwartz, Reference Blouet and Schwartz2010) including factors related to feed intake, digestion and glucose homeostasis. There is also a strong evidence suggesting the importance of the brain–gut axis in regulating pancreatic secretion (Konturek et al., Reference Konturek, Pepera, Zabielski, Konturek, Pawlik, Szlachcic and Hahn2003; Jaworek et al., Reference Jaworek, Nawrot-Porabka, Leja-Szpak and Konturek2010). Interestingly, sweet/amino acid receptors also are located in the hypothalamus (Heeley and Blouet, Reference Heeley and Blouet2016; Kohno, Reference Kohno2017) which likely are important in sensing systemic glucose and amino acid concentrations and along with neural and hormonal signals are sensed by the hypothalamus which allows for coordinated central control of metabolism, including intestinal and pancreatic function. Other hormones thought to influence pancreatic exocrine and intestinal function either directly or through neural signals include melatonin, C-natriuretic peptide, endocannabinoids and leptin to name a few (Chandra and Liddle, Reference Chandra and Liddle2009). More research is needed in ruminants to help unravel the complexities by which small intestinal digestion is regulated with the aim of developing approaches to enhance and improve the efficiency of small intestinal digestion in ruminants.
Acknowledgements
The authors thank Luiz Brito and Pablo Fanseca for helping with data search.
D. L. Harmon 0000-0001-5187-1920
Declaration of interest
Both authors declare no conflict of interest and nor competing interest.
Ethics statement
None.
Software and data repository resources
None of the data were deposited in an official repository.