Introduction
According to the World Health Organization (WHO), hypertension, a prevalent non-communicable disease worldwide, affects approximately 1.28 billion people aged 30−79 years (World Health Organization, 2023). Although preventable, this condition remains a significant cause of morbidity and mortality, particularly in older populations (Suol et al., Reference Suol, Yen, Thanh, Hao and Thang2023). Although controlling blood pressure (BP) is encouraged in hypertensive patients, one of every five patients continues to experience uncontrolled BP even with treatment (World Health Organization, 2023).
The prevalence of musculoskeletal pain is 40−60% in older populations (Li et al., Reference Li, Lin, Lu, Chung and Cheng2022; Welsh et al., Reference Welsh, Yang and Makris2020) and contributes to increased disability (Rundell et al., Reference Rundell, Patel, Krook, Heagerty, Suri, Friedly, Turner, Deyo, Bauer, Nerenz, Avins, Nedeljkovic and Jarvik2019), psychosocial impairment (Karttunen et al., Reference Karttunen, Lihavainen, Sipilä, Rantanen, Sulkava and Hartikainen2012), and sleep disturbances (Chen et al., Reference Chen, Hayman, Shmerling, Bean and Leveille2011). Chronic musculoskeletal pain coexists with other chronic diseases, including hypertension (Bae et al., Reference Bae, Shin, Lee, Kim, Park, Cho and Ha2015; Saccò et al., Reference Saccò, Meschi, Regolisti, Detrenis, Bianchi, Bertorelli, Pioli, Magnano, Spagnoli, Giuri, Fiaccadori and Caiazza2013). Approximately 31.3%–39% of individuals with musculoskeletal pain reportedly have hypertension, higher than individuals with no pain (21−25.8%) both in clinical settings and the general community (Bruehl et al., Reference Bruehl, Chung, Jirjis and Biridepalli2005; Giummarra et al., Reference Giummarra, Tardif, Blanchard, Tonkin and Arnold2020). Recent literature suggests a relationship between hypertension and pain (Alenazi & Alkhathami, Reference Alenazi and Alkhathami2023) due to decreased regulation of baroreflex activity (Saccò et al., Reference Saccò, Meschi, Regolisti, Detrenis, Bianchi, Bertorelli, Pioli, Magnano, Spagnoli, Giuri, Fiaccadori and Caiazza2013). Although chronic pain may cause high BP (Bruehl et al., Reference Bruehl, Olsen, Tronstad, Sevre, Burns, Schirmer, Nielsen, Stubhaug and Rosseland2018), most hypertension treatment guidelines focus on antihypertensive drugs and lifestyle modifications, focusing less on pain (Whelton et al., Reference Whelton, Carey, Aronow, Casey, Collins, Dennison Himmelfarb, DePalma, Gidding, Jamerson, Jones, MacLaughlin, Muntner, Ovbiagele, Smith, Spencer, Stafford, Taler, Thomas, Williams, Williamson and Wright2018). Therefore, evidence regarding the impact of pain treatment strategies on BP control in hypertensive patients is lacking.
Discussing pain management in primary care is challenging because of conflicting evidence regarding the management of multiple comorbidities (Krein et al., Reference Krein, Heisler, Piette, Butchart and Kerr2007). However, the WHO proposed a standard recommendation for pain medication prescriptions, popularly known as the ‘WHO analgesic ladder’, an evidence-based framework recognized in pain management (Anekar, Hendrix, and Cascella, Reference Anekar, Hendrix and Cascella2023). The analgesic ladder has demonstrated effectiveness and widespread usefulness in musculoskeletal disorders because it emphasizes individualized treatment plans based on pain intensity (El-Tallawy et al., Reference El-Tallawy, Nalamasu, Salem, LeQuang, Pergolizzi and Christo2021). Previous studies have established that inappropriate pain management without regard to the WHO analgesic ladder can lead to persistent pain and unnecessary side effects (Ussai et al., Reference Ussai, Miceli, Pisa, Bednarova, Giordano, Della Rocca and Petelin2015), which highlighted the importance of this guideline for pain management in hypertensive patients in primary care. Therefore, this study aimed to investigate the impact of applying the WHO analgesic ladder on resting pain score, pain severity, pain interference, and BP control in treated hypertensive patients with chronic musculoskeletal pain. The findings of this study may reveal the pain management patterns that can simultaneously control pain and BP in this population.
Methods
Study design and setting
This cross-sectional study was conducted at the Primary Care Unit, the General Practitioner Outpatient Clinic, the Orthopaedics Clinic, and the Physical Therapy Clinic of Songklanagarind Hospital, Thailand, from 1 February to 31 May 2023. The study was advertised in the hospital area. The research protocol was approved by the Faculty of Medicine, Prince of Songkla University, Thailand (Code: REC.65-489-30-2). All patients provided written informed consent before enrolling in the study. Purposive sampling was used to select 210 participants who met the eligibility criteria. The inclusion criteria were as follows: (1) age ≥ 40 years, (2) diagnosis with primary hypertension for at least 3 months, (3) good compliance with hypertensive treatment, defined as regular intake of antihypertensive drugs without any treatment lapses in the previous 7 days, and (4) experiencing musculoskeletal pain lasting at least 3 months. The exclusion criteria were as follows: (1) experiencing pain from other sources within 24 h of data collection, such as headache or abdominal pain, (2) active cancer at any bodily site, (3) fractures in the area of pain location, (4) being bedridden, and (5) neurological deficits that affect walking ability.
The sample size was calculated with the n4studies application based on previous research focusing on the association between pain and BP using two independent proportions (Bruehl et al., Reference Bruehl, Chung, Jirjis and Biridepalli2005). The proportion of hypertensive patients experiencing pain was set at 0.10, whereas those without pain were estimated at 0.26. The significance level (α error probability) was established at 0.05, and the β-error probability was set as 0.20. A sample size of 210 participants was subsequently determined.
Measurements and outcomes
BP measurements were performed by a trained physical therapist in a separate room using an autonomic BP monitor (TM-2657P; A&D Co. Ltd., Tokyo, Japan). The device was validated according to the British and Irish Hypertension Society standards (Chu et al., Reference Chu, Dong, Wong and Lee2023). After resting in a sitting position for at least 10 min, measurements were taken thrice at 1-min intervals. The mean of the second and third values was used for data analysis. This BP assessment protocol was used in several settings in previous studies. The criteria for uncontrolled BP were systolic BP of ≥ 140 mmHg and/or diastolic BP of ≥ 90 mmHg) (Olsen et al., Reference Olsen, Bruehl, Nielsen, Rosseland, Eggen and Stubhaug2013; Wieberdink et al., Reference Wieberdink, Ikram, Hofman, Koudstaal and Breteler2012).
Pain measurements were conducted using two tools for assessing the degree of pain through face-to-face interviews. Both pain assessments were administered to participants on the day of data collection and after at least 3 months of prescribed pain treatment. The first tool was the numerical pain rating scale (NPRS) to assess resting pain. Participants were asked to rate their resting pain on a scale of 0–10, with 0 denoting no pain and 10 representing the most imaginable pain. The second tool was the Thai version of the Brief Pain Inventory Short Form (BPI-SF), which comprises two subscales: pain severity and pain interference. The pain severity subscale score was derived from the mean of the participants’ current, worst, average, and least pain, experienced within a 24-h period. The pain interference subscale assesses seven functional aspects that pain may interfere with general activity, mood, walking ability, normal work, relationships with others, sleep, and enjoyment of life. The scores for each aspect range from 0 to 10, with 0 indicating no interference and 10 indicating complete interference due to pain. The mean scores across the seven aspects were calculated to measure overall pain interference (Komolsuradej et al., Reference Komolsuradej, Srikrajang, Rangsiprakarn, Wongaree, Wasusathapon, Kanjanakomut, Nukaew, Cheutalay, Kaewpipat, Hoonthong, Ung and Iamthanaporn2023; Poquet & Lin, Reference Poquet and Lin2016).
Pain management data encompassing pain treatment prescriptions were extracted from the Hospital Information System (HIS), encompassing pain treatment prescriptions. The participants were categorized into three groups based on their pain management regimens following the WHO analgesic ladder, which comprises three hierarchical steps, each corresponding to the level of pain severity.
Step 1 involved the prescription of non-opioid analgesics, such as non-steroidal anti-inflammatory drugs (NSAIDs) and acetaminophen, for managing mild pain (NPRS score 1−4). Step 2 involved the administration of weak opioids, such as codeine or tramadol, in combination with non-opioid analgesics targeting moderate pain (NPRS score 5−6). Step 3 encompassed the prescription of strong opioids, such as morphine or fentanyl, for cases of severe pain (NPRS score 7−10) that remained uncontrolled despite weaker opioids (Hirschfeld & Zernikow, Reference Hirschfeld and Zernikow2013; van Dijk et al., Reference van Dijk, Kappen, van Wijck, Kalkman and Schuurmans2012). In this study, ‘no treatment’ referred to not having received pain treatment in the previous month. ‘Partial treatment’ indicated pharmacological pain treatment below the recommended WHO analgesic ladder, whereas ‘complete treatment’ indicated pain treatment following the WHO analgesic ladder recommendation.
Procedure
The study was conducted using face-to-face interviews and retrospective data retrieved from the HIS. Participant information on general characteristics was collected using a standard questionnaire. Subsequently, the BPI-SF was used for pain evaluation, and BP was measured thrice. Finally, the history of pain treatment and drug prescriptions (type, time of consumption, and dose) within the previous 1 month was collected. The study flowchart is shown in Figure 1.

Figure 1. Flowchart of the hypertensive patients with chronic musculoskeletal pain participated in the study (n = 210).
Statistical analysis
Data management was performed using R Studio Version 3.3.0 (Public Benefit Corporation, USA, 2009). The median, mean, interquartile range, standard deviation (SD), and percentage were calculated for descriptive analyses. The differences in pain characteristics, BP level, and associated factors among three pain management groups were evaluated using the Kruskal−Wallis test and analysis of variance F-test to compare continuous variables, whereas the chi-square and Fisher’s exact test were used to compare categorical variables. Multivariate logistic regression analysis (MLRA) was used to analyse the association between the variables of interest and uncontrolled BP. The significance level was set at P < 0.05.
Results
Overall, 289 patients with hypertension were screened, and 210 had uncontrolled BP (60.48%). Of the participants, 62.4% (n = 131) were male. Most were older adults, aged > 60 years (79.5%), and all had at least one comorbidity (Table 1). There was a significant difference in the resting pain scores, pain severity subscale score, and pain interference subscale score among the pain management groups. Pain measurements reflected pain characteristics following a minimum 3-month period of pain management. Across all pain aspects (resting pain, pain severity, and pain interference), the partial treatment group consistently exhibited higher pain levels, compared with the other groups. We found that pain interference was the most common pain characteristic, reflecting lower pain in the complete treatment group, compared with partial treatment and no treatment group (P = 0.044). The following pain interference items had the highest mean (SD): general activity (4.56 [3.01]), normal work (work outside the home and household chores) (4.00 [3.23]), and walking ability (3.53 [3.37]). Overall, 78.6% (n = 105) of participants received pain treatment, with most receiving partial treatment (63.4%) (Table 2).
Table 1. Characteristics of participants according to pain management patterns according to WHO analgesic ladder (n = 210)

Abbreviations: IQR, interquartile range; BMI, body mass index; DM, diabetes mellitus; CKD, chronic kidney disease; CVA, cerebral vascular accident; DLP, dyslipidaemia.
Table 2. Differences between pain characteristics (resting pain scale, pain severity subscale, and pain interference subscale) and BP among hypertensive patients (n = 210)
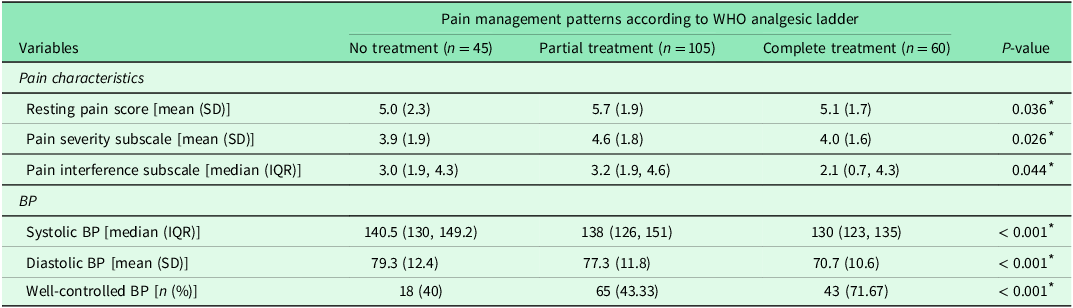
* Statistically significant at P < 0.05 (analysed using the Kruskal-Wallis test or ANOVA F-test); SD, standard deviation; IQR, interquartile range.
According to the pain medication prescriptions, the most frequently prescribed NSAIDs were celecoxib, meloxicam, and etoricoxib. The most prescribed adjuvants comprised analgesic creams, anticonvulsants (gabapentin), and muscle relaxants (tolperisone) (Figure 2).

Figure 2. Pain medication prescriptions in patients receiving partial treatment following the WHO analgesic ladder with mild, moderate, and severe pain levels (n = 105) (NSAIDS, non-steroidal anti-inflammatory drugs).
After identifying potential confounding variables, including age, sex, body mass index, underlying disease, hypertension treatment duration, and pain duration (Model 1 of three MLRA models), both the resting pain score and pain management were significantly associated with uncontrolled BP. The partial treatment group had the highest odds ratio for increased risk of uncontrolled BP (P < 0.001). However, variables such as pain severity, pain interference, area of pain, and treatment efficacy were not significantly associated with uncontrolled BP (P > 0.05) after conducting MLRA (Table 3).
Table 3. Multivariable logistic regression model for pain-related factors associated with uncontrolled BP in hypertensive patients with chronic pain (n = 210)

* Statistically significant; CI, confidence interval; OR, odds ratio; LR, likelihood ratio.
Discussion
In this study, we found that adhering to the WHO analgesic ladder guidelines for pain treatment was associated with reduced pain severity, pain interference, and improved BP control. These findings demonstrate the benefits of the WHO analgesic ladder for controlling pain and BP in hypertensive patients with chronic musculoskeletal pain, particularly in older adults.
Our study findings suggest a positive association between pain and uncontrolled BP, which aligns with the result of a larger cohort study (n = 43, 789) that showed that hypertension was associated with pain severity (Giummarra et al., Reference Giummarra, Tardif, Blanchard, Tonkin and Arnold2020). Chronic musculoskeletal pain can cause various health problems that can be associated with uncontrolled BP, such as mood disorder and anxiety (Mazza et al., Reference Mazza, Ravenni, Armigliato, Rossetti, Schiavon, Fiorini, Rigatelli, Ramazzina and Casiglia2016), sleep disturbance (Makarem et al., Reference Makarem, Alcántara, Williams, Bello and Abdalla2021), decreased physical activity and movement limitation due to pain interference (Karayannis et al., Reference Karayannis, Sturgeon, Chih-Kao, Cooley and Mackey2017) and lack of exercise adherence (Collado-Mateo et al., Reference Collado-Mateo, Lavín-Pérez, Peñacoba, Del Coso, Leyton-Román, Luque-Casado, Gasque, Fernández-Del-Olmo and Amado-Alonso2021). Particularly, we found that comprehensive pain control can lower pain interference in hypertensive patients. The result of the three most occurring pain interference in this study, pain interference with general activity, working, and walking abilities, suggests that chronic musculoskeletal pain decreased physical activity and movement in participants’ daily lives in this cohort. Several studies have also reported that reduced physical activity is associated with poorly controlled BP in treated hypertensive patients (Cherfan et al., Reference Cherfan, Vallée, Kab, Salameh, Goldberg, Zins and Blacher2020; Solomon et al., Reference Solomon, Negussie, Bekele, Getahun and Gurara2023; Zhang et al., Reference Zhang, Xu, Cai, Zheng, Zheng and Ni2023). The pathophysiological mechanism between chronic musculoskeletal pain and hypertension can be attributed to physiological changes in the endogenous and cardiovascular pain regulatory systems (Olsen et al., Reference Olsen, Bruehl, Nielsen, Rosseland, Eggen and Stubhaug2013; Saccò et al., Reference Saccò, Meschi, Regolisti, Detrenis, Bianchi, Bertorelli, Pioli, Magnano, Spagnoli, Giuri, Fiaccadori and Caiazza2013). Even in acute pain, typically, the BP rises due to the autonomic response. However, chronic pain conditions often cause an opposite effect, resulting in a diminished baroreceptor response, possibly due to reduced vagal inhibitory activity (baroreceptor desensitization) (Bruehl et al., Reference Bruehl, Chung, Ward, Johnson and McCubbin2002; Bruehl et al., Reference Bruehl, Olsen, Tronstad, Sevre, Burns, Schirmer, Nielsen, Stubhaug and Rosseland2018). This failure in the baroreceptor homeostatic control mechanisms can lead to persistent BP elevation. Additionally, chronic pain can disrupt descending inhibitory pathways while facilitating nociceptive information transmission (Kosek & Ordeberg, Reference Kosek and Ordeberg2000). This dual effect may intensify pain sensitivity and exacerbate BP elevation (Bruehl et al., Reference Bruehl, Olsen, Tronstad, Sevre, Burns, Schirmer, Nielsen, Stubhaug and Rosseland2018; Rivasi et al., Reference Rivasi, Menale, Turrin, Coscarelli, Giordano and Ungar2022).
A significant association was observed between pain management patterns and uncontrolled BP in this study (P < 0.001). Notably, the complete treatment group exhibited a higher rate of well-controlled BP and significantly lower resting pain (P = 0.036), pain severity subscale (P = 0.026), and pain interference subscale (P = 0.044) scores than did the partial treatment group. Although theoretically supporting the possible physiological effect of pain and uncontrolled BP, a few clinical studies have demonstrated the evidence of this relationship. Among them, this study demonstrates the benefits of comprehensive pain management in positively improving BP control and alleviating pain, particularly pain interference. Studies have shown that pain interference is an important measurement due to its strong association with lower quality of life (Smith, Richardson, and Cowan, Reference Smith, Richardson and Cowan2023), low conscientiousness (Judge, Meyr, and Segerstrom, Reference Judge, Meyr and Segerstrom2021), and abnormal nutritional status in older adults (Komolsuradej et al., Reference Komolsuradej, Srikrajang, Rangsiprakarn, Wongaree, Wasusathapon, Kanjanakomut, Nukaew, Cheutalay, Kaewpipat, Hoonthong, Ung and Iamthanaporn2023). Therefore, the lowest interference in the complete treatment group in this study may represent greater effects of pain control and can lead to reduced BP. This can be supported by experimental studies by Bruehl et al. that demonstrated a positive correlation of mean resting BP with pain severity level in patients with chronic benign pain (Bruehl, Burns, and McCubbin, Reference Bruehl, Burns and McCubbin1998) and chronic low back pain (Bruehl et al., Reference Bruehl, Chung, Ward, Johnson and McCubbin2002).
Regarding the drug responses, most patients in the complete treatment group experienced moderate pain. According to the WHO analgesic ladder recommendations (Figure 3), this group should receive weak opioids combined with non-opioid analgesics. Due to the potent histamine-mediated vasodilation effects of opioids and a hypersensitivity to opioid receptor agonists in hypertensive patients (Baldo and Pham, Reference Baldo and Pham2012), it could be speculated that opioids have a hypotensive effect (Zelis et al., Reference Zelis, Mansour, Capone and Mason1974), resulting in a decrease in BP in patients receiving opioids due to histamine-mediated vasodilation, which alleviates hypotension (Chen and Ashburn, Reference Chen and Ashburn2015). Furthermore, the hypotensive effects might be more significant in hypertensive patients due to their heightened sensitivity to opioid receptor agonists (Cozzolino et al., Reference Cozzolino, Sasso, Cataldo, Gruosso, Giammarco, Cavalli, Di Maggio, Renzo, Salvatore, Giugliano and Torella2005). In contrast, the most prevalent drug prescribed in the partial treatment group was NSAIDs combined with adjuvants. This treatment pattern can be attributed to increased BP because there is evidence that NSAIDs have the potential to increase BP by inhibiting prostaglandin synthesis and promoting the production of endothelin I (Snowden & Nelson, Reference Snowden and Nelson2011), ultimately leading to increased secretion of aldosterone. This results in higher water retention with the sodium and calcium components (Morgan & Anderson, Reference Morgan and Anderson2003), which may increase BP. Notably, the opioids tended not to be prescribed in this group, even for moderate pain. This may be explained by the physicians’ intention to avoid potential adverse effects in older patients (Baldo & Pham, Reference Baldo and Pham2012; Krantz et al., Reference Krantz, Palmer and Haigney2021). Moreover, 25.9–31.2% in the partial treatment group received adjuvant analgesics only, which may cause inadequate pain control and uncontrolled BP. However, we found a very low rate of non-pharmacological treatment prescriptions from physicians for pain control (5–10%), which correspond with a previous study that revealed low prevalence of non-pharmacological pain management in a specialized hospital due to factors such as limited access to pain assessment tools, favourable attitude, and age of the attending healthcare personnel (Tsegaye et al., Reference Tsegaye, Yazew, Gedfew, Yilak and Yalew2023)
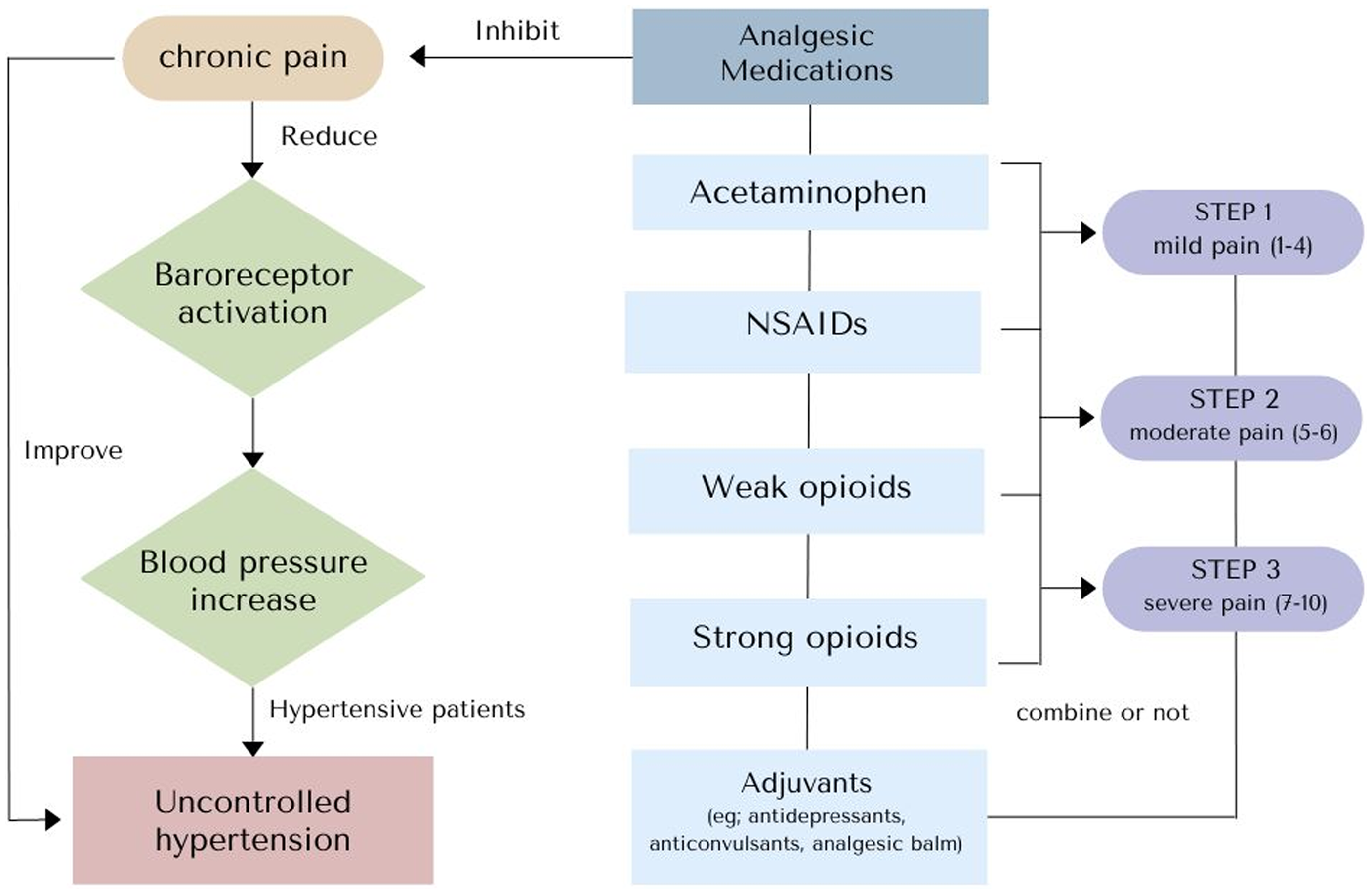
Figure 3. Effects of chronic pain and analgesic medications on blood pressure regulation (NSAIDS, non-steroidal anti-inflammatory drugs).
In managing hypertension in general practice, the latest treatment guideline, the European Society of Hypertension (ESH) guidelines (Mancia et al., Reference Mancia, Kreutz, Brunström, Burnier, Grassi, Januszewicz, Muiesan, Tsioufis, Agabiti-Rosei, Algharably, Azizi, Benetos, Borghi, Hitij, Cifkova, Coca, Cornelissen, Cruickshank, Cunha, Danser, Pinho, Delles, Dominiczak, Dorobantu, Doumas, Fernández-Alfonso, Halimi, Járai, Jelaković, Jordan, Kuznetsova, Laurent, Lovic, Lurbe, Mahfoud, Manolis, Miglinas, Narkiewicz, Niiranen, Palatini, Parati, Pathak, Persu, Polonia, Redon, Sarafidis, Schmieder, Spronck, Stabouli, Stergiou, Taddei, Thomopoulos, Tomaszewski, Van de Borne, Wanner, Weber, Williams, Zhang and Kjeldsen2023), primarily focus on drug therapy and lifestyle modifications. Although there is growing recognition of the importance of pain control in hypertension management, current guidelines only specifically mention two diseases, gouty arthritis and rheumatoid arthritis, due to their association with cardiovascular risk. Interestingly, the guideline stated that treatment for these conditions, involving NSAIDs and glucocorticoids, can potentially interfere with hypertension treatment and lead to uncontrolled hypertension (Kremer et al., Reference Kremer, Bloom, Breedveld, Coombs, Fletcher, Gruben, Krishnaswami, Burgos-Vargas, Wilkinson, Zerbini and Zwillich2009), as observed in our study where the high-dose NSAID group showed a higher incidence of uncontrolled hypertension.
The ESH 2023 guidelines emphasize the importance of managing simultaneous BP control and alleviating pain and inflammation but caution against excessive NSAID use (Hansildaar et al., Reference Hansildaar, Vedder, Baniaamam, Tausche, Gerritsen and Nurmohamed2021). However, notably, previous guidelines, including the ESH 2017 and the American College of Cardiology and American Heart Association guidelines 2018, did not specify hypertension treatment in cases of chronic pain or musculoskeletal pain. Hence, there is an emerging trend suggesting that future treatment approaches should integrate both BP control and pain management (Williams et al., Reference Williams, Mancia, Spiering, Agabiti Rosei, Azizi, Burnier, Clement, Coca, de Simone, Dominiczak, Kahan, Mahfoud, Redon, Ruilope, Zanchetti, Kerins, Kjeldsen, Kreutz, Laurent, Lip, McManus, Narkiewicz, Ruschitzka, Schmieder, Shlyakhto, Tsioufis, Aboyans, Desormais, De Backer, Heagerty, Agewall, Bochud, Borghi, Boutouyrie, Brguljan, Bueno, Caiani, Carlberg, Chapman, Cífková, Cleland, Collet, Coman, de Leeuw, Delgado, Dendale, Diener, Dorobantu, Fagard, Farsang, Ferrini, Graham, Grassi, Haller, Hobbs, Jelakovic, Jennings, Katus, Kroon, Leclercq, Lovic, Lurbe, Manolis, McDonagh, Messerli, Muiesan, Nixdorff, Olsen, Parati, Perk, Piepoli, Polonia, Ponikowski, Richter, Rimoldi, Roffi, Sattar, Seferovic, Simpson, Sousa-Uva, Stanton, van de Borne, Vardas, Volpe, Wassmann, Windecker, Zamorano, Windecker, Aboyans, Agewall, Barbato, Bueno, Coca, Collet, Coman, Dean, Delgado, Fitzsimons, Gaemperli, Hindricks, Iung, Jüni, Katus, Knuuti, Lancellotti, Leclercq, McDonagh, Piepoli, Ponikowski, Richter, Roffi, Shlyakhto, Simpson, Sousa-Uva, Zamorano, Tsioufis, Lurbe, Kreutz, Bochud, Rosei, Jelakovic, Azizi, Januszewics, Kahan, Polonia, van de Borne, Williams, Borghi, Mancia, Parati, Clement, Coca, Manolis, Lovic, Benkhedda, Zelveian, Siostrzonek, Najafov, Pavlova, De Pauw, Dizdarevic-Hudic, Raev, Karpettas, Linhart, Olsen, Shaker, Viigimaa, Metsärinne, Vavlukis, Halimi, Pagava, Schunkert, Thomopoulos, Páll, Andersen, Shechter, Mercuro, Bajraktari, Romanova, Trušinskis, Saade, Sakalyte, Noppe, DeMarco, Caraus, Wittekoek, Aksnes, Jankowski, Polonia, Vinereanu, Baranova, Foscoli, Dikic, Filipova, Fras, Bertomeu-Martínez, Carlberg, Burkard, Sdiri, Aydogdu, Sirenko, Brady, Weber, Lazareva, Backer, Sokolovic, Jelakovic, Widimsky, Viigimaa, Pörsti, Denolle, Krämer, Stergiou, Parati, Trušinskis, Miglinas, Gerdts, Tykarski, de Carvalho Rodrigues, Dorobantu, Chazova, Lovic, Filipova, Brguljan, Segura, Gottsäter, Pechère-Bertschi, Erdine, Sirenko and Brady2018). This holistic approach potentially influences future treatment strategies in general practice for BP control in treated hypertensive patients who have chronic musculoskeletal pain, particularly in older adults.
Strengths and limitations
This is the first study to investigate the benefit of the WHO analgesic ladder in controlling pain severity, pain interference, and BP in hypertensive patients with chronic musculoskeletal pain. This study has some limitations. First, we were unable to compare changes in pain over the time course of medication consumption because the pain severity at the pre-visit used to determine treatment was assessed by a physician, which may have led to inconsistencies when compared with the pain measured by the researcher during the present visit. Second, only 5−10% of physician prescriptions included non-pharmacological therapies (such as physical therapy and acupuncture). As described previously, pain treatment appears beneficial for patients with high BP. Considering that non-pharmacological treatments could be an alternative for physicians to avoid the potential side effects of NSAIDs and opioid analgesics, their use may improve the efficacy of hypertensive treatment. Further studies are required to confirm these observations. Finally, this was an observational study (cross-sectional investigation with retrospective data retrieval), and further prospective studies are needed to determine the long-term effects of each pain medication on BP control.
Conclusions
The study findings support the effectiveness of comprehensive pain treatment following the WHO analgesic ladder in managing pain severity, pain interference, and BP in treated hypertensive patients. These results highlight the importance of incorporating pain control into the management of hypertension in primary care, especially for older individuals experiencing musculoskeletal pain.
Acknowledgements
Author would like to thank Kansupak Chinsunthia for data collection and Associate Professor Rassamee Chotipanvithayakul from Faculty of Medicine, Prince of Songkla University for her valuable advice throughout the study.
Financial statement
This study was supported by research grant from Faculty of Medicine, Prince of Songkla University (grant number MR PSU-653021-146).
Competing interests
None.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional guidelines on human experimentation (please name) and with the Helsinki Declaration of 1975, as revised in 2008. Written (or Verbal) informed consent was obtained from all subjects/patients.









