Primary liver cancer (PLC) is the second leading cause of cancer-related deaths worldwide(Reference Torre, Bray and Siegel1). Despite enormous progress in the modern therapeutic era, it remains a generally incurable disease with 5-year net survival ranging from 5 % to 30 %(Reference Allemani, Matsuda and Di Carlo2). Hepatocellular carcinoma (HCC) is the most common subtype, accounting for approximately 90 % of PLC cases(Reference Llovet, Zucman-Rossi and Pikarsky3). Tumour stage, general health, hepatic function and anticancer therapy are main prognostic indicators for HCC survival(Reference Bruix, Sherman and Llovet4). However, the role of other predictive factors including diet and nutrition in HCC prognosis has been largely unexplored.
Folate, a water-soluble B vitamin, involved in one-carbon metabolism, has been related to cancer development and progression(Reference Newman and Maddocks5). Folate functions as a conveyor of one-carbon units in the formation of nucleotides and methionine, whose deficiency can impair DNA synthesis, repair and methylation and thereby promote carcinogenesis(Reference Lamprecht and Lipkin6). In addition, tumour cells require more folate than normal cells to maintain rapid DNA replication and cell proliferation, and excess folate may enhance tumour growth(Reference Hansen, Jensen and Fuchtbauer7). The liver is responsible for storage and metabolism of folate. Experimental evidence has suggested that dietary methyl deficiency in folate and other one-carbon donors can induce the development of liver cancer through induction of oxidative damage, alteration of lipid metabolism and epigenetic abnormality;( Reference Pogribny, James and Beland 8 , Reference Lai, Chen and Ho 9 ) whereas folic acid supplementation can inhibit angiogenesis during early hepatocarcinogenesis( Reference Chagas, Bassoli and de Souza 10 , Reference Guariento, Furtado and de Conti 11 ), and withdrawal of folate can suppress hepatoma cell growth(Reference Lee, Xu and Chiu12). Three case–control studies have reported an inverse association between circulating folate concentrations and the risk of HCC( Reference Cui, Quan and Piao 13 – Reference Chang, Goldstein and Mu 15 ). A prospective cohort study observed that hepatitis B surface antigen (HBsAg)-positive participants with lower folate concentrations were at increased risk of developing liver damage and HCC(Reference Welzel, Katki and Sakoda16). Another large prospective cohort study also showed a protective effect of higher folate intake on HCC incidence among 494 743 healthy adults who participated in the National Institutes of Health-American Association of Retired Persons (NIH-AARP) Diet and Health Study(Reference Persson, Schwartz and Park17).
Folate status has been linked with cancer mortality in many epidemiological studies( Reference Peng, Dong and Wang 18 – Reference McEligot, Ziogas and Pfeiffer 21 ). Specific to HCC prognosis, however, the only existing evidence found that lower plasma folate concentrations were associated with inferior overall survival (OS) among 160 HCC patients with a median plasma folate concentration of 12·2 ng/ml(Reference Yeh, Goyal and Shen22). Of note, the study was conducted in the USA, where mandatory food fortification with folic acid has been implemented since 1998(Reference Crider, Bailey and Berry23). As we all know, circulating folate concentrations vary substantially across the world due to the difference in diet, lifestyle, food-fortification practices and dietary supplement use(Reference Midttun, Theofylaktopoulou and McCann24). Asians had almost half the circulating folate concentration of Americans (12·4 v. 28·5 nmol/l; to convert to ng/ml, divide by 2·27)(Reference Midttun, Theofylaktopoulou and McCann24), which possibly places Asian populations at increased risk of cancer incidence and mortality. Given that China alone accounts for around 50 % of the total numbers of PLC cases and deaths globally(Reference Torre, Bray and Siegel1), investigation of the influence of folate status on HCC prognosis in Chinese populations represents a high-priority research area.
In the present study, we prospectively examined whether serum folate concentrations at diagnosis were associated with survival outcomes among a large sample size of Chinese patients with newly diagnosed HCC enrolled into the Guangdong Liver Cancer Cohort (GLCC) study.
Subjects and methods
Study population
The GLCC, an ongoing, prospective cohort study, was established in 2013 at Sun Yat-sen University Cancer Center (SYSUCC). It is designed to identify genetic and environmental factors that affect the progression and prognosis of PLC. As previously described(Reference Fang, Chen and Wang25), first incident PLC patients who have not yet initiated cancer treatment were enrolled within 30 d of diagnosis. PLC diagnosis was verified in the SYSUCC Clinical Information Presentation System according to the National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology: Hepatobiliary Cancers (Reference Benson, Abrams and Ben-Josef26). Written informed consent was provided by all participants. The study was approved by the institutional review board of School of Public Health at the Sun Yat-sen University.
Between September 2013 and February 2017, we enrolled 1306 eligible patients into the GLCC. After excluding 324 patients who had no available serum samples for folate measurements, were diagnosed with PLC other than HCC (e.g. intrahepatic cholangiocarcinoma (ICC) and HCC-ICC) or had the Barcelona Clinic Liver Cancer (BCLC) stage D, we finally included 982 patients with newly diagnosed, previously untreated HCC in the present study. Detailed participant selection is shown in Fig. 1. The included patients did not differ by demographics and lifestyle characteristics from the patients excluded from the analysis (Supplementary Table S1). Among the included patients, 59·6 % of the patients were pathologically confirmed.
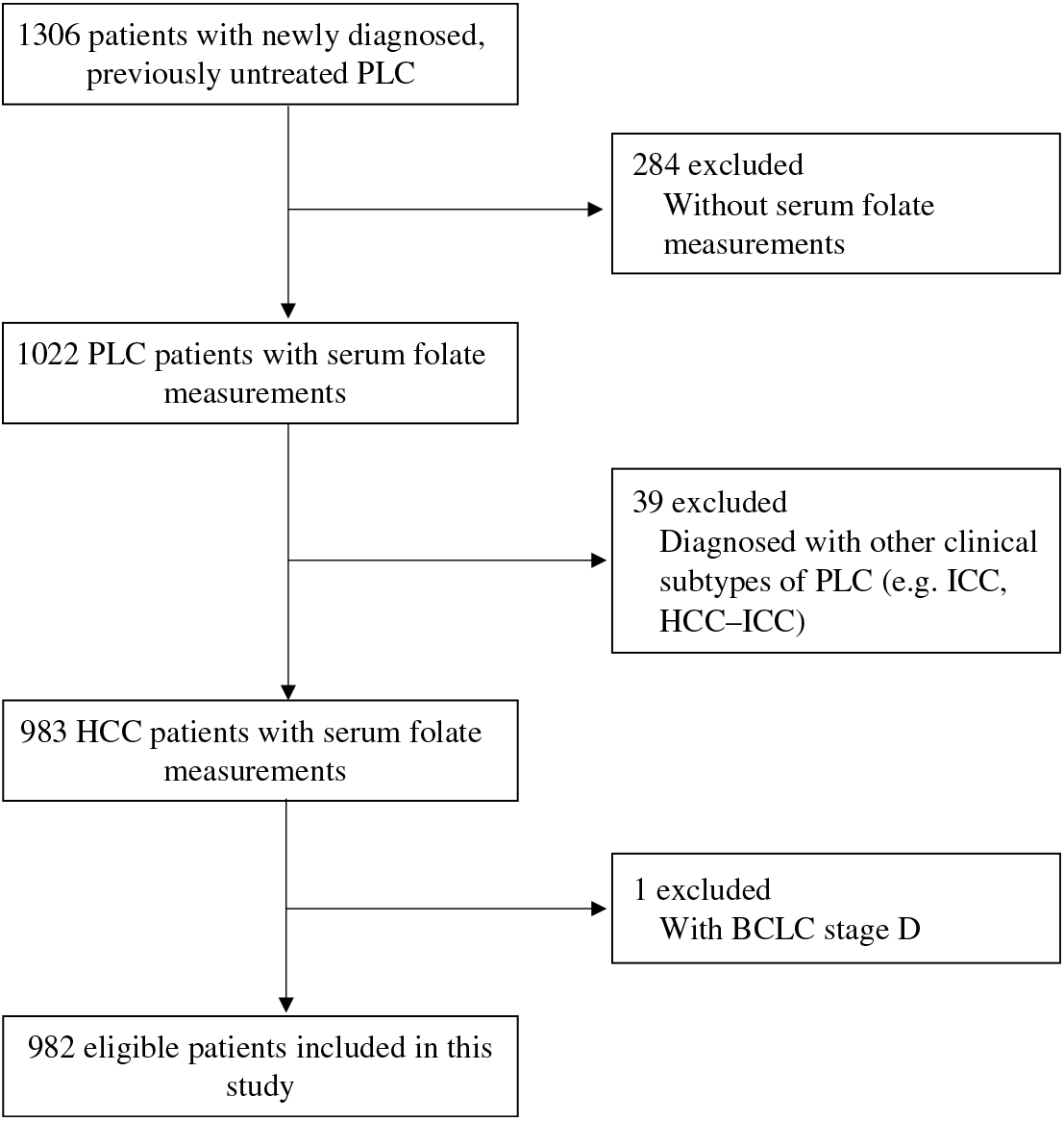
Fig. 1. Flow chart of participant selection from the Guangdong Liver Cancer Cohort study. PLC, primary liver cancer; HCC, hepatocellular carcinoma; ICC, intrahepatic cholangiocarcinoma; BCLC, Barcelona Clinic Liver Cancer.
Laboratory analyses
Peripheral venous blood was drawn after overnight fasting prior to anticancer therapy. Serum samples were separated into aliquots, and stored in −80°C freezers. Serum folate measurements were batched and made using a chemiluminescent microparticle immunoassay (ARCHITECT Folate assay, Abbott Diagnostics) at the KingMed Diagnostics Laboratory (Guangzhou, China). All laboratory personnel were blinded, and multiple masked quality control samples were interspersed among the case samples. The intra-assay CV was 7·7% for blinded, replicate, quality control samples.
Routine laboratory parameters, including HBsAg and antibodies to hepatitis C virus (anti-HCV), alanine aminotransferase (ALT), aspartate aminotransferase (AST), γ-glutamyltransferase (GGT), alkaline phosphatase (ALP), albumin, total bilirubin (TBIL), α-fetoprotein (a tumour marker) and C-reactive protein (CRP, a marker for systemic inflammation), were measured at the Clinical Laboratory of SYSUCC.
Clinical and lifestyle data collection
Detailed demographic, diagnostic and treatment information was extracted from the SYSUCC electronic clinical and administrative databases. Three predictors were employed to assess preexisting chronic liver diseases: (1) HBsAg and anti-HCV, (2) Child–Pugh score( Reference Pugh, Murray-Lyon and Dawson 27 , Reference Kamath, Wiesner and Malinchoc 28 ) and (3) liver damage score(Reference Fedirko, Duarte-Salles and Bamia29), a summary score of the number of abnormal laboratory-defined values for six liver function tests (ALT >50 U/l, AST >40 U/l, GGT >60 U/l, ALP >150 U/l, albumin <40 g/l and TBIL >20·5 μmol/l). The BCLC stage was chosen to assess tumour progression, which comprehensively considers tumour number and size, Child–Pugh score and performance status of the patient(Reference Llovet, Bru and Bruix30). Information on lifestyles, e.g. daily activity, smoking status, alcohol drinking status and multivitamin use, was obtained through baseline interviews. Daily activity was assessed by summing the products of time spent on a variety of activities (e.g. work, transportation, housework, physical exercises and leisure sedentary activity) with the mean metabolic equivalent (MET) for that activity. Dietary information was collected using a validated seventy-nine-item semi-quantitative FFQ(Reference Zhang and Ho31). Patients were asked how often (never, per year, per month, per week, or per d) they had consumed each food item on average in the previous year before diagnosis. Nutrient intakes were estimated by multiplying the frequency of consumption of each food item by its nutrient content per portion size according to the China Food Composition Database( 32 , 33 ). Dietary folate intake was standardised to 2000 kcal (8368 kJ) using the residual method(Reference Willett, Howe and Kushi34). Anthropometric measures were collected by nurses following a standard procedure with the same calibrated equipment. BMI was calculated as weight (kg) divided by height squared (m2).
Survival measurements
Survival outcomes assessed included liver cancer-specific survival (LCSS) and OS in this analysis. The former considers death due to HCC, and the latter considers death from any cause. Follow-up began at the time of HCC diagnosis until the occurrence of a death event, and a patient without an event of interest during the follow-up was censored at the last known date alive or at the time of last outcome ascertainment (26 September 2017). Deaths were ascertained through reviewing medical records and by telephone interview with next-of-kin every 6 to 12 months. Identification number or full name in combination with date of birth of persistent non-responders was searched in the National Death Registration System. The underlying cause of death was assigned from death certificates per the International Classification of Diseases-10 codes.
Statistical analysis
To investigate the association of folate status with LCSS and OS, we divided the patients into four groups according to sex-specific quartiles of serum folate concentrations. Demographic and clinical characteristics were compared across quartiles of serum folate using ANOVA for normally distributed continuous variables, the Kruskal–Wallis tests for skewed distributed continuous variables and the χ 2 test for categorical variables. Mortality rate expressed as per 10 000 person-days was calculated as the number of deaths divided by the total person-days during follow-up. Cox proportional hazards models were used to calculate hazard ratios (HR) and 95 % CI with the third quartile as the reference category. We first adjusted for non-clinical factors (age at diagnosis, sex (women, men), BMI, smoking status (never, former, current) and alcohol drinking status (never, former, current)), and then additionally adjusted for clinical prognostic factors (CRP levels (≤3·0 mg/l, >3·0 mg/l), liver damage score (0, 1–2, ≥3), BCLC stage (0, A, B, C) and cancer treatment (hepatectomy/liver transplantation, local ablation, hepatic arterial intervention, other treatments)). Other potential confounders, such as education level, residence and payment for medical care, only marginally changed the results and were thus excluded in the multivariate models. In the LCSS analysis, death from HCC was the endpoint, and death due to other causes was censored. In the OS analysis, death from any cause was the endpoint. Proportional hazard assumption was satisfied by including a time-dependent variable, which was the cross-product of folate and time. Tests for linear trend were based on the integer scores of different quartiles of serum folate (from 1 to 4). Non-linearity was tested with restricted cubic splines(Reference Desquilbet and Mariotti35), which was not significant. Covariates with missing observations were imputed by the multiple imputation method. Similar results were obtained when restricted to non-missing data.
Multiplicative interactions were assessed by entering main effect terms, a cross-product term of the serum folate quartile and the stratification variable into the model and were evaluated using likelihood ratio tests based on the models with and without the interaction terms. Selected stratification variables included sex, age at diagnosis (<45 years, 45–60 years, ≥60 years), CRP levels (≤3·0 mg/l, >3·0 mg/l), liver damage score (0, 1–2, ≥3), BCLC stage (0–A, B–C), smoking status (never, former, current) and alcohol drinking status (never, former, current). Stratified analyses were thereafter performed using the initial quartile cut-offs for the entire cohort. For statistically significant multiplicative interactions, we further conducted interactions on an additive scale to estimate the departure from additivity of effects using the relative excess risk due to interaction and the attributable proportion due to interaction(Reference Knol and VanderWeele36). The delta method was used to obtain CI for the indices(Reference Hosmer and Lemeshow37).
Statistical analyses were performed using SAS software version 9.4 (SAS Institute). A two-sided value of P < 0·05 was considered of statistical significance.
Results
Patient characteristics
Of the 982 patients, there were 868 (88·4 %) men. Mean age was 52·9 (SD 11·8) years. Median serum folate concentration was 7·13 (25th–75th percentile 5·34–9·42) ng/ml. At the time of sampling, 2·2 % of patients fulfilled the clinical nutritional criteria for severe folate deficiency (<3 ng/ml), and a further 32·2 % were classified as marginal folate deficiency (3–6 ng/ml). Among the 674 patients who had completed the FFQ, average intake of energy and energy-adjusted dietary folate were 2003 (SD 589) kcal/d (8381 (SD 2464) kJ/d) and 197·9 (SD 79·7) μg/d, respectively. Detailed demographic, clinical and treatment characteristics of the study population by sex-specific quartiles of serum folate are listed in Table 1. Patients in the highest quartile of serum folate were more educated, were less likely to be smokers, were more likely to be diagnosed with fatty liver disease and diabetes mellitus, had less severe liver damage (mainly driven by GGT, ALP and albumin) and systemic inflammation and less advanced BCLC stage, and were more likely to receive surgery in comparison with patients in the lowest quartile. An increasing trend of energy-adjusted dietary folate intake across quartiles of serum folate was observed (P = 0·01 for trend). Only 4·8 % of the patients reported using multivitamin supplements in the previous year and there was no significant difference in multivitamin use across quartiles of serum folate in 709 patients with available supplement use data. Other characteristics did not significantly differ by quartiles of serum folate.
Table 1. Baseline characteristics of participants by sex-specific quartiles (Q) of serum folate concentrations in the Guangdong Liver Cancer Cohort study
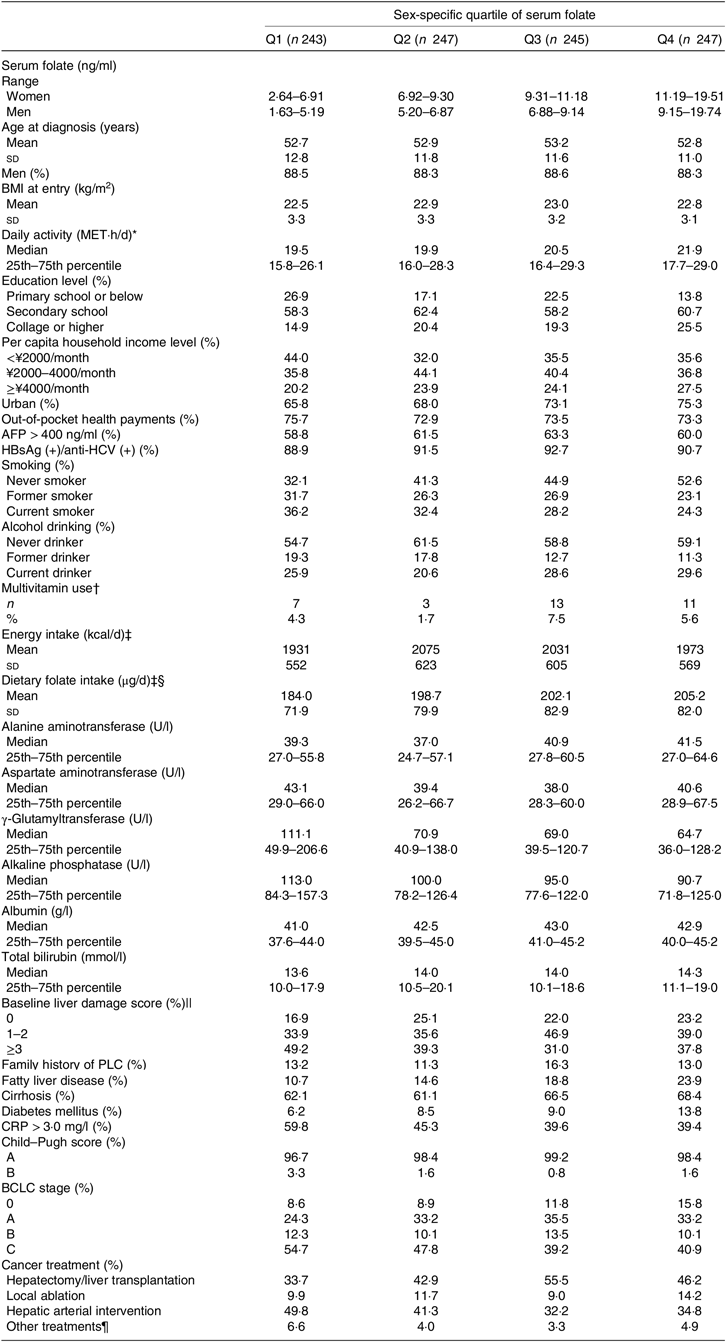
MET, metabolic equivalent; AFP, α-fetoprotein; HBsAg, hepatitis B surface antigen; anti-HCV, hepatitis C antibody; PLC, primary liver cancer; CRP, C-reactive protein; BCLC, Barcelona Clinic Liver Cancer.
* Including work, transportation, housework, physical exercises and leisure sedentary activity.
† Data were available for 709 patients.
‡ Dietary data were available for 674 patients. To convert energy in kcal/d to kJ/d, multiply by 4·184. To convert folate in μg/d to nmol/l, multiply by 2·27.
§ Dietary folate intake was standardised to 2000 kcal (8368 kJ) using the residual method.
|| A summary score of the number of abnormal laboratory-defined values for six liver function tests: alanine aminotransferase >50 U/l, aspartate aminotransferase >40 U/l, γ-glutaryl-transferase >60 U/l, alkaline phosphatase >150 U/l, albumin <40 g/l and total bilirubin >20·5 μmol/l, ranging from 0 to 6.
¶ Including radiation therapy and systemic treatment (e.g. molecular targeted therapy, systemic chemotherapy, traditional Chinese medication).
Serum folate and hepatocellular carcinoma survival
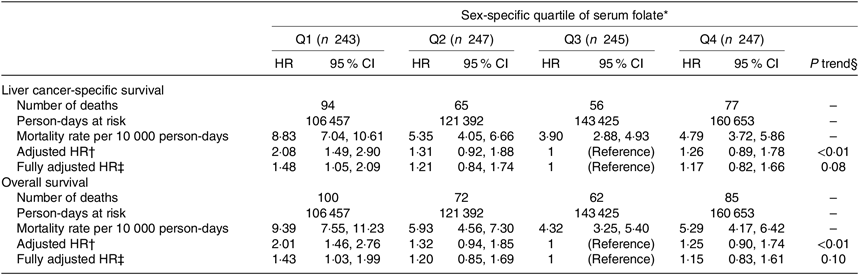
During a median of 463 (25th–75th percentile: 225–828) d of follow-up and 531 927 person-days at risk, 319 deaths (32·5 %) were documented, including 292 (91·5 %) from HCC. The associations between sex-specific quartiles of serum folate and survival outcomes are shown in Table 2. Both liver cancer-specific and overall mortality rates were highest in the first quartile and lowest in the third quartile of serum folate. In multivariable analyses with adjustment for non-clinical factors (age at diagnosis, sex, BMI, smoking and alcohol drinking status), compared with the third quartile of serum folate, the adjusted HR in the lowest quartile was 2·08 (95 % CI 1·49, 2·90) for LCSS and 2·01 (95 % CI 1·46, 2·76) for OS. The associations with LCSS and OS remained statistically significant after additional adjustment for clinical prognostic factors including CRP levels, liver damage score, BCLC stage and cancer treatment. The adjusted HR in the first (v. third) quartile of serum folate were 1·48 (95 % CI 1·05, 2·09) for LCSS and 1·43 (95 % CI 1·03, 1·99) for OS in the fully adjusted models.
Table 2. Multivariable-adjusted associations between sex-specific quartiles (Q) of serum folate concentrations and survival outcomes in the Guangdong Liver Cancer Cohort study
(Mortality rates, hazard ratios (HR) and 95 % confidence intervals)
BCLC, Barcelona Clinic Liver Cancer.
* Sex-specific quartile ranges of serum folate are: women: Q1=2·64–6·91 ng/ml, Q2 = 6·92–9·30 ng/ml, Q3 = 9·31–11·18 ng/ml, Q4 = 11·19–19·51 ng/ml; men: Q1 = 1·63–5·19 ng/ml, Q2 = 5·20–6·87 ng/ml, Q3 = 6·88–9·14 ng/ml, Q4 = 9·15–19·74 ng/ml.
† Adjusted for age at diagnosis (continuous), sex (women, men), BMI (continuous), smoking status (never, former, current) and alcohol drinking status (never, former, current).
‡ Additionally adjusted for C-reactive protein level (≤3·0 mg/l, >3·0 mg/l), baseline liver damage score (0, 1–2, ≥3), BCLC stage (0, A, B, C) and cancer treatment (hepatectomy/liver transplantation, local ablation, hepatic arterial intervention, other treatments).
§ The quartiles are treated as an ordered value in the models.
Interactions and stratified analyses
The influence of serum folate concentrations across strata of selected factors is presented in Table 3. The relation between serum folate concentrations and LCSS or OS remained similar across strata of sex, age at diagnosis, alcohol drinking status and BCLC stage (all P > 0·10 for interaction). There were statistically significant multiplicative interactions between sex-specific quartiles of serum folate and CRP levels or smoking status on associations with both LCSS and OS (all P ≤ 0·01 for interaction). When stratified by CRP levels, serum folate concentrations were inversely associated with LCSS and OS among patients who had CRP > 3·0 mg/l (all P = 0·01 for trend), but not among those with CRP ≤ 3·0 mg/l (all P > 0·05 for trend). Fig. 2 shows the joint effects of serum folate and CRP levels on HCC survival controlling for potential confounders. Compared with patients without systemic inflammation (CRP ≤ 3·0 mg/l) in the higher serum folate category (≥median), the HR were 1·91 (95 % CI 1·34, 2·71) for LCSS and 1·90 (95 % CI 1·36, 2·65) for OS in patients with systemic inflammation (CRP > 3·0 mg/l) and lower serum folate concentrations (<median). When stratified by smoking status, the inverse association of serum folate concentrations with LCSS or OS was only evident among current smokers (Table 3, all P = 0·04 for trend) rather than never or former smokers (all P > 0·05 for trend). The joint effects of serum folate concentrations and smoking status on survival outcomes are presented in Fig. 3. Current smokers in the lower serum folate category (<median) had significantly inferior LCSS (HR = 1·44, 95 % CI 1·02, 2·04) and OS (HR = 1·45, 95 % CI 1·05, 2·01) in comparison with never/former smokers with higher serum folate concentrations (≥median). According to Fig. 2 and 3, the relative excess risk due to interactions and attributable proportion due to interactions indicate positive effect modification of lower serum folate concentrations with systemic inflammation or current smoking on an additive scale and the ratio of HR suggests positive interaction on a multiplicative scale in the association with LCSS and OS. In addition, lower serum folate concentrations were associated with worse LCSS among patients who had liver damage score ≥3 (Table 3, P = 0·04 for trend), although no significant multiplicative interaction was observed between serum folate and preexisting liver damage (P = 0·09 for interaction).
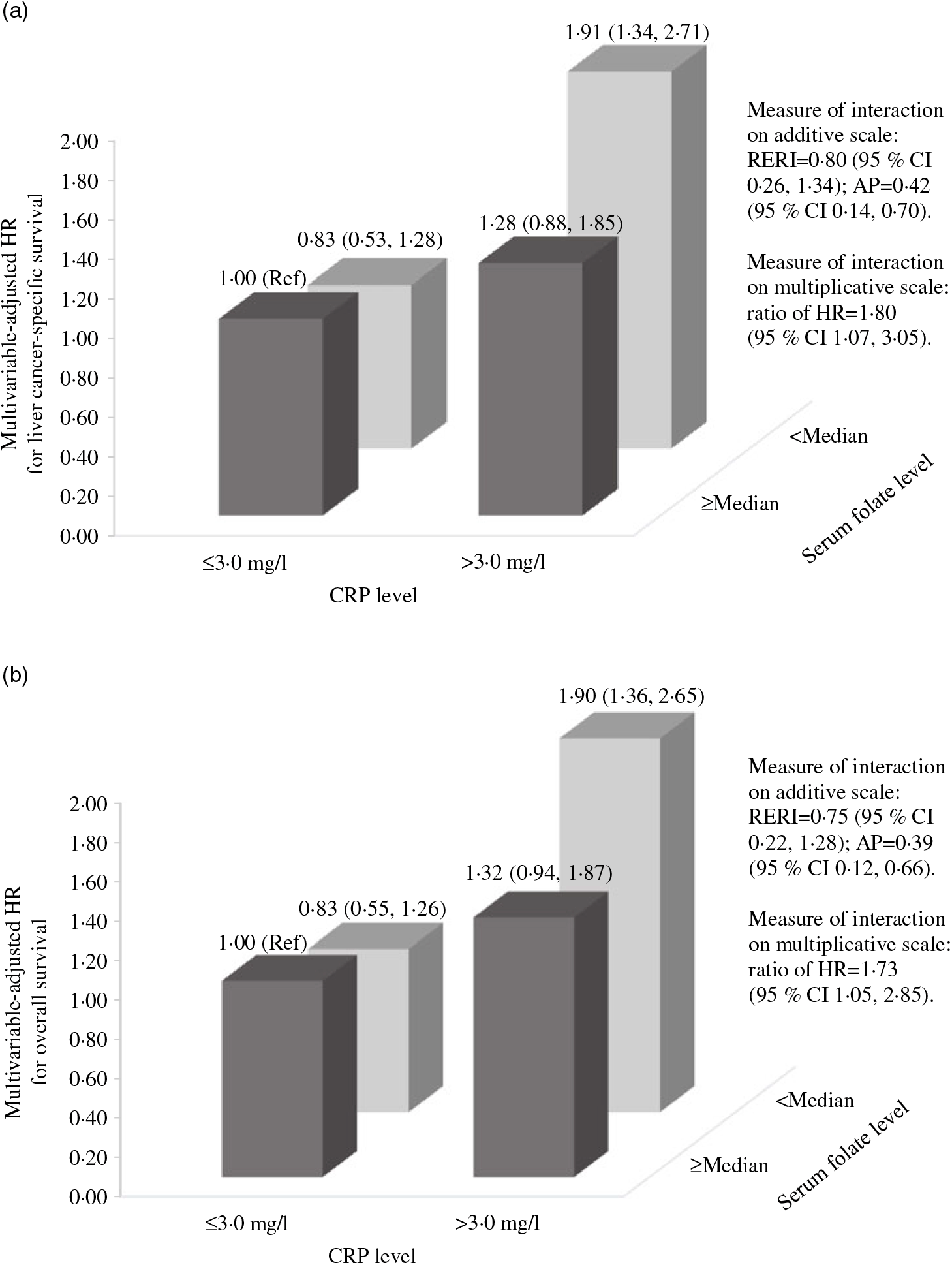
Fig. 2. Joint effects of serum folate and C-reactive protein (CRP) levels on survival outcomes in the Guangdong Liver Cancer Cohort study. (a) Liver cancer-specific survival and (b) overall survival. HR, hazard ratio; Ref, reference; RERI, relative excess risk due to interaction; AP, attributable proportion due to interaction. Measures for additive interaction and corresponding 95 % CI are estimated using the delta method. HR are estimated from Cox proportional hazards models adjusting for age at diagnosis (continuous), sex (women, men), BMI (continuous), smoking status (never, former, current), alcohol drinking status (never, former, current), baseline liver damage score (0, 1–2, ≥3), Barcelona Clinic Liver Cancer stage (0, A, B, C) and cancer treatment (hepatectomy/liver transplantation, local ablation, hepatic arterial intervention, other treatments).

Fig. 3. Joint effects of serum folate and smoking status on survival outcomes in the Guangdong Liver Cancer Cohort study. (a) Liver cancer-specific survival and (b) overall survival. HR, hazard ratio; Ref, reference; RERI, relative excess risk due to interaction; AP, attributable proportion due to interaction. Measures for additive interaction and corresponding 95 % CI are estimated using the delta method. HR are estimated from Cox proportional hazards models adjusting for age at diagnosis (continuous), sex (women, men), BMI (continuous), alcohol drinking status (never, former, current), C-reactive protein level (≤3·0 mgl, >3·0 mg/l), baseline liver damage score (0, 1–2, ≥3), Barcelona Clinic Liver Cancer stage (0, A, B, C) and cancer treatment (hepatectomy/liver transplantation, local ablation, hepatic arterial intervention, other treatments).
Table 3. Multivariable-adjusted associations between sex-specific quartiles (Q)* of serum folate concentrations and survival outcomes stratified by possible effect modifiers in the Guangdong Liver Cancer Cohort study
(Hazard ratios (HR) and 95 % confidence intervals)
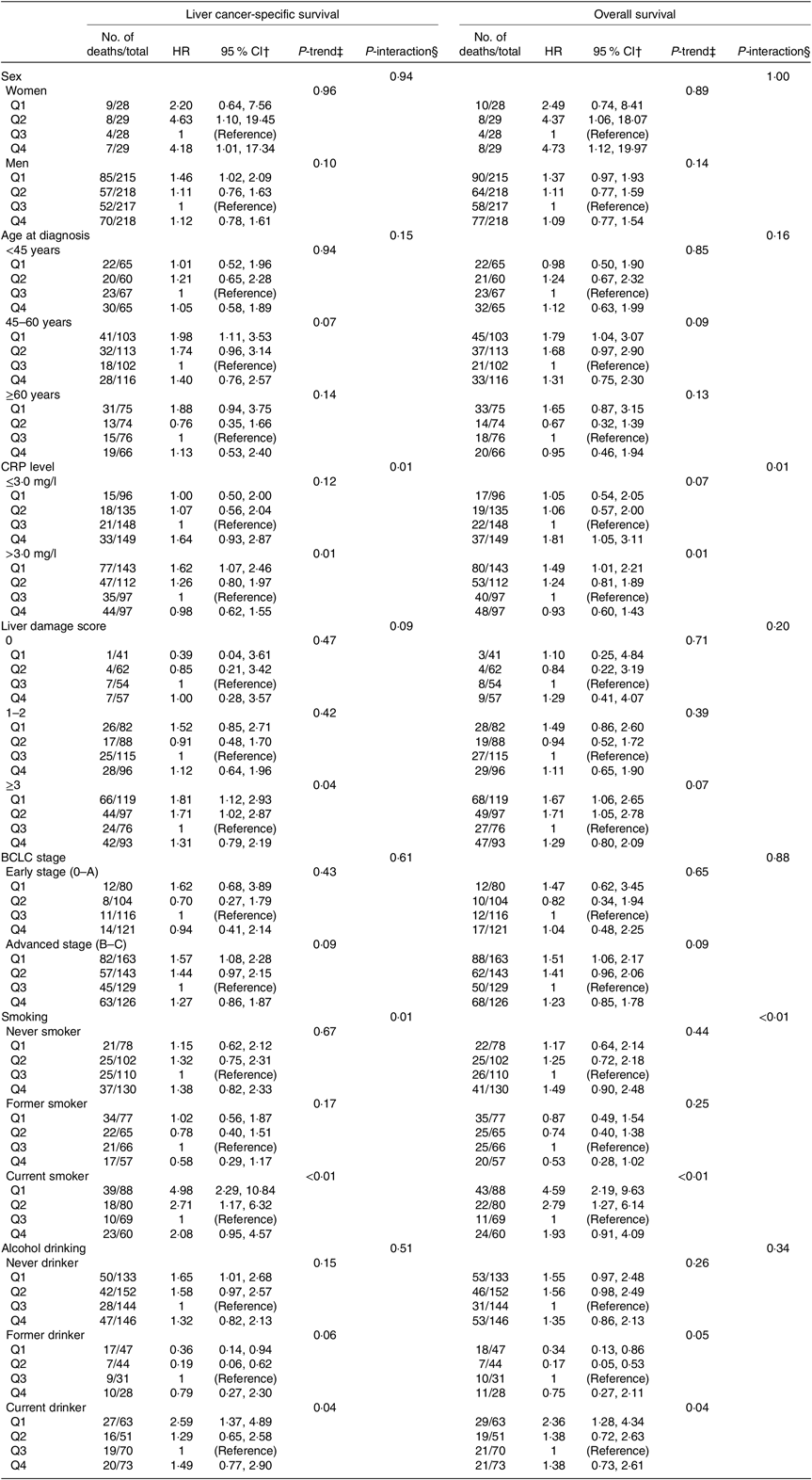
CRP, C-reactive protein; BCLC, Barcelona Clinic Liver Cancer.
* Sex-specific quartile ranges of serum folate are: women: Q1=2·64–6·91 ng/ml, Q2=6·92–9·30 ng/ml, Q3=9·31–11·18 ng/ml, Q4=11·19–19·51 ng/ml; men: Q1=1·63–5·19 ng/ml, Q2=5·20–6·87 ng/ml, Q3=6·88–9·14 ng/ml, Q4=9·15–19·74 ng/ml.
† Adjusted for age at diagnosis (continuous), sex (women, men), BMI (continuous), smoking status (never, former, current), alcohol drinking status (never, former, current), CRP level (≤3·0 mg/l, >3·0 mg/l), baseline liver damage score (0, 1–2, ≥3), BCLC stage (0, A, B, C) and cancer treatment (hepatectomy/liver transplantation, local ablation, hepatic arterial intervention, other treatments), and the corresponding variable was removed from the multivariable models when it was a stratified factor.
‡ The quartiles were treated as an ordered value in the models.
§ Interactions were assessed using likelihood ratio tests based on the models with and without the interaction terms.
Discussion
To our knowledge, this is the largest cohort study assessing folate status in relation to HCC survival. In addition, this is the first prospective study evaluating the association between serum folate concentrations and HCC prognosis in non-Caucasian populations and in populations without mandatory food fortification with folic acid and widespread folate-containing supplement use. In this prospective cohort study of 982 HCC patients, we observed that lower serum folate concentrations at diagnosis were associated with inferior LCSS and OS. These findings did not vary substantially across strata of sex, age at diagnosis, alcohol drinking status and BCLC stage. However, there were statistically significant interactions on both multiplicative and additive scales between serum folate and systemic inflammation or smoking status, and the associations of lower serum folate with worse LCSS and OS were only evident among patients with systemic inflammation or current smokers. An inverse association between serum folate and LCSS was also observed among patients with preexisting liver damage, but the interaction testing with liver damage score was not statistically significant.
Numerous epidemiological studies have examined the association between folate status and cancer mortality or survival. Mortality data from 28 845 participants included in the US National Health and Nutrition Examination Survey during 1999–2010 showed that the risk of cancer mortality decreased gradually with serum folate concentrations reaching a threshold at approximately 40 nmol/l, suggesting that lower serum folate concentrations, but not restricted to folate deficiency, are associated with an increase in cancer mortality(Reference Peng, Dong and Wang18). Rossi et al. (Reference Rossi, Hung and Beilby19) followed 1988 men and women who participated in the 1969 Busselton Health Survey for over 29 years and observed that participants with folate deficiency (<3·00 ng/ml) at baseline were at increased risk of dying from prostate cancer, but not from breast, colorectal and lung cancers, in comparison with those with sufficient folate levels (≥6·00 ng/ml). In line with the study by Rossi et al. (Reference Rossi, Hung and Beilby19), a case–control study nested within the Japan Collaborative Cohort Study also show no association of serum folic acid concentrations (geometric mean: 5·21 v. 5·46 ng/ml in men and 6·21 v. 6·63 ng/ml in women for cases v. controls) with the risk of lung cancer death(Reference Ito, Wakai and Suzuki38). In contrast, results from the Nurses’ Health Study and Health Professionals Follow-Up Study indicated that higher concentrations of prediagnostic plasma folate were related to superior colorectal cancer-specific survival and OS among 301 participants who developed colorectal cancer during follow-up with a median folate concentration of 6·9 ng/ml(Reference Wolpin, Wei and Ng20), whereas no similar associations were observed between total folate intake (from food and supplemental sources) after diagnosis and colorectal cancer-specific and overall mortality among 1550 stage I–III colorectal cancer patients consuming 978 dietary folate equivalents daily on average(Reference Lochhead, Nishihara and Qian39). McEligot et al. (Reference McEligot, Ziogas and Pfeiffer21) reported an inverse association between plasma total folate concentrations and OS after breast cancer diagnosis during an average follow-up of 6·7 years among 471 postmenopausal women (mean folate concentration: 29·9 nmol/l). Likewise, compared with patients in the lowest quartile of dietary folate intake (<190 μg/d), patients in the highest quartile (≥246 μg/d) had significantly improved breast cancer-specific survival and OS among 3116 women who developed breast cancer according to the findings from the Swedish Mammography Cohort Study(Reference Harris, Bergkvist and Wolk40). With reference to gastric cancer, a serum folate concentration <1·90 ng/ml has been linked with poor survival in 155 patients(Reference Lee, Chiang and Shih41), and higher dietary folate intakes have been suggested to be associated with reduced risk of gastric cancer mortality among susceptible MTHFR 677TT carriers(Reference Galvan-Portillo, Onate-Ocana and Perez-Perez42). However, a follow-up study of 548 participants with incident renal cell carcinoma from the European Prospective Investigation into Cancer and Nutrition Study failed to show any association between plasma folate concentrations (median: 11·86 nmol/l) and all-cause mortality(Reference Johansson, Fanidi and Muller43). Similarly, a prospective cohort study of 1270 women with invasive epithelial ovarian cancer also found little evidence that total folate intake (mean: 481 μg/d) was linked with ovarian cancer survival(Reference Dixon, Ibiebele and Protani44). Although results from the Norwegian Vitamin Trial and Western Norway B Vitamin Intervention Trial have raised the concern about the role of treatment with folic acid plus vitamin B12 in increasing cancer mortality(Reference Ebbing, Bonaa and Nygard45), none of the aforementioned studies has observed a positive association of circulating folate concentration or dietary folate intake with overall or site-specific cancer mortality/survival. The inconsistent findings of the previous studies are likely due to the difference in the timing (before/after diagnosis), dose (dietary folate intake/circulating folate concentration) and cancer type(Reference Ulrich and Potter46,Reference Lee, Willett and Fuchs47) . Most of the previous studies have measured circulating folate concentrations prior to cancer diagnosis, and few have assessed postdiagnostic folate status. It should be noted that circulating folate concentrations in the present study are comparable with or even higher than those in the Asian and US cohorts(Reference Wolpin, Wei and Ng20,Reference Ito, Wakai and Suzuki38) , in which mandatory folate fortification has not been started at the time of blood sampling (1993–1995 in Nurses’ Health Study and Health Professionals Follow-Up Study). Nevertheless, both circulating folate concentrations and total folate intake increased dramatically in American populations after the implementation of mandatory folate fortification(Reference Peng, Dong and Wang18,Reference Lochhead, Nishihara and Qian39) . Circulating folate concentrations in the European patients with renal cell carcinoma(Reference Johansson, Fanidi and Muller43) and total folate intake in the Australian patients with ovarian cancer(Reference Dixon, Ibiebele and Protani44) are higher than those in the study population as well, which is possibly due to the widespread use of folic acid-containing supplements in European and Australian populations.
Existing data are scarce on HCC. Yeh et al. (Reference Yeh, Goyal and Shen22) prospectively evaluated the association between plasma folate concentrations and OS among 160 HCC patients with a median follow-up of 1·12 years and found that patients with plasma folate <12·2 ng/ml had worse survival than those with plasma folate ≥12·2 ng/ml(HR = 1·96; 95 % CI 1·24, 3·09). We add to similar observations in Chinese populations, despite that the study by Yeh et al. was conducted in American populations who had almost twice as much circulating folate concentrations as our study population (12·2 v. 7·13 ng/ml) due to mandatory folic acid fortification and use of folate-containing supplements( Reference Crider, Bailey and Berry 23 , Reference Kantor, Rehm and Du 48 ). In our study, patients in the first quartile (range of serum folate: 2·64–6·91 ng/ml for women and 1·63–5·19 ng/ml for men) had a 48 % higher risk of liver cancer-specific mortality and a 43 % higher risk of all-cause mortality after adjustment for non-clinical and clinical prognostic factors, compared with patients who had serum folate concentrations in the third quartile (range of serum folate: 9·31–11·18 ng/ml for women and 6·88–9·14 ng/ml for men). Notably, folate status of the study population was generally good with a median serum folate concentration of 7·13 ng/ml and only 2·2 % of the patients were classified as severe folate deficiency, which may be a consequence of a relatively high dietary folate intake (197·9 v. 180·9 μg/d) and low frequencies of the MTHFR 677TT genotype (7 %; 95 % CI: 5 %, 8 %) and the MTHFR 677T allele (25 %; 95 % CI: 23 %, 27 %) in southern Chinese population( Reference He, Wang and Zhao 49 , Reference Wang, Fu and Li 50 ). Overall, the study population has comparable serum folate concentrations with participants from other Chinese cohorts (geometric mean 14·7 (95 % CI 11·4, 19·0) nmol/l)(Reference Midttun, Theofylaktopoulou and McCann24). These findings indicate that lower circulating folate concentrations, but not restricted to low folate status (<3 ng/ml), are associated with inferior survival among HCC patients. The associations were largely consistent across different strata of sex, age, alcohol drinking status and BCLC stage. Although alcohol consumption has been linked with folate deficiency and liver disease progression(Reference Persson, Schwartz and Park17,Reference Medici and Halsted51) , alcohol drinking status did not modify the relationship between serum folate and HCC survival in the current study. Similarly, the findings from the NIH-AARP Diet and Health Study also showed no interaction between folate intake and alcohol consumption on the association with liver disease mortality(Reference Persson, Schwartz and Park17). Smoking is another risk factor for both folate deficiency and HCC(Reference Thuesen, Husemoen and Ovesen52,Reference El-Serag53) . In the present study, we observed a synergy of lower folate concentrations and current smoking in the prognosis of HCC. Lower folate concentrations were significantly associated with worse HCC survival among current smokers but not among never and former smokers, suggesting that current smokers might benefit most from improving folate status. CRP, a marker of systemic inflammation, has been identified as a prognostic factor for HCC(Reference Sieghart, Pinter and Hucke54). In the study population, CRP was inversely associated with folate status and when stratified by CRP levels, an inverse association of serum folate concentrations with survival outcomes was pronounced only among HCC patients with systemic inflammation (CRP >3·0 mg/l). Elevating serum folate concentrations may prolong the survival time of HCC patients with systemic inflammation. In addition, folate status has been reported to be related to liver damage(Reference Welzel, Katki and Sakoda16). According to our observations, lower folate concentrations were strongly linked with worse LCSS in patients with preexisting liver damage (liver damage score ≥3). However, these findings should be interpreted with caution because interaction testing with liver damage score was not significant.
Although it remains obscure how lower folate status exactly contributes to inferior HCC survival, several potential reasons can be considered. Folate, as a methyl donor, is involved in DNA methylation, as well as DNA synthesis and repair(Reference Newman and Maddocks5). Given the crucial role of folate in DNA methylation, inadequate folate would lead to epigenetic changes in human tumours: global DNA hypomethylation and site-specific CpG island promoter hypermethylation(Reference Crider, Yang and Berry55). Long interspersed nucleotide elements-1 hypomethylation in the plasma and white blood cells, a surrogate marker for global DNA hypomethylation, has been reported to be associated with poor prognosis in HCC patients(Reference Yeh, Goyal and Shen22). In addition, folate deficiency can disturb hepatic methionine metabolism, increase DNA strand breaks and promote liver damage(Reference Halsted, Villanueva and Devlin56). Nevertheless, it must be noted that poor survival is not restricted to patients with low-folate status, but even extended to those with marginal folate deficiency.
Another concern is that excess folate may promote cancer progression after the development of neoplastic foci. Rapid tumour cell proliferation requires higher rates of DNA replication, which in turn, needs enough folate and other one-carbon donors for nucleotide synthesis. In animal models of breast cancer, a high dose of folic acid has been reported to enhance tumour growth(Reference Hansen, Jensen and Fuchtbauer7), while withdraw of folate is thought to cause a reduction in growth rate of hepatoma cells(Reference Lee, Xu and Chiu12). A Taiwan study observed that higher erythrocyte folate levels were associated with inferior survival among 232 HCC patients who had a median erythrocyte folate level of 688 ng/ml(Reference Kuo, Huang and Kuo57). Notably, our observations also suggest that patients in the top quartile of serum folate tend to experience an increased risk of liver cancer-specific and overall mortality, although not statistically significant. Nevertheless, erythrocytes and serum folate concentrations are not the same indicators, additional efforts are needed to verify the hypothesis in a broader range of circulating folate concentrations.
Several strengths of this study lend credibility to its findings. First, we prospectively enrolled a homogeneous patient population with first incident HCC and collected blood samples within 30 d of diagnosis to eliminate potential confounding by receiving anticancer therapy. Second, serum folate concentrations were centrally measured in the same laboratory with strict quality control. Third, we collected detailed covariate information, including demographics, lifestyle, clinical characteristics and cancer treatment, thereby minimising the possibility of residual confounding. Fourth, the sample size is large for an outcome study of this kind. And finally, both OS and LCSS were used as endpoints in our analyses, although HCC is a highly lethal cancer.
We also acknowledge several limitations in our study. We have only one measurement of serum folate made at the time of diagnosis. Changes in diet and lifestyle, cancer progression and tumour-targeting therapy, especially receiving chemotherapy inhibiting the folate circle, may influence circulating folate concentrations following diagnosis. Yet we do not have information on biomarkers of other one-carbon nutrients (e.g. vitamin B2, vitamin B6, vitamin B12, choline and betaine). Additionally, we cannot rule out the possibility of reverse causality, given the fact that serum folate reduced as HCC progressed( Reference Cui, Quan and Piao 13 , Reference Lin and Yin 58 , Reference Kuo, Lin and Wu 59 ). However, the inverse associations remained evident after further adjusting for clinical prognostic factors including preexisting liver damage, BCLC stage and cancer treatment. Moreover, both preexisting liver damage and BCLC stage were not significant effect modifiers for the association between serum folate and HCC survival in our study. In addition, we cannot entirely exclude the possibility that excess folate may adversely influence HCC survival since serum folate concentrations in our patients were relatively low and within a narrow range. Lastly, caution should be taken when generalisation of the conclusion to racially diverse patient populations since all our study participants are of Asian descent.
Conclusions
In summary, the findings from this prospective cohort study suggest that lower serum folate concentrations at diagnosis, but not restricted to severe folate deficiency, are associated with worse survival in HCC patients, especially in those with systemic inflammation and current smokers. Given that both food fortification and supplement use are not yet popular in China, a future trial of folate supplementation seems to be promising in HCC patients with lower folate status.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0007114519000734
Acknowledgments
The authors thank the office and field staff of SYSUCC for data collection, processing and preparation. We thank all GLCC study participants for their contributions to this study.
This work was supported by the National Natural Science Foundation of China (A. P. F., grant number 81803219; H. L. Z., grant number 81472966); the Medical Research Foundation of Guangdong Province, China (A. P. F., grant number A2018402); the Natural Science Foundation of Guangdong Province, China (A. P. F., grant number 2018A030310335) and the Fundamental Research Funds for the Central Universities, China (A. P. F., grant number 17ykpy14). The funders had no role in the design, analysis or writing of this article.
A. P. F., M. S. C., Y. J. Z. and H. L. Z. designed the research; A. P. F., Z. Y. L., G. C. L., P. Y. C., X. Y. W., D. M. Z., Y. L., J. A. L., R. H. Z., Z. G. Z., Y. J. X., X. J. X., W. H. L., M. S. C., Y. J. Z. and H. L. Z. conducted the research; Z. G. Z., Y. J. X., X. J. X., M. S. C. and Y. J. Z. provided the essential materials; A. P. F. analysed the data; A. F. P. wrote and revised the paper; A. P. F., Y. J. Z. and H. L. Z. had primary responsibility for the final content. All authors read and approved the final manuscript.
There were no conflicts of interest.









