When exploring relationships between a nutrient and disease outcome, both prospective observational and randomised controlled trial (RCT) evidence needs to be considered. The report from a conference that focused on setting intake targets for the long-chain n-3 (omega-3) fatty acids (FA) EPA and DHA, concluded, ‘Thus, evidence is most robust when studies of both designs provide concordant results, as is the case for EPA + DHA consumption and CHD’(Reference Harris, Mozaffarian and Lefevre1). The purpose of this review is to summarise current evidence regarding the effects of increased intakes/blood levels of EPA and DHA on CVD outcomes and risk for death from any cause. We will examine both the epidemiological evidence as well as the findings from RCT, and we will end with a summary of potential mechanisms of action.
Epidemiology: n-3 blood levels and risk for fatal CHD
Three major prospective studies have demonstrated that a higher level of n-3 FA in the blood (especially in the erythrocyte membranes) is associated with reduced risk for death from any cause (Table 1). These three studies, and several more, have been condensed into a meta-analysis as this has become the preferred method of summarizing the results of multiple studies with similar designs. In the n-3 and CVD arena, the most recent meta-analysis was reported by Del Gobbo et al. and included results from nineteen separate cohorts(Reference Del Gobbo, Imamura and Aslibekyan2). In each, circulating levels of four separate n-3 FA (EPA, DHA, α-linolenic acid and docosapentaenoic acid) were linked with risk for fatal CHD. For a 1-sd increase in FA level, all four of these n-3 species were associated with a significant, 9 and 10 % reduction in risk. Using data from this meta-analysis to estimate erythrocyte EPA + DHA levels (the Omega-3 Index)(Reference Harris and von Schacky3) across ten cohorts for which data were available revealed that the risk for fatal CHD was reduced by 35 % comparing quintiles 1–5. The Omega-3 Index values for these quintiles were 4·2 and 8·3 %, respectively (EPA + DHA expressed as a per cent of total erythrocyte FA). These findings corroborated the cut-points for high- and low-risk for fatal CHD of 4 and 8 %, respectively, originally proposed for this metric 14 years ago(Reference Harris and von Schacky3). Hence, evidence is strong for a beneficial effect of higher n-3 blood levels on risk for CHD death.
Table 1. Major epidemiological and randomised clinical trials testing the effects of n-3 fatty acids on disease outcomes
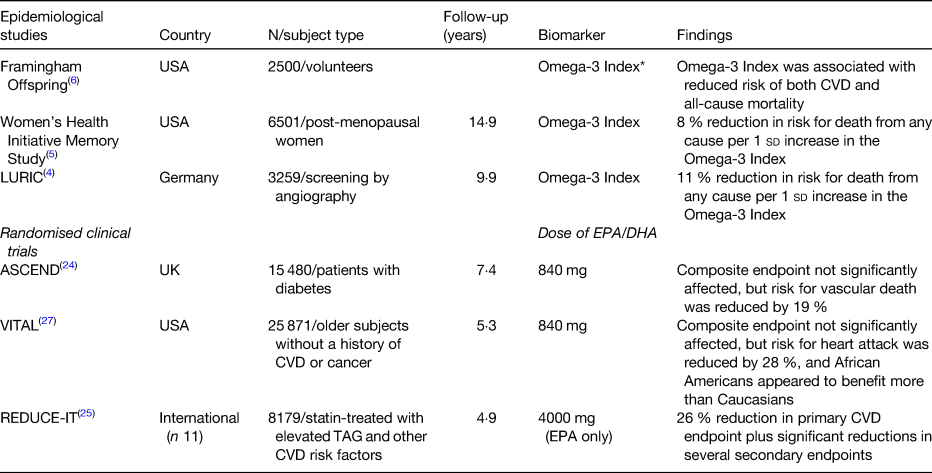
* Erythrocyte EPA + DHA expressed as a per cent of total erythrocyte fatty acids(Reference Harris and von Schacky3).
In addition to these relationships with fatal CHD, higher Omega-3 Index levels have been significantly associated with lower risk for death from any cause(Reference Kleber, Delgado and Lorkowski4–Reference Harris, Tintle and Etherton6) (Table 1). Interestingly, the Omega-3 Index was a better predictor of risk for all-cause mortality and total CVD events than serum cholesterol, a classic risk factor for CHD. This suggests, not that cholesterol is not an important risk factor, but that the Omega-3 Index, as a relatively new biomarker, should be seriously considered as well when evaluation a patient's risk for future CVD events. A novel approach exploring the link between n-3 levels and health was reported by Lai et al. who asked if n-3 levels were associated with ‘healthy ageing’.(Reference Lai, de Oliveira Otto and Lemaitre7) The latter was defined as the absence after age 65 years, of chronic diseases and disabilities (e.g. CVD, cancer, kidney or lung disease, loss of cognitive function or physical abilities) or death from other causes. They found that, comparing the highest to the lowest quintile of plasma n-3 levels, there was an 18 % lower risk for ‘unhealthy ageing’. This study adds yet another stone to the growing wall of evidence for the health-giving effects of these marine n-3 FA. Since blood levels are largely determined by the intake of oily fish and/or fish oil supplements,(Reference Block, Harris and Pottala8) these findings support the hypothesis that, over the long-term, higher intakes of EPA + DHA are not only cardioprotective, but good for overall health.
Randomised controlled trials: does changing n-3 levels by supplementation reduce risk?
The epidemiological evidence summarised earlier strongly points to a link between higher n-3 tissue levels and lower risk for adverse outcomes of ageing. But perhaps n-3 levels are simply a marker of an overall healthy lifestyle. Researchers are keenly aware of this possibility and do their best to control for these other factors in their statistical analyses, but still the concern remains. Indeed, in a study in acute coronary syndrome patients, those individuals who began taking n-3 supplements after discharge (whether advised to do so by their doctors or on their own) differed from those who did not in many ways suggesting that they were more health conscious.(Reference Harris, Kennedy and Maddox9) Thus, as noted earlier, when studying nutritional links with disease, both epidemiological studies (which capture long-term relationships between nutrient levels and disease outcomes in ‘real life’) and interventional studies (which, although randomised and thus unencumbered with doubts about non-random effects, nevertheless can only study effects in a relatively short window of time in a patient population that may not be representative) must be given consideration.
It was a string of remarkably successful clinical trials between 1989 and 2007(Reference Burr, Fehily and Gilbert10–Reference Yokoyama, Origasa and Matsuzaki13) that cemented the cardioprotective effects of n-3 FA in the medical (and consumer) mind. However, over the past decade, several null studies were reported(Reference Roncaglioni, Tombesi and Avanzini14–Reference Kromhout, Giltay and Geleijnse16), casting doubt on these earlier findings, and including these studies in meta-analyses naturally led to conclusions that n-3 treatment does not reduce risk for CVD(Reference Rizos, Ntzani and Bika17, Reference Aung, Halsey and Kromhout18). A variety of commentaries have pointed out the problems with these meta-analyses(Reference Maki and Dicklin19–Reference Harris21) including the inclusion of studies that recruited patients regardless of baseline n-3 levels who were also treated with multiple other CHD medications and were given low doses of EPA + DHA (<1 g) and were treated for relatively short periods of time. Under these combined conditions, it is difficult to see how a positive outcome could even have been expected.
However, what has been a consistent finding, even in these null trials, is a reduction in the risk for cardiovascular or cardiac death(Reference Maki, Palacios and Bell22, Reference Alexander, Miller and Van Elswyk23). This was confirmed yet again in a recent RCT A Study of Cardiovascular Events in Diabetes (ASCEND)(Reference Bowman, Mafham and Wallendszus24) (Table 1). This study provided (again) about 840 mg/d EPA + DHA v. placebo to about 14 500 diabetic patients and followed them for serious vascular events over 7·4 years. This endpoint consisted of a combination of non-fatal heart attack or stroke, transient ischaemic attack or vascular death. There was no effect of treatment on the first two components of this endpoint, but there was a 19 % reduction (P < 0·05) in vascular fatalities. Since the originally defined, combined endpoint was not met, the authors concluded that n-3 treatment had ‘no effect’, but clearly there was an effect, and a very important one that has been seen time and time again. Nevertheless, most press reports of the ASCEND trial labelled it as (yet another) failed fish oil study, but the only ‘failure’ in the study was that of the investigators to pick the right endpoint at the beginning, and cardiovascular death would have made the most sense based on past research findings.
Results from two other major n-3 RCT have also been released recently (Table 1). The first was a study called REDUCE-IT(Reference Bhatt, Steg and Miller25), and it included about 8000 individuals, all statin-treated, with mildly elevated TAG levels and other risk factors for CHD(Reference Bhatt, Steg and Brinton26). The trial lasted 4·9 years. Patients were randomised to either 4 g/d icosapent ethyl (EPA ethyl esters, Amarin Corp.) or a mineral oil placebo. The investigators reported that the primary endpoint, major adverse cardiovascular events, was reduced by 25 % (P < 0·001). This is by far the highest dose n-3 study yet performed, and its remarkable success in a statin-treated population suggests that the failures of past studies have been the failure to give enough n-3. This should not be surprising; if you give too little active agent, you are unlikely to see an effect.
While REDUCE-IT was the highest dose n-3 study ever conducted, VITAL was the largest(Reference Manson, Cook and Lee27). VITAL (which studied the effects of n-3 and vitamin D on CVD and cancer outcomes) included over 25 000 subjects, all without known CVD or cancer at baseline. It was the first truly primary prevention study in this area. VITAL provided, as so many other smaller studies have done, one capsule of Lovaza (Omacor) which delivered 840 mg EPA + DHA ethyl esters daily. The study lasted about 5 years. Although the study was reported as a no-effect trial (because, again, the combined primary endpoint was not significantly reduced), there were important secondary endpoint findings. For example, risk for a heart attack was reduced by 28 %, risk for any CVD event was 17 % lower, and risk for a fatal heart attack was 50 % lower in the n-3 group. Not surprisingly, it was people who reported lower background fish intake that benefited the most. A surprising finding, that needs to be followed-up on, is that African American subjects had a 77 % reduction in heart attacks when taking an n-3 supplement.
These three studies: ASCEND, REDUCE-IT and VITAL have breathed new life into the n-3 field, such that now one can no longer say, ‘fish oils don't work’. They do work if the right dose is given and if the study is large enough to be able to detect the beneficial effects. As has always been the case, the n-3 FA are completely safe to take, with or without other medications, and can be relatively inexpensive. The benefit:risk ratio for these important nutrients continues to be very favourable.
Mechanisms of action
The mechanisms responsible for the beneficial cardiovascular effects, especially on fatal events, are not clear at this point. In particular, why fatal, more often than non-fatal, events are reduced is puzzling. Obviously, a heart attack that might have been fatal were it not for the increase in n-3 would now be classified as non-fatal, increasing the count in that category as the count in the fatal category decreased. So ‘no effect’ on non-fatal events in the face of reduced fatal events may partly explain why this pattern has been seen. But this is a statistical, not a pathophysiological, explanation. Space does not permit a full discussion of the biological effects of n-3 that have been observed over the years, but a short list would include the following: improved cardiac remodelling after a heart attack(Reference Heydari, Abdullah and Pottala28), blood pressure-lowering(Reference Miller, Van Elswyk and Alexander29), heart rate reduction(Reference Mozaffarian, Geelen and Brouwer30), TAG reduction(Reference Balk, Lichtenstein and Chung31), alteration of membrane physiochemical properties(Reference Shaikh, Kinnun and Leng32) and inhibition of platelet function(Reference Wachira, Larson and Harris33). The molecular bases for these effects (and others) are summarised in Fig. 1. How these forces might work together in as yet unknown ways to affect metabolic and biochemical processes is a topic for future research.
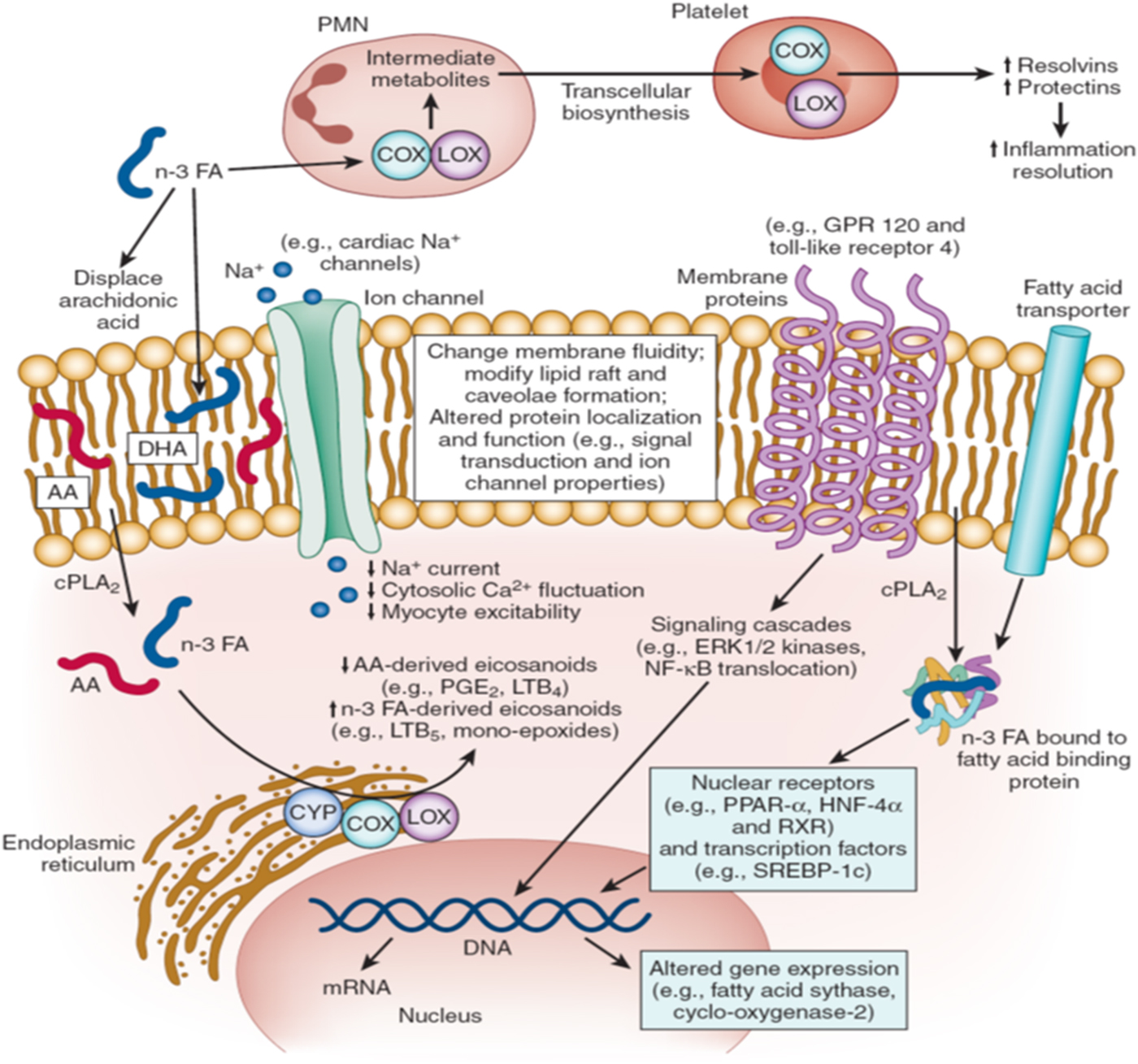
Fig. 1. Molecular mechanisms of n-3 fatty acid (FA) action. AA, arachidonic acid; COX, cyclooxygenase; CPLA, cytosolic phospholipase A; CYP, cytochrome P450; GPR, G-protein-coupled receptor; HNF, hepatic nuclear factor; LOX, lipo-oxygenase; LTB, leukotriene B; PLA, phenylactic acid; PMN, polymorphonuclear leucocytes; RXR, retinoid X receptors; SREBP, sterol regulatory element-binding protein.
n-3 FA modulate multiple molecular pathways that together contribute to their physiologic effects. First, the physicochemical properties of cellular and organelle membranes are influenced by n-3 FA lipid composition (centre). Incorporation of n-3 FA into these membranes alters membrane fluidity and biophysics of lipid rafts, effects that modulate protein function and signalling events. For example, enrichment of cellular membranes with n-3 FA disrupts dimerisation and recruitment of toll-like receptor 4, which may contribute to anti-inflammatory effects by downregulation of NF-κB activation. Ion channels, such as sodium (Na+), L-type calcium (Ca2+), and Na–Ca exchangers may be similarly modulated by n-3 FA incorporation into lipid membranes. Secondly, n-3 FA seem to interact directly with membrane channels and proteins (centre). For example, direct modulation of ion channels or G-protein-coupled receptor 120 may contribute to antiarrhythmic or anti-inflammatory effects, respectively. Thirdly, n-3 FA directly regulate gene expression via nuclear receptors and transcription factors (lower right). n-3 FA are natural ligands of many key nuclear receptors in multiple tissues, including PPAR (α, β, γ and δ), hepatic nuclear factors (4α and γ), retinoid X receptors (α, β and γ) and liver X receptors (α and β). Interactions between n-3 FA and nuclear receptors are modulated by cytoplasmic lipid-binding proteins (e.g. FA-binding proteins) that transport the FA into the nucleus. n-3 FA also alter function of transcription factors, such as sterol regulatory element-binding protein-1c. Such genetic regulation contributes to observed effects of n-3 FA on lipid metabolism and inflammatory pathways. Fourthly, after release from phospholipids by cytosolic phospholipase A2, n-3 (and n-6) FA are converted to eicosanoids by cyclooxygenase, lipo-oxygenase and cytochrome P450 enzymes (lower left). n-3 FA displace arachidonic acid in membrane phospholipids, reducing the production of arachidonic acid-derived eicosanoids (e.g. PGE2) while increasing those generated from n-3 FA. This altered eicosanoid profile may influence inflammation, thrombosis and vascular function. Fifthly, emerging evidence suggests that n-3 FA play an important role in inflammation resolution via specialised pro-resolving mediators, including resolvins or protectins that are n-3 FA metabolites derived from the actions of cyclooxygenase and lipo-oxygenase (top). Biosynthesis of specialised pro-resolving mediators seems to require involvement of two or more cell types (‘transcellular biosynthesis’), with one cell type converting the n-3 FA into metabolic intermediates and the second cell type converting these into the specialised pro-resolving mediators. n-3 FA-derived specialised pro-resolving mediators seem to be key drivers of inflammation resolution programmes that reduce chronic inflammation in a wide range of animal models. The roles of each of these molecular pathways in the cardiovascular protection of n-3 FA represent promising areas for future investigation. (Modified and reprinted with permission from Mozaffarian et al.(Reference Mozaffarian and Wu34)).
Conclusion
The strong epidemiological evidence base for n-3 FA may reflect the very long-term effects of having higher v. lower levels of EPA and DHA in tissues. Most RCT cannot mimic this situation (except perhaps by providing much higher than nutritional doses), and thus they cannot test the long-term effects of nutrients such as n-3. Consideration of the findings from both kinds of studies sheds the most light on the potential cardiovascular benefits of the long-chain marine n-3 FA.
Acknowledgements
We are indebted to the Africa Nutritional Epidemiology Conference under the auspices of the African Nutrition Society for granting us the platform to present. We are also grateful to Sheila Gautier for her untiring efforts without which this paper may not have been written.
Financial Support
None.
Conflict of Interest
W. S. H. is the owner of a laboratory that offers the Omega-3 Index test to researchers, clinicians and consumers. F. B. Z. has no conflicts to disclose.
Authorship
The authors had joint responsibility for all aspects of preparation of this paper.




