Vitamin D is usually synthesised in skin that is exposed to UV radiation, which has led to the term ‘sunshine vitamin’(Reference Borradale and Kimlin1). Traditionally, the primary role of vitamin D is related to calcium absorption and bone health. Children and adults with vitamin D deficiency have an increased risk of developing rickets or osteomalacia(Reference Holick, Binkley and Bischoff-Ferrari2). Recently, a resurgence of childhood rickets has highlighted the need for adequate vitamin D status in many parts of the world(Reference Robinson, Hogler and Craig3–Reference Prentice5). Furthermore, mounting evidence from epidemiological studies indicates that vitamin D status is inversely associated with the risk of CVD, cancers and diabetes(Reference Borradale and Kimlin1, Reference Holick and Chen6), although there is some uncertainty about what defines an adequate vitamin D status(7).
Vitamin D deficiency is prevalent and is considered a serious issue throughout the world(Reference Forrest and Stuhldreher8–Reference Cashman and Kiely10), even in sunnier climates such as Australia and New Zealand(Reference Nowson, McGrath and Ebeling11). Recently, the Scientific Advisory Committee on Nutrition(7) reported that in the UK, 22–24 % of adults aged 19–64 years, and 17–24 % of those ≥65 years were vitamin D deficient (plasma 25-hydroxyvitamin D3 (25(OH)D3) <25 nmol/l). There are several factors that have contributed to the low vitamin D status commonly seen today, such as lifestyle changes (increased indoor lifestyle, sun screens use) and human characteristics (e.g. ageing, clothing, increased obesity, low-fat diet trend)(Reference Holick12). Therefore, foods that contribute to vitamin D intake have become more important than before. However, there are only a few foods naturally rich in vitamin D, such as oily fish and egg yolks(Reference Schmid and Walther13).
The aim of this review is first to critically evaluate the existing evidence on whether the vitamin D content of animal-derived foods can be increased by feeding cholecalciferol (vitamin D3) and/or calcifediol (25(OH)D3) supplements to laying hens, fish and cows. Second, the present review summaries evidence from the human randomised controlled trials (RCT), which include the effects of 25(OH)D3 supplementation on increasing serum/plasma 25(OH)D3 concentration.
Vitamin D absorption, synthesis and metabolism
Generally, the term vitamin D refers to both vitamin D2 and vitamin D3. Vitamin D2 is produced by fungi, while vitamin D3 is produced by human subjects and animals(Reference O'Mahony, Stepien and Gibney14). Human subjects usually synthesise vitamin D3 in the skin(Reference Holick, MacLaughlin and Clark15) where 7-dehydrocholesterol in the epidermis is converted to pre-vitamin D3 when skin is exposed to sunlight. Then, pre-vitamin D3 undergoes a temperature-dependent isomerisation to vitamin D3 over a period of approximately 3 d(Reference Holick and Chen6). Vitamin D (vitamin D2 or vitamin D3) can also be obtained from the diet(Reference Holick, MacLaughlin and Clark15) and it is absorbed with long-chain TAG in the small intestine(Reference Haddad, Matsuoka and Hollis16). It is then incorporated into chylomicrons and transported in lymph to the blood and into the general circulation(Reference Dueland, Pedersen and Helgerud17).
After entering the circulation, there are two hydroxylation reactions to convert vitamin D to the biologically active form(Reference Holick and Chen6). The first hydroxylation reaction is in the liver where vitamin D is hydroxylated to 25(OH)D by the vitamin D-25-hydroxylase enzyme. The second hydroxylation reaction is in the kidney where 25(OH)D is converted to 1,25(OH)2D by 25-hydroxyvitamin D-1α-hydroxylase(Reference Holick and Chen6), and the 1,25(OH)2D metabolite is the biologically active form of vitamin D(Reference Jones, Strugnell and DeLuca18).
Foods of animal origin as dietary sources of vitamin D
Vitamin D content of vitamin D-enriched foods can differ considerably between food retailers. One US retail study analysed the vitamin D content of egg yolks collected from twelve individual retail supermarkets across the country and reported a broad range of vitamin D3 and 25(OH)D3 concentrations of 9·7–18 and 4·3–13·2 µg/kg, respectively(Reference Exler, Phillips and Patterson19). In addition, our recent UK retail study(Reference Guo, Kliem and Lovegrove20) showed vitamin D3 and 25(OH)D3 concentrations of eggs were significantly different depending on the egg production systems. Egg yolks produced by birds kept in indoor systems had much lower concentrations (40·2 (se 3·1) µg/kg) of vitamin D3 than the egg yolks produced from outdoor systems (57·2 (se 3·2) µg/kg), while 25(OH)D3 concentrations of the eggs were higher in organic eggs only. Similarly, the vitamin D contents of fish have been shown to vary according to the production systems. The study of Lu et al. (Reference Lu, Chen and Zhang21) indicated the vitamin D3 content of wild salmon to be three times higher than that of farmed salmon; however, the 25(OH)D3 content of the salmon was not measured. In addition, other studies(Reference Mattila, Piironen and Uusi-Rauva22, Reference Bilodeau, Dufresne and Deeks23) have shown the 25(OH)D3 content of several species of marine and freshwater fish to be <0·02 µg/100 g. Therefore, foods generally regarded as rich sources of vitamin D may not be sustainable vitamin D contributors for the general population, due to variability in vitamin D content, which in turn may be influenced by production systems or different species (genotype). Furthermore, the National Diet and Nutrition Survey of the UK(Reference Bates, Lennox and Bates24) reported that the average daily intake of vitamin D for adults was 3·1 µg for men and 2·6 µg for women, which is much lower than the UK vitamin D reference nutrition intake of 10 µg/d(7). Therefore, vitamin D-enriched or fortified foods are needed to ensure an adequate vitamin D intake for the general population.
Enrichment of animal-derived foods as dietary sources of vitamin D
Vitamin D-enriched eggs
In general, there are two main methods to enrich the vitamin D content of eggs: increased sunlight exposure and vitamin D supplementation of the birds’ diet. Because hens can synthesise vitamin D from natural sunlight exposure, free-range egg production system may be an inexpensive way to increase their vitamin D content. A study by Kuhn et al. assigned laying hens to a free-range treatment or an indoor treatment for over 4 weeks and found that eggs from the free-range group, which were exposed to sunlight, had significantly higher vitamin D3 content (mean 14·3 µg/100 g DM) than eggs from the indoor group (mean 3·8 µg/100 g DM)(Reference Kuhn, Schutkowski and Kluge25). Furthermore, there are several studies which have shown that the vitamin D3 content of eggs can be enhanced by feeding vitamin D3 supplements to the hens (Table 1)(Reference Mattila, Lehikoinen and Kiiskinen26–Reference Duffy, Rajauria and Clarke32). The results of all studies revealed that egg yolk vitamin D3 concentration was efficiently increased by vitamin D3 dietary supplementation. The study of Yao et al. showed a linear dose–response relationship existed between vitamin D3 dietary supplementation and vitamin D3 concentrations of egg yolks(Reference Yao, Wang and Persia30). Moreover, as 25(OH)D3 is a metabolite of vitamin D3, the 25(OH)D3 content in eggs can also be enhanced by supplementing the birds’ diet with vitamin D3. However, the response in 25(OH)D3 content of egg yolk is much less than that of vitamin D3. Browning and Cowieson(Reference Browning and Cowieson31) showed that a 4-fold increase in vitamin D3, and a 2-fold increase in 25(OH)D3 in egg yolk resulted from a 4-fold increase in the vitamin D3 in the diet (62·5–250 µg/kg). Similarly, evidence from another study showed that the vitamin D3 in egg yolk was increased approximately 7-fold as a result of feeding a diet with a 3·5-fold higher vitamin D3 content (from 62·4 to 216 µg/kg), while the corresponding increase in 25(OH)D3 content was only about 1·5-fold(Reference Mattila, Lehikoinen and Kiiskinen26).
Table 1. Summary of enrichment studies investigating the impact of adding vitamin D to the diet of laying hens on the vitamin D content of egg yolks
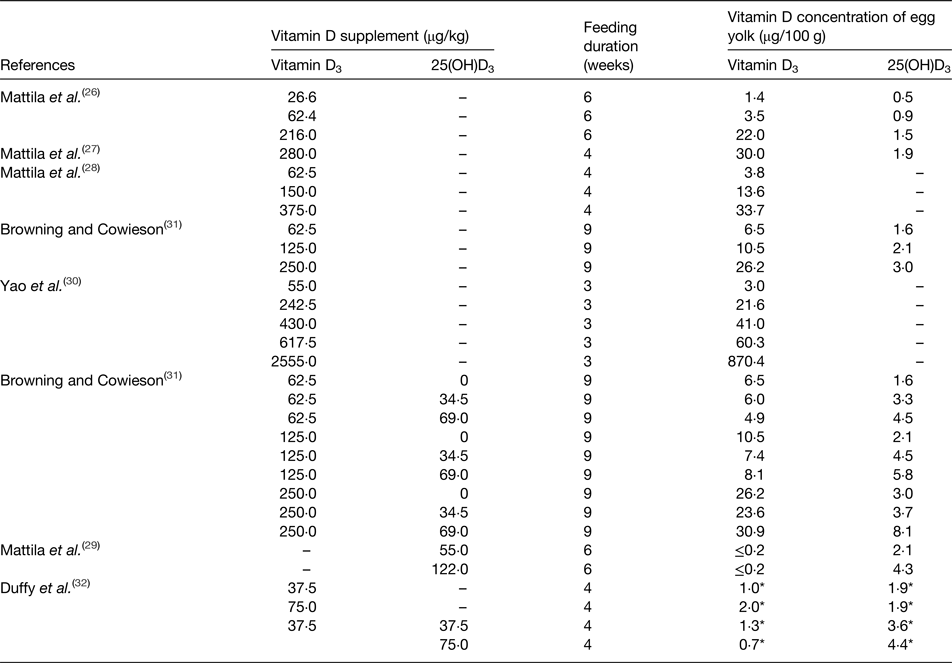
25(OH)D3, 25-hydroxyvitamin D3.
* Vitamin D content per egg.
There are only a few studies(Reference Mattila, Vakonen and Valaja29, Reference Browning and Cowieson31, Reference Duffy, Rajauria and Clarke32) examining the effect of feeding birds with diets supplemented with 25(OH)D3. In the EU, 25(OH)D3 has only recently been authorised for addition to poultry diets, and the maximum content of the vitamin D3 and 25(OH)D3 combination for laying hens is 80 µg/kg(33, 34). It is of note that most of vitamin D supplementation studies(Reference Mattila, Lehikoinen and Kiiskinen27–Reference Browning and Cowieson31), summarised in Table 1, had higher vitamin D doses than the EU diet limit(33), thus, the potential for increasing vitamin D in eggs by adding vitamin D to the diet of laying hens is limited by EU regulations. Browning and Cowieson(Reference Browning and Cowieson31) and Duffy et al. (Reference Duffy, Rajauria and Clarke32) both showed an addition of 25(OH)D3 to the vitamin D3 supplement resulted in the elevation of the 25(OH)D3 content of the egg yolk, but there was no significant increase in the vitamin D3 content of the egg yolk. Other studies investigated dietary supplementation with 25(OH)D3(Reference Mattila, Vakonen and Valaja29, Reference Duffy, Rajauria and Clarke32), and showed that only egg yolk 25(OH)D3 was increased, but not vitamin D3. Therefore, we speculate that 25(OH)D3 in the diet can be absorbed directly by laying hens without transfer to vitamin D3 in the circulation.
Vitamin D-enriched fish
There are very few studies on enriching the vitamin D content of fish (Table 2)(Reference Horvli, LIE and Aksnes35–Reference Graff, Hoie and Totland38). Mattila et al. fed rainbow trout with different doses of vitamin D3 supplements up to 539 µg/kg, but no significant differences in the vitamin D3 content of the fish fillet were observed(Reference Mattila, Piironen and Hakkarainen37). In contrast, the study of Horvli et al. with Atlantic salmon showed a dose–response relationship between the vitamin D3 in the diet up to 28·68 mg/kg and vitamin D3 in the fish meat(Reference Horvli, LIE and Aksnes35). Similar high vitamin D3 supplementation doses were reported in another two studies(Reference Vielma, Lall and Koskela36, Reference Graff, Hoie and Totland38), which also showed that elevated vitamin D3 content of the fish liver or whole fish had been achieved by supplemental vitamin D3 in the diet. However, 25(OH)D3 contents of the enriched fish were not measured in these studies(Reference Horvli, LIE and Aksnes35–Reference Graff, Hoie and Totland38), and the lack of evidence on the effects by feeding fish with 25(OH)D3 on the vitamin D content of the fish warrants further research. Again, supplement doses of the listed studies(Reference Horvli, LIE and Aksnes35–Reference Graff, Hoie and Totland38) in Table 2 were over the EU diet limit for farmed fish of 75 µg/kg(33), which will limit application in the market.
Table 2. Summary of enrichment studies investigating the impact of vitamin D supplemental fish feeding on vitamin D content of fish

* Estimated from graph.
Vitamin D-enriched milk
A few studies have investigated the longer term effect of supplemental vitamin D3 on the vitamin D content of the milk; the summary of these studies is presented in Table 3(Reference Hollis, Roos and Draper39–Reference Weiss, Azem and Steinberg42). Hollis et al. showed a 10-fold enhancement of vitamin D3 intake from 100 to 1000 µg/d resulted in a 7·5-fold increased vitamin D3 concentration of the milk and a 2-fold increase in 25(OH)D3(Reference Hollis, Roos and Draper39). Moreover, McDermott et al. compared three different doses of vitamin D3 with a control diet, and showed an increased concentration of vitamin D3 and 25(OH)D3 in the milk(Reference Mcdermott, Beitz and Littledike41). However, the relationship between increasing dietary vitamin D3 doses and milk vitamin D3 or 25(OH)D3 concentrations were not linear. Furthermore, the study of Weiss et al. investigated the effect of feeding 450 µg/d vitamin D3 to pre-calving cows for 13 d which resulted in concentrations of vitamin D3 and 25(OH)D3 in the milk ranging from 0·33–0·45 to 0·36–1·02 µg/l, respectively(Reference Weiss, Azem and Steinberg42). In addition, the study included a diet treatment of 6 mg vitamin D3 with a cation–anion difference of −138 mEq/kg daily for 13 d; the concentrations of 25(OH)D3 in the milk were increased but the treatment effect disappeared after 28 d. Therefore, evidence from the limited number of studies(Reference Hollis, Roos and Draper39–Reference Weiss, Azem and Steinberg42) demonstrated that milk vitamin D concentrations can be increased by feeding dairy cows with vitamin D supplements. However, it is of note that the highest milk vitamin D3 and 25(OH)D3 concentrations were 0·47 and 3·69 µg/l, respectively (Table 3), which for one typical milk serving of 200 ml only contributes 0·09 and 0·74 µg vitamin D3 and 25(OH)D3, respectively, well below the current UK vitamin D reference nutrition intake of 10 µg/d(7). Furthermore, the doses of vitamin D in those studies(Reference Mcdermott, Beitz and Littledike41, Reference Weiss, Azem and Steinberg42) were much higher than the maximum allowed vitamin D content in EU (0·01 mg/kg diet at 880 g DM/kg approximately equivalent to 2·27 mg/d)(34), which imposes an even greater restriction on the possibility of increasing vitamin D in milk by adding vitamin D supplements in the diet of dairy cows.
Table 3. Summary of enrichment studies investigating the impact of vitamin D supplementation to the diet of dairy cows on vitamin D content of milk
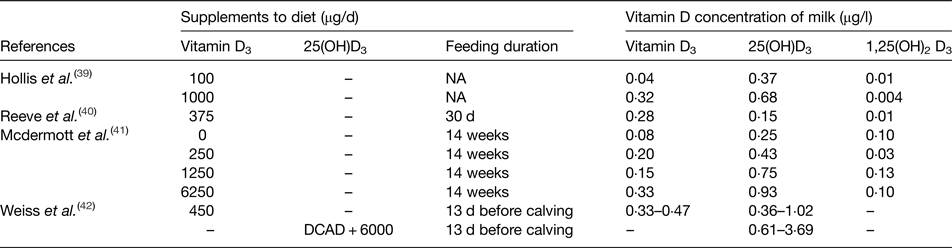
25(OH)D3, 25-hydroxyvitamin D3; 1,25(OH)2D3, 1,25 dihydroxyvitamin D3; DCAD, dietary cation–anion difference of −138 mEq/kg.
Evidence from human dietary intervention studies with vitamin D-enriched animal-derived foods
Despite numerous animal-based vitamin D-enrichment studies on vitamin D in eggs, fish and milk, there are few RCT on the effect of consuming vitamin D-enriched foods on the vitamin D status of the consumer. To our knowledge, only one recent study has investigated the weekly effect of consuming seven vitamin D3 or seven 25(OH)D3-enriched eggs on vitamin D status compared with commercial eggs of ≤2 egg/week(Reference Hayes, Duffy and O'Grady43). After 8 weeks follow-up in winter, the results showed that while the serum 25(OH)D of the subjects who consumed commercial eggs decreased from a baseline of 41 (sd 14·1) nmol/l to 35 (sd 11·4) nmol/l, the serum 25(OH)D of subjects who consumed vitamin D3-enriched eggs or 25(OH)D3-enriched eggs was maintained. The serum 25(OH) D concentrations of subjects who consumed vitamin D3- or 25(OH)D3-enriched eggs were 50 (sd 21·4) nmol/l and 49 (sd 16·5) nmol/l, respectively. However, there was no significant difference between vitamin D3- and 25(OH)D3-enriched egg consumption on serum 25(OH)D concentrations.
Although there are a limited number of human dietary intervention studies on vitamin D-enriched foods, the study of Mattila et al. (Reference Mattila, Vakonen and Valaja29) demonstrated that the effect of foods enriched with either vitamin D3 or 25(OH)D3 on human vitamin D status depended on their relative effectiveness of raising serum or plasma 25(OH)D concentrations. A previous study(Reference Jakobsen44) indicated that there was no consensus on the relative effectiveness of 25(OH)D3 compared with vitamin D3 for raising human serum or plasma 25(OH)D3 concentrations. Furthermore, UK food composition tables(Reference McCance and Widdowson45) indicate that there is no certainty on the relative potency of 25(OH)D3 compared with vitamin D3, although it was assumed that 25(OH)D3 had a potency of five times that of vitamin D3 for calculating the total vitamin D of foods(Reference McCance and Widdowson45).
Human intervention studies on the relative effects of calcifediol and cholecalciferol supplementation on vitamin D status
Heterogeneity of intervention studies
Eleven RCT that investigated the effects of 25(OH)D3 relative to vitamin D3 were identified(Reference Hahn, Halstead and Teitelbaum46–Reference Navarro-Valverde, Sosa-Henriquez and Alhambra-Exposito56) (Table 4). Nine studies administered 25(OH)D3 supplementation only, except two studies which provided a combination supplement of 25(OH)D3 and calcium(Reference Hahn, Halstead and Teitelbaum46, Reference Cavalli, Cavalli and Marcucci49). Five of the eleven studies(Reference Barger-Lux, Heaney and Dowell47, Reference Cavalli, Cavalli and Marcucci49–Reference Jetter, Egli and Dawson-Hughes52) supplemented 25(OH)D3 to generally healthy subjects, whereas the other six studies(Reference Hahn, Halstead and Teitelbaum46, Reference Jean, Terrat and Vanel48, Reference Banon, Rosillo and Gomez53–Reference Navarro-Valverde, Sosa-Henriquez and Alhambra-Exposito56) supplemented 25(OH)D3 to clinical patients. Most studies reported the serum or plasma 25(OH)D concentration at both the beginning and end of the treatment, except one study(Reference Ortego-Jurado, Callejas-Rubio and Rios-Fernandez55), which only reported the 25(OH)D concentration at the end of the treatment. In terms of the vitamin D status measurement, most studies measured total 25(OH)D concentration, except two studies(Reference Cavalli, Cavalli and Marcucci49, Reference Jetter, Egli and Dawson-Hughes52), which measured 25(OH)D3. For the characteristics of the investigated subjects, five studies included both men and women(Reference Hahn, Halstead and Teitelbaum46, Reference Jean, Terrat and Vanel48, Reference Cashman, Seamans and Lucey51, Reference Banon, Rosillo and Gomez53, Reference Ortego-Jurado, Callejas-Rubio and Rios-Fernandez55), while the other studies only included men or women. In addition, most studies reported the age and BMI of the subjects, except two studies(Reference Hahn, Halstead and Teitelbaum46, Reference Jean, Terrat and Vanel48) that did not report the BMI range.
Table 4. Summary of study details and serum 25, hydroxyvitamin D (25(OH)D) concentration in long-term randomised controlled trials with calcifediol (25 hydroxyvitamin D3 (25(OH)D3)) supplementation in adults (order by year)
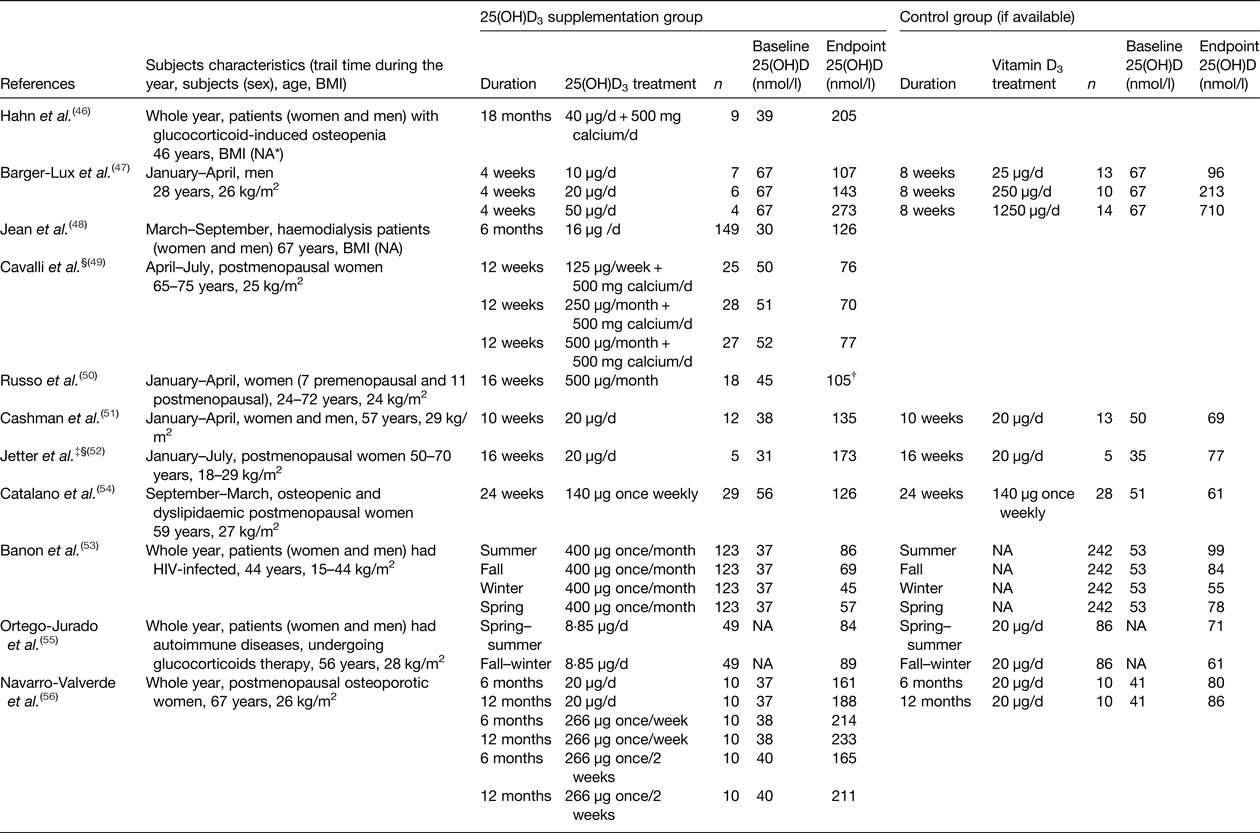
* NA, not available.
† Estimated from graph.
‡ Same study of (Jetter et al. (Reference Jetter, Egli and Dawson-Hughes52)) and (Bischoff-Ferrari et al. (Reference Bischoff-Ferrari, Dawson-Hughes and Stocklin62)).
§ Study has measured vitamin D status as 25(OH)D3.
Acute pharmacokinetic action of cholecalciferol and calcifediol
An early study provided meals with single doses of 25(OH)D3 of 1·5, 5 or 10 µg/kg body weight to generally healthy subjects and showed that the peak serum 25(OH)D3 concentration was reached within 4–8 h after ingestion(Reference Haddad and Rojanasathit57). A later study by Jetter et al. compared the pharmacokinetic absorption of vitamin D3 and 25(OH)D3 by providing a single dose of 20 µg vitamin D3 or 20 µg 25(OH)D3 to postmenopausal women(Reference Jetter, Egli and Dawson-Hughes52). The time to reach maximum plasma 25(OH)D3 concentration was 22 and 11 h for vitamin D3 and 25(OH)D3, respectively. In addition, the peak concentration of plasma 25(OH)D3 (44 nmol/l) from 25(OH)D3 supplementation was higher than vitamin D3 supplementation (35 nmol/l), although they were not significantly different. This study further compared the effect of a higher single dose of 140 µg vitamin D3 and 140 µg 25(OH)D3 with the time to reach peak plasma 25(OH)D3 being 21 and 4·8 h for vitamin D3 and 25(OH)D3 supplementation, respectively(Reference Jetter, Egli and Dawson-Hughes52). In addition, the maximum plasma concentration of 25(OH)D3 for 25(OH)D3 treatment (100 nmol/l) was significantly higher than for vitamin D3 treatment (44 nmol/l). These results suggest that 25(OH)D3 was absorbed more quickly than vitamin D3 possibly because 25(OH)D3 has higher solubility in aqueous media than vitamin D3 due to its more polar chemical structure(Reference Cianferotti, Cricelli and Kanis58). Furthermore, as this metabolite of vitamin D3 is produced in the liver, the hepatic metabolism of vitamin D3 to 25(OH)D3 is circumvented and consequently the conversion from vitamin D3 to 25(OH)D3 would be negligible(Reference Heaney, Armas and Shary59). In patients with liver disease who had an impaired ability to synthesise 25(OH)D3 from vitamin D3(Reference Nair60), the study of Sitrin and Bengoa(Reference Sitrin and Bengoa61) verified that 25(OH)D3 could be absorbed more efficiently than vitamin D3 after oral supplementation. Therefore, supplementation with 25(OH)D3 is not only more efficient at increasing vitamin D status in generally healthy people, but may also have a specific role in tackling lower vitamin D status in patients who are suffering from liver diseases.
Chronic effects and relative effectiveness of cholecalciferol and calcifediol treatments
Regarding the expected higher biological effect of 25(OH)D3 in raising serum or plasma 25(OH)D level after long-term administration, several studies have confirmed that oral consumption of 25(OH)D3 is highly effective in raising serum or plasma 25(OH)D level (Table 4)(Reference Hahn, Halstead and Teitelbaum46–Reference Navarro-Valverde, Sosa-Henriquez and Alhambra-Exposito56). However, the majority of the evidence in support of a higher impact of 25(OH)D3 supplementation compared with vitamin D3 on serum or plasma 25(OH)D3 level is from only four studies(Reference Cashman, Seamans and Lucey51, Reference Jetter, Egli and Dawson-Hughes52, Reference Catalano, Morabito and Basile54, Reference Navarro-Valverde, Sosa-Henriquez and Alhambra-Exposito56) where both 25(OH)D3 and vitamin D3 treatments were included in the same study (Table 5). The study of Barger-Lux et al. (Reference Barger-Lux, Heaney and Dowell47) provided three different doses of vitamin D3 (25, 250, 1250 µg/d) or 25(OH)D3 (10, 20, 50 µg/d) to the participants for 8 and 4 weeks, respectively. However, the effects of 25(OH)D3 and vitamin D3 treatments were not directly comparable as the interventions were not at the same dose or treatment time. Thus, the study of Barger-Lux et al. (Reference Barger-Lux, Heaney and Dowell47) was excluded from the relative effectiveness analysis. In order to compare the relative effectiveness of 25(OH)D3 and vitamin D3 supplementation on raising serum or plasma 25(OH)D concentrations, a dose–response factor was calculated for each μg of orally consumed 25(OH)D3 or vitamin D3 in four studies(Reference Cashman, Seamans and Lucey51, Reference Jetter, Egli and Dawson-Hughes52, Reference Catalano, Morabito and Basile54, Reference Navarro-Valverde, Sosa-Henriquez and Alhambra-Exposito56). The dose–response factors of 25(OH)D3 and vitamin D3 were calculated by using endpoint 25(OH)D concentration minus baseline 25(OH)D concentration, divided by the dose of the supplementation (dose–response factor = Δ serum/plasma (mmol/l)/dose (μg)). Then, the relative effectiveness of 25(OH)D3 to vitamin D3 was calculated by dividing the dose–response factor of 25(OH)D3 by that of vitamin D3.
Table 5. Summary of randomised controlled trials with both calcifediol (25 hydroxyvitamin D3 (25(OH)D3)) and vitamin D3 in adults to calculate the relative effectiveness of 25(OH)D3 and vitamin D3 supplementation in raising serum 25, hydroxyvitamin D (25(OH)D) level
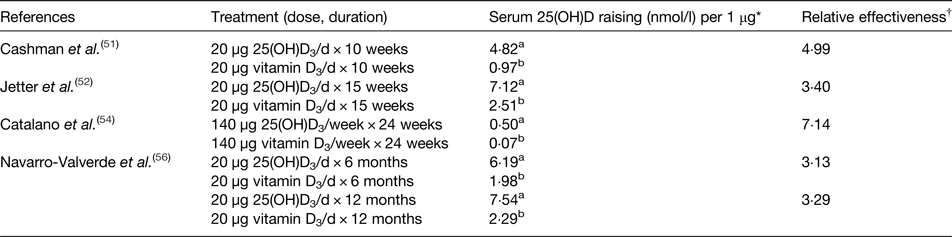
* Dose–response factor = Δ serum/plasma (mmol/l)/dose (μg).
† Relative effectiveness = a/b within same study.
The highest relative effectiveness was found in the study by Catalano et al. (Reference Catalano, Morabito and Basile54). Weekly treatment of 140 µg 25(OH)D3 or 140 µg vitamin D3 supplements was provided to osteopenic and dyslipidaemic postmenopausal women for 24 weeks. Supplementation with 25(OH)D3 raised serum 25(OH)D from a baseline of 56–126 nmol/l, while vitamin D3 treatment increased serum 25(OH)D to a lower extent, from baseline 51 to 61 nmol/l. Thus, the relative effectiveness factor derived from this study was 7·14, i.e. dietary 25(OH)D3 was 7·14 times more effective at increasing serum 25(OH)D than dietary vitamin D3.
Vitamin D dietary recommendations are generally between 10 and 20 µg/d(Reference Cashman and Kiely10), yet, there are few studies which have compared the effectiveness of dietary 25(OH)D3 and vitamin D3 using doses of 20 µg in their treatments. Cashman et al. (Reference Cashman, Seamans and Lucey51) provided daily supplements of 20 µg vitamin D3 or 20 µg 25(OH)D3 to adult men and women with a mean age of 57 years and with baseline serum 25(OH)D of 28·9 nmol/l during winter. After 10 weeks of supplementation, the subjects’ serum 25(OH)D increased to 135 and 69 nmol/l for the 25(OH)D3 and vitamin D3 treatments, respectively. A relative effectiveness factor of 4·99 was calculated representing the relative effectiveness of each μg of dietary 25(OH)D3 relative to dietary vitamin D3 for raising serum 25(OH)D concentration. However, lower relative effectiveness factors were achieved in other studies using the same dose of 20 µg vitamin D3 and 25(OH)D3. Jetter et al. supplemented healthy postmenopausal women with 20 µg 25(OH)D3 or 20 µg vitamin D3 for 16 weeks during the winter(Reference Jetter, Egli and Dawson-Hughes52). They found that for the 25(OH)D3 treatment, plasma 25(OH)D3 increased to 173 nmol/l from a baseline of 31 nmol/l, whereas for the vitamin D3 treatment, plasma 25(OH)D3 increased to 77 nmol/l from a baseline level of 35 nmol/l. The relative effectiveness factor of each μg of 25(OH)D3 was 3·40 compared with vitamin D3 in raising plasma 25(OH)D3 level. A similar low relative effectiveness factor was found in another study where post-menopausal osteoporotic women were given either 20 µg vitamin D3 or 20 µg 25(OH)D3 over 6 or 12 months(Reference Navarro-Valverde, Sosa-Henriquez and Alhambra-Exposito56). The serum concentration of 25(OH)D for the 25(OH)D3 treatment reached 161 and 188 nmol/l from a baseline of 37 nmol/l after 6 or 12 months administration, respectively, while the comparable values for the vitamin D3 treatment were an increase to 80 and 86 nmol/l from a baseline of 41 nmol/l. So the relative effectiveness factor of 25(OH)D3 relative to vitamin D3 treatment at 6 and 12 months were 3·13 or 3·29, respectively.
In summary, of the studies reviewed, the relative effectiveness of 25(OH)D3 to vitamin D3 for raising vitamin D status (Table 5), ranged from 3·13 to 7·14. Previous studies have demonstrated that the season may have influences on vitamin D status(Reference Schmid and Walther13, Reference O'Mahony, Stepien and Gibney14). There were two studies conducted during the winter which may have minimised any confounding influence of cutaneous vitamin D synthesis from UV radiation (Reference Barger-Lux, Heaney and Dowell47, Reference Cashman, Seamans and Lucey51). Other studies have longer intervention periods of 6 months or more, which could not have avoided some cutaneous synthesis. Furthermore, baseline status may be another factor that influences the relative effectiveness factor. The study of Catalano et al. had the highest factor of 7·14 in the present review, and the baseline concentration of 25(OH)D of the study participants was higher (>50 nmol/l) than the others(Reference Catalano, Morabito and Basile54). Therefore, the different relative effectiveness seen in different studies may be due to the different characteristics or genotypes of the subjects, or different study designs.
Overall, evidence suggests that dietary 25(OH)D3 can more effectively increase serum 25(OH)D concentrations than vitamin D3 and may also be absorbed faster reaching a serum or plasma 25(OH)D plateau earlier than vitamin D3 supplementation. Furthermore, supplementation with 25(OH)D3 may also have more benefits to human health compared with vitamin D3 in a general healthy population. Bischoff-Ferrari et al. reported that 20 µg 25(OH)D3 supplementation over 4 months led to a 5·7 mmHg decrease in systolic blood pressure and improvements in several markers of innate immunity in healthy postmenopausal women(Reference Bischoff-Ferrari, Dawson-Hughes and Stocklin62).
For patients with different diseases and receiving long-term medication, studies(Reference Mehrotra, Kermah and Budoff63–Reference Griz, Bandeira and Diniz65) showed that several drugs (e.g. antiepileptic agents, glucocorticoids, antiretroviral or anti-oestrogen drugs) interfered with vitamin D metabolism, which resulted in patients being more likely to have low vitamin D status. Thus, it is not only important to increase vitamin D status in the generally healthy population but also in patients with specific illnesses and receiving certain medication. Therefore, the studies using 25(OH)D3 treatments in patients were also summarised in Table 4(Reference Hahn, Halstead and Teitelbaum46, Reference Jean, Terrat and Vanel48, Reference Banon, Rosillo and Gomez53–Reference Navarro-Valverde, Sosa-Henriquez and Alhambra-Exposito56), and those studies consistently reported that chronic 25(OH)D3 supplementation effectively increased serum 25(OH)D concentrations. For example, Ortego-Jurado et al. showed a lower daily dose of 8·85 µg 25(OH)D3 to be more effective than a 20 µg dose of vitamin D3 for increasing vitamin D status in patients with autoimmune disease who were treated with a low dose of glucocorticoids throughout the year(Reference Ortego-Jurado, Callejas-Rubio and Rios-Fernandez55). Similarly, the study of Banon et al. showed that a monthly dose of 400 µg 25(OH)D3 was safe and effective at improving vitamin D status of HIV-infected patients throughout the year(Reference Banon, Rosillo and Gomez53).
Furthermore, supplementation with 25(OH)D3 may have additional benefits on patients’ health. Previously, 25(OH)D3 was recommended for patients with kidney disease since 25(OH)D3 has a direct action on bone metabolism(Reference Torregrosa, Bover and Cannata66). Hahn et al. provided a daily 40 µg 25(OH)D3 and 500 mg calcium supplement to patients who had glucocorticoid-induced osteopenia for 18 months(Reference Hahn, Halstead and Teitelbaum46). The treatment markedly increased vitamin D status from 39 to 205 nmol/l. In addition, this study showed that the 25(OH)D3 treatment improved mineral and bone metabolism. Jean et al. also offered haemodialysis patients who suffered from vitamin D deficiency with a daily dose of 16 µg 25(OH)D3 for 6 months; vitamin D status reached 126 nmol/l from 30 nmol/l, at the same time 25(OH)D3 supplementation corrected the excess bone turnover(Reference Jean, Terrat and Vanel48). Similarly, a study by Catalano et al. (Reference Catalano, Morabito and Basile54) provided 140 µg 25(OH)D3 supplements for 24 weeks to osteopenic and dyslipidaemic postmenopausal women, and results showed that 25(OH)D3 improved plasma lipid levels (increased HDL-cholesterol (P = 0·02) and decreased LDL-cholesterol (P = 0·02)) in osteopenic and dyslipidaemic postmenopausal women when added to an ongoing atorvastatin treatment.
As an alternative to vitamin D-enriched foods, vitamin D fortification of foods may also be an option for tackling vitamin D deficiency throughout the world. In general, fortification of foods refers to mandatory and voluntary fortification. The contribution of vitamin D-fortified foods to vitamin D intake by the public varies considerably between countries as there are different food standard policies(Reference Cashman and Kiely10), and in practice, vitamin D2 or vitamin D3 are used for fortification. Evidence from one previous meta-analysis of RCT showed that vitamin D3 supplementation is more effective at raising vitamin D status than vitamin D2(Reference Tripkovic, Lambert and Hart67). However, a further comprehensive systematic review and meta-analysis of thirty-three RCT(Reference Shab-Bidar, Bours and Geusens68) showed that the effect of vitamin D3 supplement on serum 25(OH)D3 response was limited by the supplemental dose, duration, age of subjects and baseline level. In addition, the meta-analysis showed a greater serum or plasma 25(OH)D increase when the intervention study used a dose of 20 µg/d vitamin D3 or even higher, with subjects aged >80 years and an administration period of at least 6–12 months or subjects had lower baseline 25(OH)D status (<50 nmol/l) than subjects aged <80 years, administration period <6 months or subjects had higher baseline 25(OH)D status (≥50 nmol/l)(Reference Shab-Bidar, Bours and Geusens68). Therefore, better strategies are needed to raise vitamin D status of the public throughout life, and 25(OH)D3-fortified foods warrant further research.
Conclusions
Vitamin D insufficiency has become a world problem, especially where sunlight exposure is limited by geographic reasons (latitude), personal characteristics (skin pigmentation, ageing) or behaviour (sunscreen use, cultural reasons). However, there are a few natural foods rich in vitamin D. Thus, vitamin D-enriched foods produced through a food chain approach such as feeding animals vitamin D supplements or vitamin D-fortified foods are needed to guarantee an adequate dietary intake of vitamin D by the general population.
The present review summarised the available and limited number of RCT investigating the effect of 25(OH)D3 supplementation on serum or plasma 25(OH)D concentration. We concluded that it is difficult to get consensus on the effectiveness of 25(OH)D3 supplementation relative to vitamin D3 for raising vitamin D status, due to various influencing factors such as different person characteristics (age, BMI), baseline vitamin D status and time of the year. However, it is unquestionable that 25(OH)D3 supplementation is more efficient at raising serum 25(OH)D concentrations and also appears to be absorbed faster by than the same dose of vitamin D3. Second, by reviewing available evidence on vitamin D-enriched eggs, fish or milk, it is practical and possible to increase the vitamin D content of eggs, fish or milk by addition of vitamin D supplements to the diet of poultry, fish or dairy cows. However, the limitations of adding vitamin D to animal feed should be considered in future enrichment studies. Furthermore, there are a few RCT investigating the impact of these vitamin D-enriched foods on improving vitamin D status. Therefore, 25(OH)D3-enriched or fortified foods should be further explored in the future, and additional RCT should be conducted to investigate the effect of 25(OH)D3-enriched or fortified foods on vitamin D status of the general population and patients with long-term health conditions.
Financial Support
This review received no specific grant from any funding agency in the public, commercial or not-for-profit sectors. J. G. was supported by the Barham Benevolent Foundation studentship.
Conflicts of Interest
None.
Authorship
J. G. conceived and wrote the manuscript. All authors critically reviewed and approved the final version of the manuscript.







