Dissociative identity disorder (DID)Reference Gillig1 is probably the most disputed psychiatric disorder.Reference Dalenberg, Brand, Loewenstein, Gleaves, Dorahy and Cardeña2, Reference Brand, Vissia, Chalavi, Nijenhuis, Webermann and Draijer3 DID is the most severe of the dissociative disorders and involves two or more dissociative personality states as well as recurrent gaps in the recall of everyday events, important personal information and/or traumatic events that are inconsistent with ordinary forgetting and is not related to substance misuse or general medication. For decades the disorder has been officially recognised in the DSM (in the DSM-III (1980) as Multiple Personality Disorder), but many patients with DID share a history of years of misdiagnoses, inefficient pharmacological treatment and several admissions to hospital.4 To date, many clinicians and scientists still question the validity and even the very existence of DID.Reference Piper and Merskey5, Reference Pope, Barry, Bodkin and Hudson6 Unfamiliarity with the spectrum of dissociative disorders, lack of knowledge and appreciation of its epidemiology,Reference Sar7 the existence of imitative DID as well as the reluctance of individuals with DID to present their dissociative symptoms, often due to feelings of shame, may lead to the validity concerns and under- and misdiagnosis of DID. Years of incorrect treatment results in protracted personal suffering and high direct and indirect societal costs.Reference Lloyd8, Reference Myrick, Webermann, Langeland, Putnam and Brand9 Structural brain imaging holds promise to aid disease diagnosis by providing objective biomarkers at the single-subject level,Reference Wolfers, Buitelaar, Beckmann, Franke and Marquand10 thereby paving the way for a fast and correct diagnosis of individuals with DID. The current study presents a first step towards an automated classification of individuals with DID by investigating whether individuals with DID can be separated from healthy controls on the basis of neuroimaging markers, thus informing DID's neuroanatomical basis in terms of grey and white matter of the brain.
Sceptics of DID assume that dissociative symptoms can easily be simulated by normal healthy individuals, which comprises the Fantasy Model of DID.Reference Vissia, Giesen, Chalavi, Nijenhuis, Draijer and Brand11 Several pioneering functional brain imaging studies from our group have shown that individuals with genuine DID can be distinguished from healthy controls simulating DID,Reference Schlumpf, Nijenhuis, Chalavi, Weder, Zimmermann and Luechinger12–Reference Reinders, Willemsen, Vissia, Vos, den Boer and Nijenhuis14 but critics could still argue that people can manipulate their brain activity.Reference Zotev, Phillips, Young, Drevets and Bodurka15, Reference Kim and Birbaumer16 As neuroanatomical data is unlikely to be subject to cognitive manipulation, in this study we investigate whether people with DID can be separated from healthy controls at the individual level on the basis of neuroanatomical biomarkers by employing a multivariate data-driven pattern recognition approach. We used a probabilistic pattern recognition approachReference Wolfers, Buitelaar, Beckmann, Franke and Marquand10 that allowed us to investigate the diagnostic value of grey and white matter of the brain in a comparatively large sample of individuals with DID as compared with healthy controls, and to quantify the degree to which the brain phenotype of people with DID can be distinguished from those of healthy people. This study is important in two ways: (a) it provides evidence as to whether genuine DID can be distinguished from normal healthy individuals on the basis of their brain morphology, thereby addressing the core of the Fantasy Model for DID; and (b) it provides neuroanatomical biomarkers that could support the development of pattern recognition methodologies to be used as a clinically useful aid for the diagnostic accuracy of DID.
Method
Participants
Magnetic resonance imaging (MRI) data from 75 participants were obtained in the Netherlands in the University Medical Centre in Groningen (UMCG) and the Amsterdam Medical Centre (AMC), and in Switzerland at the University Hospital in Zurich. Details of these two samples have previously been published elsewhere: Chalavi et al Reference Chalavi, Vissia, Giesen, Nijenhuis, Draijer and Barker17, Reference Chalavi, Vissia, Giesen, Nijenhuis, Draijer and Cole18 (Dutch sample), Schlumpf et al Reference Schlumpf, Nijenhuis, Chalavi, Weder, Zimmermann and Luechinger12, Reference Schlumpf, Reinders, Nijenhuis, Luechinger, van Osch and Jäncke13 (Swiss/German sample) and Reinders et al Reference Reinders, Chalavi, Schlumpf, Vissia, Nijenhuis and Jäncke19 (combined). All participants gave informed written consent in accordance with the Declaration of Helsinki and as dictated by ethics approval which was obtained from the Medical Ethical Committees of UMCG (Reference number: METC2008.211) and the AMC (Reference number: MEC09/155), and by the cantonal ethical commission of Zurich (Kantonale Ethikkommission Zürich; reference number: E-13/2008).
Overall, 32 women with DID were recruited from private practitioners of psychiatry and psychotherapy and psychiatric out-patient departments. They were initially diagnosed according to DSM-IV criteria for DID.20 The clinical diagnosis was subsequently confirmed by independent expert clinicians using the Structural Clinical Interview for DSM-IV Dissociative Disorders (SCID-D),Reference Boon and Draijer21, Reference Steinberg22 specifically to avoid the inclusion of imitative DID.Reference Draijer and Boon23 DID is known to be highly comorbid.Reference Rodewald, Wilhelm-Göling, Emrich, Reddemann and Gast24, Reference Bozkurt, Duzman Mutluer, Kose and Zoroglu25 In the current study post-traumatic stress disorder (PTSD) comorbidity was debriefed using the SCID-D and it was found that out of the 32 DID individuals, 29 had comorbid PTSD and 3 had PTSD in remission. The following information concerning other comorbid disorders was obtained based on DSM-IV (1994) classification from the participants and/or their personal therapists (N = 29): no other comorbid disorders (N = 13), somatoform disorder (N = 2), depression (chronic N = 1, recurrent N = 10), dysthymic disorder (N = 1), specific phobias (N = 3), panic disorder (N = 3), anxiety disorder (N = 1), obsessive–compulsive disorder (N = 1), personality disorders (not otherwise specified (N = 2), mixed (N = 2), borderline personality disorder (N = 5), dependent and histrionic (N = 1)), eating disorder (N = 3), sleeping disorder (N = 2), catalepsy (N = 1), psychogenic seizures (N = 1) and attention deficit disorder (N = 1). Of note, since approximately half of the DID participants did not have any other comorbidities apart from PTSD and other comorbidities were more randomly distributed across the sample, it is unlikely that these contribute to the classification in a systematic manner. Nevertheless, if this were to influence classification then our results are likely to represent an underestimation of classification strength and a more homogeneous sample would increase the sensitivity and specificity.
The DID and healthy control group were carefully matched for demographics including age, gender (all female), years of education and Western European ancestry. As previously shown,Reference Reinders, Chalavi, Schlumpf, Vissia, Nijenhuis and Jäncke19 we did not find any significant differences between patients and healthy controls with respect to age or education (see Supplementary Table 1 available at https://doi.org/10.1192/bjp.2018.255). As part of the inclusion criteria, it was confirmed that all healthy controls were free of medication and psychiatric disorders. Furthermore, they scored below a critical cut-off of 25 on the Dissociative Experiences ScaleReference Bernstein and Putnam26 and 29 on the Somatoform Dissociation Questionnaire.Reference Nijenhuis, Spinhoven, Van Dyck, Van der Hart and Vanderlinden27 Depersonalisation symptoms were assessed using the Cambridge Depersonalisation Scale.Reference Sierra and Berrios28 Traumatic experiences were measured with the Traumatic Experience Checklist.Reference Nijenhuis, Van der Hart and Kruger29 As expected and previously reported,Reference Reinders, Chalavi, Schlumpf, Vissia, Nijenhuis and Jäncke19 psychoform and somatoform dissociative symptoms and depersonalisation scores were significantly higher in the DID group as compared with healthy controls (all P-values <0.001). Individuals with DID also scored significantly higher compared with the controls for traumatic experiences (all P-values <0.001) on all five adverse life event categories, namely emotional neglect, emotional abuse, physical abuse, sexual abuse and sexual harassment (see Supplementary Table 1).
MRI
Data acquisition
All data were obtained on 3-T Philips whole-body MRI scanners (Philips Medical Systems, Best, Netherlands) in one of the three participating centres. An optimised structural MRI protocolReference Chalavi, Simmons, Dijkstra, Barker and Reinders30 with high reproducibility between centres was used at all three centres and T1-weighted anatomical MR scans were acquired (3D MPRAGE, repetition time 9.95 ms, echo time 5.6 ms, flip-angle 8°, 1 × 1 × 1 mm3 voxels, number of slices 160, total scan time 10 m 14 s).
DID patients and healthy controls were scanned interleaved (i.e. alternating between patients and healthy controls) within a short time interval. Final samples were distributed relatively equally over the three centres, with 10 patients and 17 healthy controls included from the UMCG, 7 patients and 11 healthy controls from the AMC and 15 patients and 15 healthy controls from Zurich. The number of participants in each group also did not differ across centres (χ2 = 1.01, P = 0.603).
Image preprocessing
After quality control checks, structural images were preprocessed using the SPM8 software (http://www.fil.ion.ucl.ac.uk/spm/, release 4667). They were first segmented into different tissue types via the ‘new segment’ tool,Reference Ashburner and Friston31 which is part of the voxel based morphology (VBM) processing pipeline. Rigidly aligned grey and white matter maps, down-sampled to 1.5 mm isotropic voxel size, were then used to diffeomorphically register all participants to their common average (i.e. study-specific template) by using a matching term that assumed a multinomial distribution.Reference Ashburner and Friston32 Registration involved estimating a set of initial velocities from which the deformations were computed by a geodesic shooting procedure.Reference Ashburner and Friston33 Classification was based on a set of ‘scalar momentum’ image features derived from this registration, which describe anatomical variability among participants.Reference Singh, Fletcher, Preston, Ha, King and Marron34 These images contain all information necessary to reconstruct the original images (in addition to the template) and therefore provide a parsimonious representation of shape difference. The scalar momentum images for grey and white matter were spatially smoothed with an isotropic 10 mm Gaussian kernel and concatenated prior to classification. This choice of feature construction method and smoothing level was based on previous work, where smoothed scalar momentum images yielded greater accuracy than a range of alternative features (including Jacobian determinants, rigidly aligned grey matter, spatially normalised grey matter and Jacobian scaled spatially normalised grey matter) for predicting participant age and gender in a publicly available data setReference Marquand, Filippone, Ashburner, Girolami, Mourao-Miranda and Barker35 and for discriminating neurological disorders from structural MRI.Reference Marquand, Filippone, Ashburner, Girolami, Mourao-Miranda and Barker35
Pattern recognition
Binary Gaussian process classifiers (GPCsReference Rasmussen and Williams36) were used as the primary analytical approach for this study. GPCs are a supervised pattern recognition approach that assigns a predictive probability of group membership to each individual based on a set of ‘training’ data. They are kernel classifiers similar to the widely used Support Vector Machines and have shown high levels of performance for neuroimaging data.Reference Marquand, Howard, Brammer, Chu, Coen and Mourão-Miranda37–Reference Schrouff, Rosa, Rondina, Marquand, Chu and Ashburner39 Moreover, GPCs have advantages over Support Vector Machines in that the probabilistic predictions they provide encode a measure of predictive confidence that quantifies diagnostic uncertainty and can capture variability within clinical groups. More importantly, probabilistic predictions can be easily adjusted to compensate for the prior frequency of diagnostic classes in experimental populations. This means that inference remains coherent in classification scenarios where the frequency of each class in the test set is different from the frequencies observed in the training set and is useful to accommodate variations in disease prevalenceReference Hahn, Marquand, Plichta, Ehlis, Schecklmann and Dresler40 and to compensate for unbalanced training data setsReference Lim, Jung, Ahn, Won, Hahn and Lee41 as outlined below. These properties are particularly important for the present study because the classes are unbalanced and the number of samples available for training is small.
Linear binary GPCs were used to discriminate people with DID from healthy controls. Technical details surrounding GPC inference have been presented elsewhere. A leave-one-participant-out cross-validation approach was used to assess classifier generalisability, whereby a single participant was excluded to comprise the test set, and all parameters were inferred from the remaining data (training set), before applying this classifier to predict the labels for the test samples. Prior to classification, a t-test was used to select a set of discriminant voxels, using a fixed threshold of P < 0.001. This was repeated, excluding each participant once. Importantly, feature selection was performed using the training data only, which ensures the classifier remains unbiased.
Classifier performance was evaluated using receiver operating characteristic curves derived from the probabilistic predictions and classification accuracy, which measures the classifier performance in a categorical sense. To derive categorical predictions from the probabilistic predictions obtained from the GPC, a threshold according to the frequency of classes in the training set was applied to the probabilistic predictions (i.e. 0.5 if the classes are balanced). Classifier assessment metrics included the sensitivity and specificity in addition to the positive and negative predictive value. Finally, the (balanced) accuracy was computed as the mean of sensitivity and specificity, which quantifies the overall categorical classification performance of the classifier in a way that accommodates potential class imbalance in the data.
Forward maps of regional class differences
To understand the relative importance of different brain regions underlying the classification decision, we employed a forward mapping strategy.Reference Haufe, Meinecke, Gorgen, Dahne, Haynes and Blankertz42 This has advantages over the more commonly used strategy of directly mapping the weight vector.Reference Mourão-Miranda, Bokde, Born, Hampel and Stetter43 Specifically, a voxel may have a high weight because of an association with the class labels or as a result of high collinearity between voxels, where the high weight may help to cancel out noise or mismatch in other voxels. In contrast, the coefficients from the forward modelling approach proposed by Haufe and colleaguesReference Haufe, Meinecke, Gorgen, Dahne, Haynes and Blankertz42 represent the group differences between classes, which are more often of interest when interpreting a trained classifier. In this case, stronger positive values in a forward map indicate a stronger association of a region with the DID (‘favouring DID’), i.e. increased volume of a brain area in DID; whereas negative values indicate a stronger association with healthy controls (‘favouring healthy controls’), i.e. decreased volume of a brain area in DID. Note that only voxels surviving the univariate feature selection step are included in the map and that the GPC discriminates data on the basis of the whole pattern. Hence, local inferences based on these approaches should be made with caution. To report results in standard Montreal Neurological Institute (MNI) coordinates, the DARTEL group tissue template (grey matter [GM], white matter [WM] and cerebrospinal fluid [CSF]) was coregistered to an SPM8 new_segment segmentation of the FSL MNI152_T1_1 mm template brain. Advanced normalisation tools (ANTS version 2.1.0Reference Avants, Tustison, Song, Cook, Klein and Gee44) were used to calculate a non-linear transformation employing the SyN algorithm (antsRegistrationSyN.sh, default parameters) using multimodal information from the three channels (CSF, GM and WM). The results of the registration were visually inspected and it appeared to be a significant improvement on a simple affine registration. We selected the cluster peaks of the forward maps in terms of x-, y- and z-coordinates on the basis of the maximum (absolute) coefficient. Anatomical labels were derived by A.A.T.S.R., S.C. and Y.R.S. from a consensus of different atlases.
Results
Classification
Using the imaging data, the classifier discriminated between the DID and healthy control groups with high sensitivity (71.88%) and specificity (73.81%), yielding an overall balanced accuracy of 72.84%, which was significantly higher than the accuracy expected by chance (P < 0.01, permutation test). Receiver operating characteristic analysis (see Fig. 1) further demonstrated that classification performance was above chance across all decision thresholds and yielded an area under the curve of 0.74. The forward maps for the grey and white matter components are displayed in Fig. 2. Brain areas contributing to the classification are listed in Table 1.
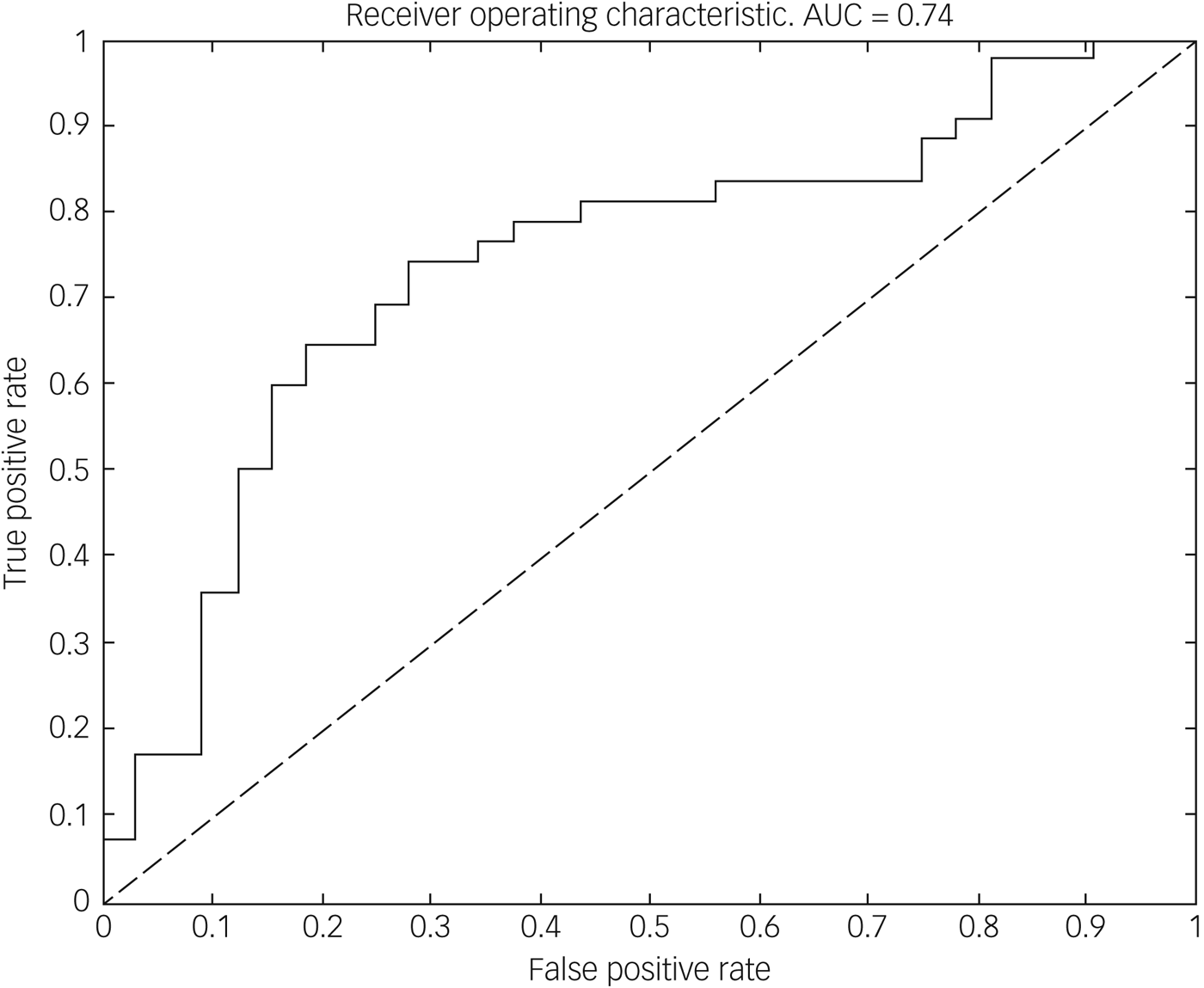
Fig. 1 Receiver operating characteristic curve for discriminating between people with dissociative identify disorder (DID) and healthy controls. The dotted line indicates chance level and the solid line is constructed by varying the decision threshold smoothly between 0 and 1, plotting true-positive rate (sensitivity) against false-positive rate (1 − specificity). Points northwest of the dotted line indicate above chance discrimination. The area under the curve (AUC) summarises classifier performance across all decision thresholds.
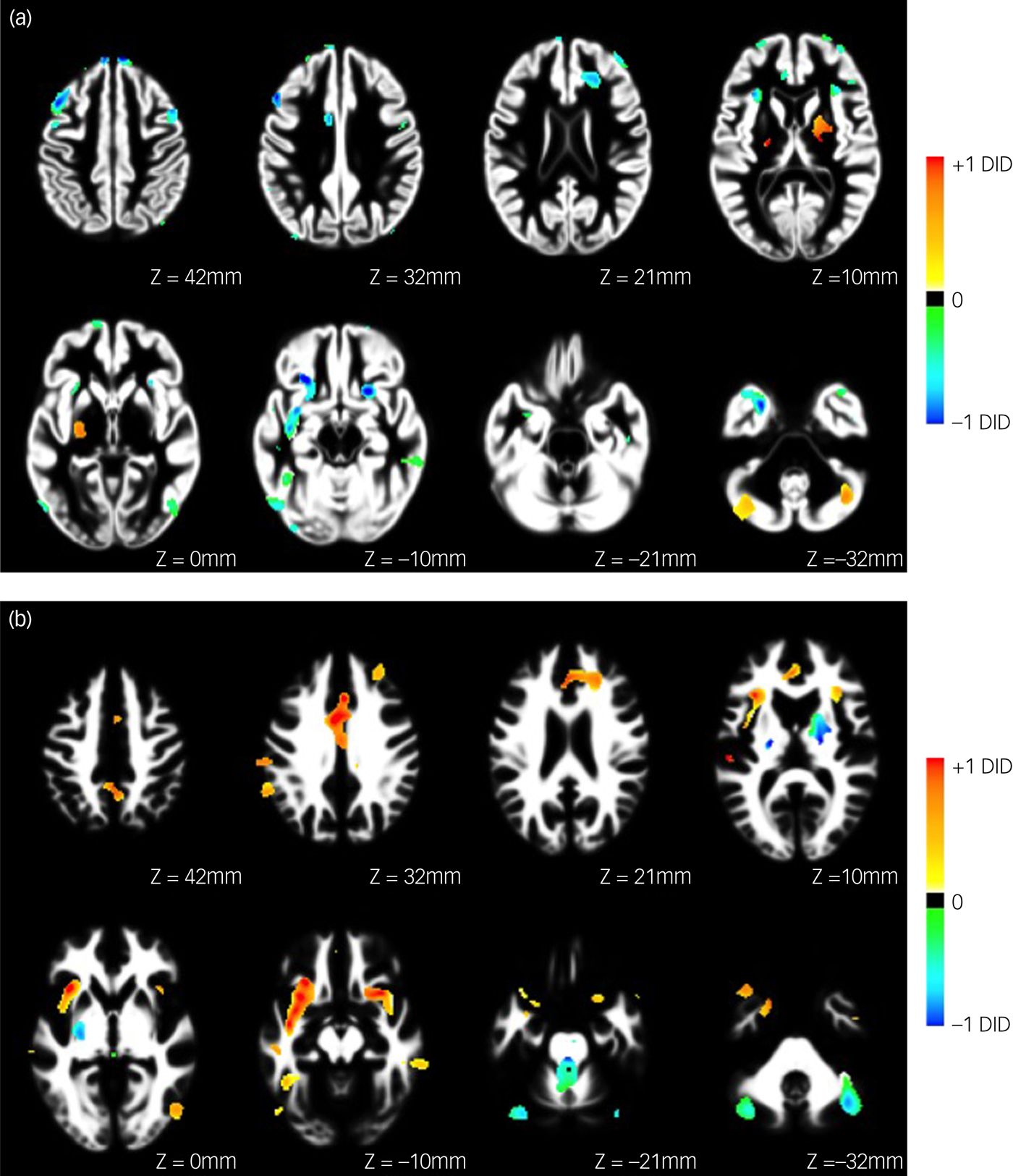
Fig. 2 Forward map showing the underlying pattern of abnormality differentiating people with dissociative identity disorder (DID) from normal healthy controls for (a) grey and (b) white matter. Coefficient images are overlaid on the study-specific anatomical templates in axial views. Images are scaled such that the maximum value in each image was equal to one, and only regions surviving the feature selection step are shown. Positive coefficients indicate a positive association in favour of DID, whereas negative coefficients indicate a positive association in favour of healthy controls.
Table 1 Grey and white matter patterns of affected brain regions

Only clusters larger than an arbitrary threshold of eight voxelsReference Reinders, Willemsen, Vissia, Vos, den Boer and Nijenhuis14 are shown. L, left; R, right; BA, Brodmann area; x, y, z, coordinates in MNI space; DID, dissociative identity disorder; Max, weight vector score; I., inferior; M., middle; S., superior.
a. Favouring healthy controls: negative values in a forward map indicate a stronger association with healthy controls, i.e. a relative decreased volume of a brain area in DID.
b. Favouring DID: positive values in a forward map indicate a stronger association of a region with the DID, i.e. relative increased volume of a brain area in DID.
Relative decrease in regional volume in the DID group
For grey matter, the pattern of voxels favouring healthy controls, i.e. a relative increase for the healthy control group or a relative decrease in the DID group, included bilateral middle, superior and dorsolateral frontal gyrus; left medial and right orbito-frontal gyrus; bilateral anterior cingulate gyrus; bilateral middle temporal gyrus; bilateral fusiform gyrus; right inferior temporal gyrus; left inferior parietal lobule and supramarginal gyrus and bilateral superior occipital gyrus.
For white matter, the pattern of voxels favouring healthy controls included the bilateral inferior fronto-occipital tract, the left corticospinal tract, and the right superior and left inferior longitudinal fasciculus. In addition, the white matter of the right inferior, bilateral middle and superior frontal regions; bilateral temporal, cerebellar and lateral occipital regions; the left amygdala–hippocampal junction and (anterior) cingulate were also included in the distinguishing pattern.
Relative increase in regional volume for the DID group
For grey matter, the pattern of voxels favouring DID was found to be far less pronounced than that of favouring healthy controls, and included left superior frontal gyrus, left medial parietal lobule and bilateral cerebellum.
The pattern of voxels in the white matter of the brain favouring DID included left inferior and superior longitudinal fasciculus, the left inferior fronto-occipital fasciculus and the right corticospinal tract. In addition, the white matter of bilateral (anterior) cingulate and insula regions, bilateral inferior, medial and superior frontal regions, left parietal regions and putamen, right inferior and middle temporal regions and bilateral cerebellum were included.
Discussion
Main findings
This is the first study to demonstrate that individuals with DID and healthy controls can be differentiated at the individual level with a high level of accuracy on the basis of their neuroanatomical markers. This discrimination was based on neuroanatomical data in the largest sample of individuals with DID included in a brain imaging study to date. We found widespread grey and white matter spatially dependent patterns of abnormal brain morphology in individuals with DID as compared with healthy controls. These findings are important because they provide evidence for a biological basis for distinguishing between genuine DID and healthy controls. The current study also provides support for the development of pattern recognition methodologies as a clinically useful diagnostic tool in DID, thereby paving the way for future studies with a diagnostic aim in distinguishing between genuine DID and other psychopathologies.
Applying pattern recognition methods to brain imaging data of people with DID offers a biomarker approach that can complement, aid and improve clinical decision making. This could reduce misdiagnoses, treatment time, treatment costs and ultimately improve the patient's quality of life.
Comparison to other studies
Despite the reported high prevalence of DID of approximately 6% in psychiatric out-patientsReference Foote, Smolin, Kaplan, Legatt and Lipschitz45 and 1–3% of the general population,Reference Sar, Akyüz and Doğan46 neurobiological studies on DID are scarceReference Chalavi, Vissia, Giesen, Nijenhuis, Draijer and Barker17, Reference Reinders, Chalavi, Schlumpf, Vissia, Nijenhuis and Jäncke19, Reference Dorahy, Brand, Sar, Krüger, Stavropoulos and Martínez-Taboas47 and depend on mass univariate data-analysis approaches, which provide inferences at the group level. Mass univariate data-analyses ignore information encoded by spatially distributed patterns of morphological abnormalities throughout the brain and have limited ability to provide inferences at the level of the individual. In contrast, multivariate data-analysis techniques using pattern classification methodology allow for the study of underlying patterns of effects and the distribution across brain regions and, more importantly, they provide predictions that quantify group separation at the individual level on the basis of patterns of abnormality in the data, which may be clinically useful.Reference Wolfers, Buitelaar, Beckmann, Franke and Marquand10
Pattern recognition methods are becoming widely used for neuroimaging-based psychiatric diagnostics.Reference Wolfers, Buitelaar, Beckmann, Franke and Marquand10 Our study shows that people with DID can be distinguished from healthy controls at an individual level with a sensitivity of 71.88% and specificity of 73.81%. This level of accuracy is comparable to what has been demonstrated for most psychiatric disorders,Reference Wolfers, Buitelaar, Beckmann, Franke and Marquand10 including psychosis.Reference Mourao-Miranda, Reinders, Rocha-Rego, Lappin, Rondina and Morgan48
Neuroanatomical biomarkers
According to the Trauma Model,Reference Vissia, Giesen, Chalavi, Nijenhuis, Draijer and Brand11 DID is an early-onset form of PTSD.Reference Vissia, Giesen, Chalavi, Nijenhuis, Draijer and Brand11, Reference Chalavi, Vissia, Giesen, Nijenhuis, Draijer and Barker17, Reference Chalavi, Vissia, Giesen, Nijenhuis, Draijer and Cole18 Early life stressors may have long-lasting detrimental effects on neurobiology due to altered stress reactivity following childhood trauma.Reference Reinders, Chalavi, Schlumpf, Vissia, Nijenhuis and Jäncke19 Earlier neuroanatomical studies in DID have found a smaller hippocampal volume, which seems to be the result of exposure to stress hormones due to antecedent traumatisation. Although it is currently unknown how early traumatisation affects the development of the brain,Reference Reinders, Chalavi, Schlumpf, Vissia, Nijenhuis and Jäncke19 it is not surprising that long-lasting trauma results in widespread patterns of affected grey and white matter brain regions. We found affected brain regions in different lobules in the brain, but most prominently in the frontal grey and white matter regions, supporting findings from previous studies using univariate data-analysis approaches to investigate the neuroanatomical correlates of dissociative symptoms across disorders.Reference Chalavi, Vissia, Giesen, Nijenhuis, Draijer and Barker17, Reference Nardo, Högberg, Lanius, Jacobsson, Jonsson and Hällström49–Reference Daniels, Frewen, Theberge and Lanius51 Interestingly, the pattern of affected grey matter structural regions in the current study shows overlap with regions found in functional brain imaging studies involving dissociation, which included different patient samples and different paradigms – such as resting state in dissociative PTSD,Reference Lanius, Vermetten, Loewenstein, Brand, Schmahl and Bremner52 script-driven imagery in DIDReference Reinders, Willemsen, Vissia, Vos, den Boer and Nijenhuis14 or emotion processing in depersonalisation disorderReference Lemche, Anilkumar, Giampietro, Brammer, Surguladze and Lawrence53 – but consistently reported functional aberrations in frontal regions of the brain. Models of trauma-related dissociationReference Reinders, Willemsen, Vissia, Vos, den Boer and Nijenhuis14, Reference Lanius, Vermetten, Loewenstein, Brand, Schmahl and Bremner52 propose important roles for the cingulate gyrus, medial prefrontal cortex and superior frontal regions in emotion under-/overregulation and dissociation. The current multivariate study in the most severe form of dissociative disorders found that structural aberrations of these brain regions are part of the identified spatially distributed pattern. We therefore propose that future research into dissociation and DID focuses on these specific prefrontal areas, as well as the middle and dorsolateral prefrontal cortex, as important candidate regions for identifying neurobiomarkers of dissociation and specifically of DID.
Limitations
Our study has several strengths and limitations. First, although the findings show that a person with DID can be distinguished from healthy controls at an individual level with a high sensitivity and specificity, future studies are needed to unravel how individuals with DID differ from their most common misdiagnosed psychopathologies, such as borderline personality disorder and schizophrenia. We recommend that such future studies carefully debrief past psychiatric and neurological history, including depressive and/or psychotic episodes, history of epilepsy and duration of treatment, because these factors are likely to improve the ability for differential diagnostics of the classifier. The validation of the complex pattern recognition system with respect to these factors is important for clinical use and will need large patient samples of a wide variety of psychopathology. Furthermore, we recommend that future studies include a PTSD group without dissociative symptoms to investigate how PTSD comorbidity affects the classification, both at the level of the clinical presentation and biology. However, it is important to note that there is negligible overlap between results from the only multivariate study in PTSDReference Gong, Li, Tognin, Wu, Pettersson-Yeo and Lui54 and our results, indicating that the multivariate patterns found in the current study are likely to be dissociation-specific neuroanatomical biomarkers. Second, we employed a multicentre acquisition protocol to overcome single-site recruitment limitations, but carefully matched MRI acquisition parameters across sites using a study-optimised scanning sequence.Reference Chalavi, Simmons, Dijkstra, Barker and Reinders30 We further accounted for inter-site effects during the data analyses by standardising the data within each site separately prior to classification, which speaks against the possibility that inter-site effects can be fully attributed to site effects. Third, only women volunteered to participate in the study and therefore the results cannot be extended to all people with DID. Most of these women were currently using medication or had used medication in the past (see Supplementary Table 1). However, because medication use was highly variable across participants it is unlikely that the effects on grey and white matter will be consistent, as previously reported in a separate study of our group.Reference Chalavi, Vissia, Giesen, Nijenhuis, Draijer and Barker17 Nevertheless, it is recommended that future studies systematically investigate whether neuroanatomical changes are related to psychotropic medication.
Supplementary material
Supplementary material is available online at https://doi.org/10.1192/bjp.2018.255.
Funding
This article represents independent research part funded by the National Institute for Health Research Biomedical Research Centre at South London and Maudsley National Health Service Foundation Trust and King's College London. The views expressed are those of the author(s) and not necessarily those of the National Health Service, the National Institute for Health Research or the Department of Health. A.A.T.S.R. was supported by the Netherlands Organisation for Scientific Research (www.nwo.nl), NWO-VENI grant number 451-07-009. S.C. is supported by a David Caul graduate research grant from the International Society for the Study of Trauma and Dissociation (http://www.isst-d.org/about/awards.htm). A.F.M. gratefully acknowledges support from the King's College London Centre of Excellence in Medical Engineering, funded by the Wellcome Trust and Engineering and Physical Sciences Research Council and from a VIDI fellowship from the Netherlands Organisation for Scientific Research (grant number 016.156.415). Y.R.S. was supported by the Forschungskredit of the University of Zurich.
Acknowledgements
The authors thank all the participants and their therapists. We thank Nel Draijer, Mechteld Giesen, Ekaterina Weder and Eva Zimmermann for participant inclusion and scanning, as well as Andrew Lawrence for coordinate transformation into MNI space and for preparing the Tables.





eLetters
No eLetters have been published for this article.