The rapidly increasing prevalence of obesity necessitates the development of strategies for weight management and control. As the gastrointestinal tract elicits various signals that influence hunger and food intake(Reference Badman and Flier1), the gut is considered a strategic target organ.
The use of reduced energy diets, such as low-energy meal replacers, may result in body-weight reduction(Reference Truby, Baic and deLooy2). However, compliance to these diets is difficult to achieve, partly due to hunger pangs, thereby seriously limiting their success. Food-based approaches that employ gastrointestinal mechanisms for an enhanced and prolonged reduction in hunger and food intake may be very helpful in this respect.
It is well known that the satiating effect of nutrients varies between the different segments of the intestine, where nutrients are delivered(Reference Maljaars, Peters and Mela3–Reference Strader, Vahl and Jandacek13). For instance, in animal studies, surgical transposition of an ileal segment to a proximal site results in an increase in ileal nutrient exposure and a significant reduction in food intake.
This has been confirmed in human studies. Infusion of fat in the ileum more potently reduces hunger feelings compared with infusion of the same dose of fat in the duodenum(Reference Maljaars, Haddeman and Peters14). Welch et al. (Reference Welch, Sepple and Read9) compared infusion of a high dose of fat in the ileum v. the jejunum. The reduction in food intake was larger after jejunal fat infusion compared with ileal fat infusion(Reference Welch, Sepple and Read9). It should be noted that the doses of fat employed in that study were supra-physiological (1547 kJ (370 kcal)). Therefore, fat would have spilled over into the ileum after jejunal infusion, expanding the intestinal area exposed to fat in the jejunal experiment. These human data appear to confirm the results from animal studies that increasing the small-intestinal surface area exposed to nutrients increases the impact on food intake(Reference Meyer, Tabrizi and DiMaso5). Differences exist between small-intestinal segments with regard to gut peptide secretion(Reference Knutson, Koenders and Fridblom15). Furthermore, increasing the surface area exposed to nutrients has been shown to affect the secretion of glucagon-like peptide-1 (GLP-1) in human subjects(Reference Maljaars, Symersky and Kee16).
In previous studies, we have shown in human subjects that fat delivery to the ileum via an ileal tube results in a significant reduction in hunger score. Targeted delivery of nutrients to the ileum is difficult to achieve in daily life non-invasively. However, development of substances or capsules that slowly release fat during intestinal transport appears technically feasible(Reference Knutson, Koenders and Fridblom15). The present study was undertaken to explore alternative strategies of intestinal fat delivery, in order to compare and eventually optimise their effects on hunger and food intake.
In the present study, there were four study arms. The first arm was the control arm, in which fat was ingested orally. The second arm was the ileum arm, in which the effects of ileal fat infusion were tested. The third study arm was the sequential treatment, in which gradual fat delivery was mimicked by a sequential release of fat (first duodenum, then jejunum, followed by ileum). The fourth study arm was the simultaneous treatment, in which an identical total amount of fat was simultaneously distributed over the duodenum, jejunum and ileum. This arm was added as animal studies have shown that simultaneous exposure of different small-intestinal segments increases the effects on food intake. Inclusion of the sequential and simultaneous experiments will allow us to differentiate between the effects due to exposure of a more potent segment of the small intestine and the effects due to simultaneous feedback from different the small-intestinal segments.
We hypothesised that increasing the intestinal area exposed to a standard dose of fat may reproduce effects on hunger and food intake comparable with those observed when the same amount of fat is administered to the ileum only when the increased surface area is exposed simultaneously.
Experimental methods
Subjects
Healthy volunteers aged between 18 and 55 years with a BMI between 18 and 29 kg/m2 were recruited by advertisement. Restrained eaters (as assessed by the Dutch eating behaviour questionnaire) and subjects on a weight-reduction diet or a medically prescribed diet were excluded. Medication influencing appetite or sensory function was not allowed. Subjects with metabolic or endocrine disease, gastrointestinal disorders or after abdominal surgery were excluded. The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects/patients were approved by the Medical Ethics Committee of the University Hospital Maastricht. Written informed consent was obtained from each individual. A total of seventeen subjects met the inclusion criteria. During the study, two volunteers dropped out during the study: one due to discomfort during catheter positioning and one due to failure to position the tip of the catheter beyond the ligament of Treitz (flexura duodenojejunalis). Thus, fifteen healthy volunteers completed the protocol.
Catheter
The catheter for small-intestinal intubation was a 290 cm long, rubber Si, nine-channel (eight-lumen, one balloon inflation channel, 3·5 mm in diameter) catheter custom-made by Dentsleeve International Limited (Mississauga, ON, Canada). The functional length was 240 cm with a 50 cm connection segment. The catheter contains side holes at 80, 95, 110, 125, 210, 220, 230 and 240 cm from the proximal junction and had an inflatable balloon (maximum inflation capacity 10 ml) at the distal tip. The presence of the different side holes enabled targeted exposure of a specific small-intestinal segment to fat.
Experimental protocol
The meal replacer that we used contains 6 g fat. (For further details on the contents of the meal replacer, see ‘liquid meal’.) This meal replacer is expected to reduce hunger for a 3–4 h period, until the next meal. Reducing hunger in the between-meal period and reducing food intake were specific goals in the present study design. The 60 min period between the end of the infusion and the ad libitum lunch was scheduled to assess how the study treatments affect between-meal hunger compared with a regular meal replacer. As these meal replacers contain 6 g fat, this is the amount that we tested in the present study. Amounts as low as 3 g have been shown to affect ingestive behaviour when delivered to the ileum(Reference Maljaars, Symersky and Kee16).
The experimental protocol is shown in Fig. 1. In the present single blind, placebo-controlled, cross-over study, four treatments were scheduled. On each test day, a liquid meal replacer (see below for details) was ingested at t = 0 min, followed by an intestinal infusion from t = 30 to 120 min in all treatments. During the control treatment, a liquid meal replacer containing 6 g fat was ingested at t = 0 min, followed by a saline infusion. In the other three treatments, a fat-free liquid meal replacer was ingested at t = 0 min, followed by intestinal infusions with variations in location, but all with delivery of 6 g fat. As a positive control, an emulsion containing 6 g fat was given in the ileum: ‘ileum’ treatment. In the sequential treatment, representing slow release of fat during intestinal delivery, 2 g fat were given in the duodenum (t = 30–60 min), followed by 2 g in the jejunum (t = 60–90 min), followed by 2 g in the ileum (t = 90–120 min): ‘sequential’ treatment. The ‘simultaneous’ treatment was given to optimise the surface area exposed to nutrients. In the present study, 2 g fat were given in the duodenum, 2 g in the jejunum and 2 g in the ileum continuously, for 90 min, i.e. from 30 to 120 min.

Fig. 1 Study outline for the experiment. The study consisted of four treatments. Order of treatments was randomised. Each subject started each test day by consuming a breakfast at t = 0 min. In the control treatment, a fat-containing drink (832 kJ (199 kcal)) was provided as breakfast. This breakfast meal was then followed by a saline infusion from t = 30 to 120 min. On the treatment days, a fat-free breakfast (606 kJ (145 kcal)) was ingested at t = 0 min, followed by an intestinal infusion of 6 g fat from t = 30 to 120 min. In the simultaneous treatment, 2 g fat were given in the duodenum, 2 g in the jejunum and 2 g in the ileum continuously for 90 min. During the ileum treatment, 6 g fat were given in the ileum only. Finally, in the sequential treatment, 2 g fat were given in the duodenum (t = 30–60 min), followed by 2 g in the jejunum (t = 60–90 min), followed by 2 g in the ileum (t = 90–120 min). Throughout the test day, blood was sampled for gut peptide analysis, and satiety was measured by visual analogue scale (VAS) questionnaire. On all test days, an ad libitum meal was consumed at t = 180 min.
Catheter positioning
On Monday, subjects arrived at 12.00 hours after a light breakfast (ingested before 09.00 hours). Through an anaesthetised nostril, the catheter was introduced into the stomach and allowed to pass through the pylorus to the ileum by peristalsis. After passing the ligament of Treitz, a small balloon at the tip was inflated to facilitate passage of the catheter to the ileum. During the day, subjects were offered small snacks and sugared tea or coffee to stimulate peristalsis. The tip was placed in the ileum (at least 120 cm distal from the pylorus(Reference Spiller, Trotman and Higgins17)), so three infusion ports were available in the ileum. The most distal port was used for infusion. In the present study, we perfused the ileum at at least 175–195 cm from the nose. During the positioning and before every test day, the position of the catheter was checked fluoroscopically.
Test day
On each study day (Tuesday until Friday), subjects arrived at the gastrointestinal research laboratory unit at 08.00 hours following an overnight fast. After checking the position of the ileal catheter, a venous catheter was placed in a forearm vein for collection of blood samples. At 08.45 hours, a basal visual analogue scale scores for hunger feelings and a basal blood sample were collected. After this, the experiment was started. At 09.00 hours (t = 0 min), the liquid meal replacer was ingested. The infusions started at t = 30 min. Infusion rate was 0·67 ml/min (2·5 kJ/min (0·6 kcal/min)) for 90 min for all treatments. At 12.00 hours (t = 180 min), the ad libitum lunch was served. At 12.30 hours, the intravenous cannula was removed, and subjects were allowed to go home. They received an evening meal and snackbox from which they were allowed to consume freely until 22.00 hours.
Hunger
Scores for hunger feelings (fullness, hunger, appetite for a meal and appetite for a snack) were measured using electronic visual analogue scales anchored at the low end with the most negative or lowest intensity feelings (e.g. extremely and not at all) and with opposing terms at the high end (e.g. not at all and very high)(Reference Flint, Raben and Blundell18). Volunteers were asked to indicate on a 64 mm line on the iPAQ which on the scale best reflects their feeling at that moment. Measurements were taken during the test day every 30 min and every 15 min during infusion of emulsion.
Food intake
On each test day, an ad libitum lunch was served to measure food intake. Each lunch was offered in excess and consisted of a pasta meal (per 100 g: 430 kJ, 5 g protein, 2 g fat and 16 g carbohydrates). During the ad libitum lunch, subjects were not allowed to watch television, listen to the radio or read, as this could have influenced the amount eaten.
Liquid meal
In the present study, two different liquid meal replacers were used: a liquid meal replacer with fat and a liquid meal replacer without fat. The regular, fat-containing liquid meal, which was given during the control treatment, was a liquid meal replacer (325 ml, 832 kJ (199 kcal), 10·0 g protein, 7·3 g fat and 24·3 g carbohydrates of which 5·1 g fibre; Slim-Fast Optima; Unilever, Vlaardingen, The Netherlands) containing 6 g of high-oleic rapeseed oil. The fat-free version of the same liquid meal replacer (325 ml, 832 kJ (199 kcal) was given during the other treatments. These are vitamin- and mineral-fortified meal replacement products, used by consumers primarily in order to aid in weight loss and/or the prevention of weight gain.
Emulsions
Emulsions were used for small-intestinal infusions, and consisted of 10 % oil in water. K-caseinate (2·5 %) was used as an emulsifier. A very small amount of xanthan gum (0·1 %) and guar gum (0·1 %) was used as a stabiliser. NaCl was added to obtain iso-osmotic solutions (0·8 % NaCl). Total infused volume was 60 ml, containing 6 g fat. Energy load was 226 kJ (54 kcal). The pH of the emulsions ranged from 6·7 to 6·8. Saline was administered to the ileum as a control infusion.
Chemical analyses
Blood samples were drawn at regular intervals throughout the test day. After collection, the blood was kept on ice. Total peptide YY (PYY, PYYT-66HK; Linco Research, St Charles, MO, USA), cholecystokinin (CCK) (RB302; Lucron Bioproducts, Ovenberg, Milsbeek, The Netherlands) and GLP-1 (GLP1A-35HK; Linco Research) were measured by sensitive and specific commercially available RIA. The detection limit for the PYY assay is 10 pmol/l. The intra-assay CV ranges from 1·8 to 15·6 %, and the inter-assay CV from 9·5 to 27·8 %. The detection limit for the CCK assay is 0·3 pmol/l. The intra-assay CV ranges from 2·0 to 5·5 %, and the inter-assay CV from 4·1 to 13·7 %. The detection limit for the GLP-1 assay is 3 pmol/l. The intra-assay CV ranges from 21·2 to 30·3 %, and the inter-assay CV from 12 to 34 %. Blood for GLP-1 analysis was collected in ice-chilled tubes, to which a dipeptidyl peptidase (DPP)-IV inhibitor was added (DPP4; Linco Research). All gut peptides were measured in a randomly selected subset of nine subjects.
Statistical analysis
Results are presented as least square means with their standard errors of the mean, unless otherwise specified. Satiety visual analogue scale scores are expressed as percentages of the maximal score (0 mm equaled 0 % and 64 mm equaled 100 %) and as incremental cumulative areas under the curve, with the value at t = 0 min as a covariate. The incremental area under the curve (AUC) was calculated using the trapezoid rule.
A randomly selected subset of nine subjects was used for evaluation of gut peptide secretion. This group size of nine was based on a power calculation, with a predicted difference in peptide release of at least 20 %, a power of 0·8 and an overall α of 0·05 (taking into account multiple comparisons, two-sided).
All parameters were analysed using ANOVA, with subjects as blocks and treatment as factor. A Dunnett test was used to compare each treatment with control. A P value of 0·05 or less was considered significant.
Results
Food intake
Food intake (Fig. 2) was significantly reduced after the ileum v. control treatment (422 v. 499 (sem 40) g, P < 0·01), whereas no significant differences were observed after the simultaneous and sequential treatments (458 g for the simultaneous treatment and 480 g for the sequential treatment (sem 40)).

Fig. 2 Effect of 6 g fat infusion into different regions of the small intestine on food intake. Values are least square means, with their standard errors represented by vertical bars. * Mean values were significantly different (P < 0·05). ■, Control; ![]() , simultaneous;
, simultaneous; ![]() , ileum;
, ileum; ![]() , sequential.
, sequential.
Hunger
We measured hunger, fullness and appetite for a meal and appetite for a snack. As hunger, appetite for a meal and appetite for a snack produced identical results, we only show hunger scores.
Over the period t = − 15 to 180 min, hunger AUC (Fig. 3(a)) was significantly reduced after both simultaneous and ileum treatments v. control, but not after the sequential treatment. No significant differences between the four experiments were observed for fullness AUC (Fig. 3(c)).

Fig. 3 Effect of 6 g fat infusion into different regions of the small intestine on fullness and hunger. Values are least square means, with their standard errors represented by vertical bars. (a) Results for hunger area under the curve (AUC). * Mean values were significantly different (P < 0·05). ■, Control; ![]() , simultaneous;
, simultaneous; ![]() , ileum;
, ileum; ![]() , sequential. (b) Results for hunger scores. * Mean values were significantly different for ileum v. control. (P < 0·05).
, sequential. (b) Results for hunger scores. * Mean values were significantly different for ileum v. control. (P < 0·05). ![]() , Control;
, Control; ![]() , simultaneous;
, simultaneous; ![]() , ileal fat;
, ileal fat; ![]() , sequential. (c) Results for fullness AUC. ■, Control;
, sequential. (c) Results for fullness AUC. ■, Control; ![]() , simultaneous;
, simultaneous; ![]() , ileum;
, ileum; ![]() , sequential. (d) Results for fullness.
, sequential. (d) Results for fullness. ![]() , Control;
, Control; ![]() , simultaneous;
, simultaneous; ![]() , ileal fat;
, ileal fat; ![]() , sequential. Evas, electronic visual analogue scale.
, sequential. Evas, electronic visual analogue scale.
At the start of each test day, hunger (Fig. 3(b)) and fullness (Fig. 3(d)) scores did not differ between the treatments. Hunger was significantly reduced after the ileum treatment v. control at 150 min. No differences were observed for fullness.
Gut peptide secretion
Fasting plasma concentrations of CCK, GLP-1 and PYY did not differ between the treatments. With regard to the 0–180 min AUC integrated secretion (AUC 0–180 min), significant differences were not observed between the four treatments either for CCK, PYY or GLP-1 (Table 1).
Table 1 Secretion of gut peptides in response to the different treatments, as measured as area under the curve* (t=0–180 min)
(Least square (LS) mean values with their standard errors)
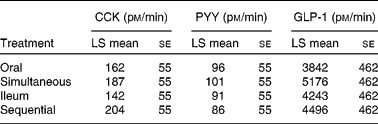
CCK, cholecystokinin; PYY, peptide YY; GLP-1, glucagon-like peptide-1.
* No significant differences were observed.
Discussion
In the present study, we have shown that infusion of a low, physiological dose of fat into the ileum resulted in a significant and clinically relevant reduction in food intake when compared with oral ingestion of the same dose of fat. When an equienergetic fat dose was spread sequentially over the small intestine, mimicking slow release of fat during intestinal transit, neither hunger scores nor food intake were significantly affected. On the other hand, optimisation of the exposed intestinal area to fat with the simultaneous delivery of fat along the whole length of the small intestine resulted in a significant reduction in hunger scores compared with oral fat but had no effect on food intake. The observed differences in satiety and food intake were not reflected by changes in serum gut peptide levels.
The concept of the ileal brake has been introduced by Spiller et al. (Reference Spiller, Trotman and Higgins17), and Read et al. (Reference Read, McFarlane and Kinsman19) initially described it as a reduction in the small-intestinal transit in response to ileal nutrient infusions. Additionally, ileal brake effects on satiety and food intake have since been demonstrated(Reference Maljaars, Peters and Mela3).
Welch et al. (Reference Welch, Sepple and Read9, Reference Welch, Saunders and Read20) were the first to show that ileal infusion of large doses of fat reduced food intake when compared with a control saline infusion or with intravenous infusion of fat. In a previous study, we compared infusion of fat into the ileum v. the duodenum on hunger levels and food intake(Reference Maljaars, Haddeman and Peters14). The ileal fat infusions significantly increased satiety and reduced hunger scores compared with duodenal fat infusion(Reference Maljaars, Haddeman and Peters14). In a recent review on ileal brake-mediated satiety, it was stated that activation of the ileal brake has profound effects on satiety(Reference Maljaars, Peters and Mela3). However, with respect to the effect of the ileal brake on food intake, mixed results have been obtained(Reference Maljaars, Peters and Mela3). Data from animal studies have focused on another potential small-intestinal target to increase satiety and reduce food intake. Lin et al. (Reference Lin, Zhao and Wang21, Reference Lin, Doty and Reedy22) have demonstrated that when the small-intestinal surface area exposed to nutrients was increased, this led to a more pronounced effect on gastric emptying and small-intestinal transit. Meyer et al. (Reference Meyer, Hlinka and Tabrizi4–Reference Meyer, Hlinka and Khatibi6) have expanded these data with observations on food intake. These authors have demonstrated that the food intake-reducing effect of maltose was larger when maltose had access to the whole length of the small intestine compared with only a jejunal loop comprising one third of the total small-intestinal length(Reference Meyer, Tabrizi and DiMaso5). Based on these experiments, the authors have concluded that simultaneous exposure of different segments of the small intestine potentiates the effect of intestinal nutrients on food intake in animals(Reference Meyer, Tabrizi and DiMaso5). It was hypothesised that the generated intestinal satiety signals are affected by integrated activity of mucosal sensors along the total length of gut exposed to the stimuli and the contact intensity of stimuli with receptors(Reference Meyer, Tabrizi and DiMaso5).
In human subjects, the effects of spatial fat distribution on food intake have not been studied in detail. However, Little et al. found that the length of the small intestine exposed to nutrients influenced the secretion of GLP-1, a potent inhibitor of food intake. When a glucose infusion was confined to the proximal 60 cm of the small intestine, no effects on GLP-1 secretion were observed. However, when this duodenal infusion was combined with a glucose infusion distal to the 60 cm point, an increase in GLP-1 concentrations was observed. These findings suggest that in human subjects, increasing the small-intestinal exposure to nutrients may potentiate the effects on satiety and food intake by increasing plasma GLP-1 concentrations.
After ingestion of a small-sized meal, the delivery and spread of nutrients over the small intestine depend on the rate of gastric emptying of a meal and on the rate of absorption of the nutrients contained in the meal(Reference Meyer, Hlinka and Tabrizi4–Reference Meyer, Hlinka and Khatibi6). Knutson et al. (Reference Knutson, Koenders and Fridblom15) demonstrated that postprandial lipid levels after a regular meal peaked at 60 min after the start of meal ingestion. Appearance of the meal marker phenolsulfonphtalein (PSP) was observed in the ileum between 30 and 120 min after the start of the ingestion of a meal, whereas others have shown that PSP levels peaked simultaneously in all three segments(Reference Keller, Runzi and Goebell23). These data showed that nutrients (including fats) do spread along the entire length of the small intestine, and that, to a large extent, all segments are exposed simultaneously. Animal studies on the effects of increasing the small-intestinal surface area exposed to nutrients have suggested that spreading the nutrients more equally over the small intestine, in order to optimise the exposure of all three segments, may improve the satiety response to a meal. An advantage of this strategy over increased ileal exposure may be that increasing the spread of nutrients along the length of the small intestine technically is more easy to accomplish. In the present study, we compared normal oral ingestion of a meal with ileal infusion of fat and with optimised distribution of a meal along the length of the small intestine. In the fourth arm, we mimicked another slow-release model, which results in sequentially exposing the duodenum, jejunum and ileum to fat.
We observed that ileal fat infusion reduced hunger and food intake compared with control. The simultaneous treatment, which resulted in the optimal exposure of the small intestine to fat, did reduce hunger but did not significantly affect food intake compared with control. The observation that simultaneous exposure of the small intestine did reduce hunger whereas the sequential experiment did not indicate that the increased effect of the simultaneous treatment is not due to increased exposure of a more potent small-intestinal segment, but due to simultaneous feedback from all three small-intestinal segments. These data confirm the hypothesis by Meyer et al. (Reference Meyer, Tabrizi and DiMaso5).
Ileal fat infusion reduced food intake by about 15 %, which at first sight may seem to be a rather small effect. However, obesity usually reflects a long-term accumulation of relatively small daily ‘energy gaps’(Reference Hill, Wyatt and Reed24). Hill et al. (Reference Hill, Wyatt and Reed24) described that by reducing energy intake by 226 kJ/d (50 kcal/d), weight gain could be prevented in 90 % of the population. This suggests that the 15 % reduction in food intake that we observed represents a meaningful and significant contribution to treating and preventing obesity and overweight.
In the small intestine, the secretion of several gut peptides varies depending on the intestinal segments that are activated(Reference Maljaars, Peters and Masclee25). CCK is predominantly released from the proximal small intestine, although CCK-secreting cells have also been demonstrated in the terminal ileum(Reference Buffa, Solcia and Go26). PYY- and GLP-1-secreting L-cells reside predominantly in the distal small intestine and colon(Reference Maljaars, Peters and Masclee25). We hypothesised that modulating the distribution of fat over the small intestine would affect peptide secretion in line with effects on satiety scores and food intake. However, no differences were observed in peptide secretion. It should be taken into consideration that only small amounts of fat were administered, and that peptides may exert their effect by paracrine or neurocrine routes v. endocrine routes (secretion into the bloodstream)(Reference Maljaars, Peters and Masclee25). However, in previous studies with an equal amount of fat, significant effects were found on the secretion of CCK and PYY. In these studies, a higher rate of infusion was used compared with the present study. Previous studies have demonstrated that infusion rate is a key determinant for peptide secretion after intestinal fat infusion(Reference Feltrin, Little and Meyer27).
Furthermore, discrepancies between peptide secretion and effects on satiety and food intake have been shown previously. A team of experts recently concluded that none of the currently known gut peptides is an adequate and reliable biomarker for satiety. Additionally, studies have shown that nutrient-induced release of 5HT3 reduces food intake, suggesting a potential role for direct activation of neural (vagal) afferents in the effects of small-intestinal fat on satiety and food intake(Reference Savastano, Hayes and Covasa28, Reference Hayes and Covasa29).
In conclusion, we confirm that ileal fat infusion significantly reduces food intake compared with oral fat ingestion. Further optimising the exposure of the small intestine to fat through simultaneous fat delivery did result in a reduction in hunger scores, but no effects on food intake were observed. These results point to the ileal brake as most potent small-intestinal control mechanism for hunger and food intake. The ileal brake is therefore an interesting target for food-based strategies in weight management.
Acknowledgements
The authors' responsibilities were as follows: P. W. J. M. participated in the design of the study, data collection, data analysis and writing of the manuscript; A. K. and M. G. performed the chemical and hormonal analyses; F. J. T. supervised the chemical and hormonal analyses; E. H. participated in the design of the study, data collection and analysis; H. P. F. P. participated in the design of the study, assisted with the data analysis and in drafting the manuscript; A. A. M. M. participated in the design of the study, and guided during all aspects of the experimental protocol, data analysis and writing of the manuscript. All of the authors participated in a critical review and in the final approval of the manuscript. The liquid meal replacers and financial support were provided by Unilever Research and Development, Vlaardingen, The Netherlands. None of the authors had a personal or financial conflict of interest.






