INTRODUCTION
Enterococci are the second most common cause of nosocomial bloodstream infections in the USA [Reference Hidron1] and vancomycin resistance occurs in nearly one third of isolates. Vancomycin-resistant Enterococcus bloodstream infections (VRE-BSI) are associated with increased morbidity, mortality, and healthcare expenditures for hospitalized patients [Reference Deshpande2–Reference DiazGranados12]. Fortunately, novel antimicrobials with activity against VRE (daptomycin, linezolid, quinupristin-dalfopristin, tigecycline) provide a therapeutic option for physicians.
The impact of newer antimicrobial agents on predictors of mortality from VRE-BSI needs further investigation [Reference Erlandson5, Reference Warren13–Reference Mave16]. While previous research has identified factors associated with poor outcomes from VRE-BSI [Charlson comorbidity index (CCI), the number of positive blood cultures, parenteral nutrition, severity of illness, renal function, liver disease, malignancy, prior VRE infection] [Reference Vergis4, Reference Erlandson5, Reference Gearhart7, Reference Mave16–Reference Bhavnani18], most of these studies were conducted prior to widespread use of novel antimicrobials with activity against VRE. Herein, we explore outcomes of patients with VRE-BSI at a tertiary-care medical centre, focusing on antimicrobial therapy and factors associated with mortality for VRE-BSI.
PATIENTS AND METHODS
Clinical data
We conducted a retrospective study of patients with nosocomial VRE-BSI at University Hospital in Birmingham, Alabama between 1 January 2005 and 1 August 2008. All patients who met CDC criteria for nosocomial bloodstream infections with vancomycin-resistant Enterococcus spp. were included in the study [Reference Horan, Andrus and Dudeck19]. All blood cultures were analysed with an automated culture system (BacT/ALERT, bioMérieux Industries, USA) and automated susceptibility testing (Microscan Walkaway 96 SI, Siemens Healthcare Diagnostics, USA). Rates of VRE-BSI were calculated as number of patients with bacteraemia/1000 patient-days of care.
Medical records were reviewed for demographic information, presence of comorbidities, laboratory data, antimicrobial treatment, and outcomes. The following definitions were used: cancer (any active malignancy); liver disease (cirrhosis due to any cause, chronic viral hepatitis, or transaminase levels at least four times the upper limit of normal); recent surgery (surgery within the pevious 60 days); transplant (receipt of a haematopoietic stem cell or solid organ transplant); leukocytosis [white blood cell (WBC) count >20 000 cells/mm3]; neutropenia (absolute neutrophil count <500 neutrophils/mm3); malnutrition (serum albumin <2·0 mg/dl); concurrent bloodstream infection (positive blood culture for bacteria or fungi with associated findings of infection within 14 days before or after the first positive VRE culture). Renal insufficiency was defined as creatinine >2 g/dl or haemodialysis at time of VRE culture. Immunosuppression was defined as receipt of any immunosuppressive medication, excluding anti-neoplastic agents for the treatment of cancer, during the index hospitalization. Corticosteroid doses equivalent to <10 mg prednisone were not included as immunosuppression. An aggregate measure of underlying illness was measured using the CCI [Reference Charlson20]. The CCI is predictive of mortality for a patient who may have a range of comorbid conditions such as heart disease, HIV/AIDS, or cancer (a total of 22 conditions). Each condition is assigned a score from 1 to 6 depending on the risk of dying associated with the condition. The CCI is the sum of these scores and a higher score is associated with a greater likelihood of death.
Primary antimicrobial therapy for VRE was defined as receipt of daptomycin, linezolid, quinupristin-dalfopristin, or tigecycline for at least 3 days of the initial 4 days of VRE treatment. Time to initiation of antibiotics was determined from collection of the first blood culture that subsequently grew VRE to the start date of antibiotics, measured in days. Microbiological failure was defined as persistently positive blood cultures while on appropriate therapy for at least 24 h or recurrence of positive blood cultures within 1 week of completing therapy. Antimicrobial stewardship guidelines were in place throughout the hospital for the entire course of the study. Daptomycin and linezolid were unrestricted for the treatment of VRE, but quinupristin-dalfopristin and tigecycline required verbal approval from an infectious disease (ID) specialist. ID consultation was recommended, but not required for all VRE infections. The primary outcome of interest was all-cause mortality at 30 days following the first positive culture for VRE.
Statistical analyses
Frequencies of categorical variables and means, medians and standard deviations of continuous variables were calculated for the overall population and for patients who received appropriate treatment. For analysis of the relationships of variables to survivors and non-survivors, univariate analyses were performed using χ2 or Fisher's exact methods for categorical variables and Student's t test or the Wilcoxon rank sum test for continuous variables. The primary model for factors associated with mortality was created using stepwise multiple logistic regression analysis. Models using all-cause mortality as the dependent variable were determined for the overall population, and then for patients who received appropriate treatment. All variables significant at α=0·20 in univariate analyses were considered as possible predictor variables for the multivariable analyses. Age was entered into the final model as a continuous variable. The criterion for entry into the models was significance at α=0·20, while the criterion for remaining in the model was significance at α=0·05. Odds ratios (OR) and corresponding 95% confidence intervals (CI) were calculated. Model fit was assessed using the Hosmer–Lemeshow goodness-of-fit statistic, and it was determined that all models fit the data well. A potential interaction between intensve-care unit (ICU) stay and ventilator use was evaluated by incorporating an interaction term into the final model. In addition to the primary models, where comorbidities were entered individually, models were constructed using the CCI as a continuous variable.
All statistical tests were two-tailed and were performed using a 0·05 significance level. Statistical analyses were conducted using SAS version 9.1 (SAS Institute Inc., USA). This study was approved by the University of Alabama at Birmingham Institutional Review Board.
RESULTS
Of 237 patient episodes of VRE-BSI that occurred during the study period, 235 had outcome data available and were included in analyses. Over the study period, rates of VRE-BSI increased significantly from a baseline of 0·06 infections/1000 patient-days to 0·17 infections/1000 patient-days (P=0·03, Fig. 1). Mean age of patients with VRE-BSI was 53·7 years and 42·3% were male. Enterococcus faecium caused 227 (96·6%) VRE-BSI, with the remainder due to E. faecalis. Patient characteristics for survivors and non-survivors are shown in Table 1. Frequent underlying illnesses present at the time of VRE-BSI included malnutrition (50·2%), concurrent bloodstream infection (51%), renal insufficiency (48·5%), mechanical ventilation (29·3%), and cancer (30·2%). More than one third (39·2%) of patients were located in an ICU when the index blood cultures were drawn. Medical comorbidities in patients were common; with a CCI score ⩾6 in 149 (63·4%) patients.
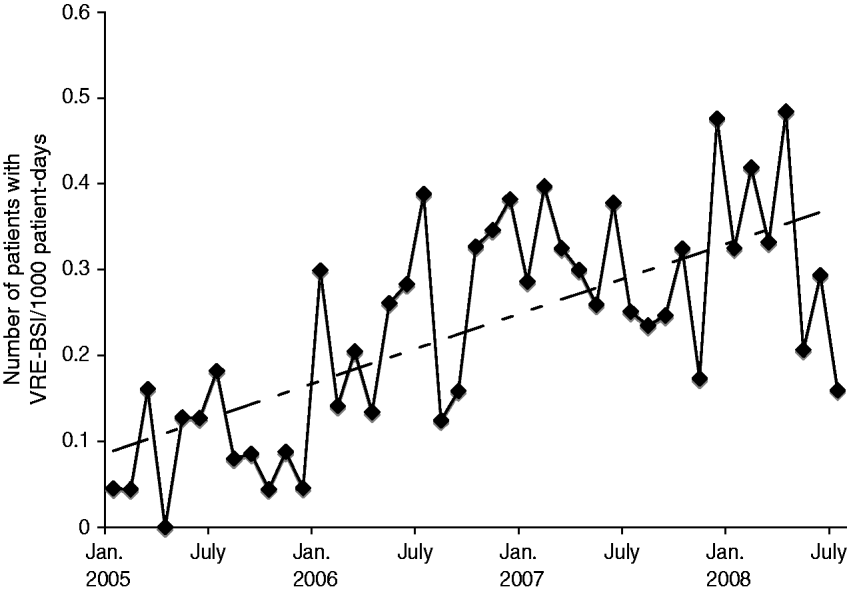
Fig. 1. The number of patients with vancomycin-resistant Enterococcus bloodstream infections (VRE-BSI)/1000 patient-days of care plotted per month at the University Hospital in Birmingham, Alabama. Linear trend added.
Table 1. Characteristics of 235 patients with vancomycin-resistant Enterococcus bloodstream infections with comparison of survivors vs. non-survivors using t test or χ2 analysis
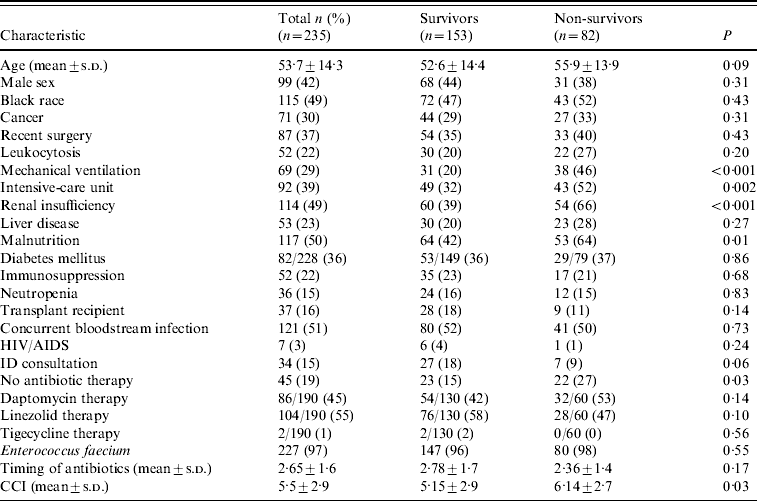
CCI, Charlson comorbidity index, HIV/AIDS, human immunodeficiency virus/acquired immunodeficiency syndrome; ID, infectious diseases; s.d., standard deviation.
All-cause mortality at 30 days was 34·9% (82/235). Significant differences between survivors and non-survivors in univariate analysis included presence of mechanical ventilation (20·3% vs. 46·3%, P<0·001); ICU location (32·0% vs. 52·4%, P=0·002); renal insufficiency (39·2% vs. 65·9%, P<0·001); malnutrition (41·8% vs. 64·6%, P=0·01); receipt of antibiotics active against the VRE isolate (85·0% vs. 73·2%, P=0·03); and mean CCI (5·15 vs. 6·14, P=0·03).
Independent predictors of mortality
Predictors of mortality in the overall population were explored in a multivariable logistic regression model (Table 2). Factors independently associated with 30-day mortality included mechanical ventilation (OR 3·32, 95% CI 1·7–6·6, P=0·007), renal insufficiency (OR 3·2, 95% CI 1·6–6·3, P=0·007) and malnutrition (OR 2·0, 95% CI 1·0–4·0, P=0·046). There was an association with receipt of VRE-active antibiotics and increased survival (OR 0·53, 95% CI 0·2–1·2, P=0·13), but this was not statistically significant. In a model with age, sex, race, timing of antibiotics, transplant and CCI (instead of individual comorbidities), CCI was an independent predictor of mortality (OR 1·13, 95% CI 1·03–1·25, P=0·01).
Table 2. Multivariable logistic regression analysisFootnote * of factors related to 30-day mortality in the overall population (n=235)
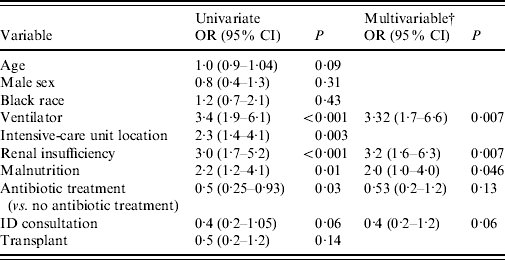
OR, Odds ratio; CI, confidence interval.
* Logistic regression analysis. Variables with P<0·20 on univariate analysis were included in a multivariable stepwise regression model in addition to variables for race and sex. Results are listed for variables with P<0·20. P values obtained are two-tailed.
† Each of the multivariable odds ratios are adjusted for all of the other variables remaining in the final model.
VRE antimicrobial therapy
Linezolid was the primary therapy for VRE-BSI in 104 (44%) patients, daptomycin in 86 (37%) patients and tigecycline in two (1%) patients. No patient received quinupristin-dalfopristin. The remaining 43 (18%) did not receive VRE-specific therapy. Of those who did not receive specific antimicrobial therapy, 22 (51%) out of 43 died by day 30. Eighteen (42%) out of 43 died before blood culture results were identified as VRE.
Microbiological failure occurred in 18 (17·5%) patients treated with linezolid and 25 (29%) patients treated with daptomycin. No failure occurred in two patients treated with tigecycline. There were 28 (27%) deaths in patients treated with linezolid, 32 (37·2%) deaths in patients treated with daptomycin and no deaths in patients treated with tigecycline. Concurrent bloodstream infection was noted in 50/86 (58%) patients in the daptomycin group and 54/104 (52%) patients in the linezolid group.
A multivariable logistic regression analysis was conducted to identify independent predictors of mortality for those patients who received VRE-active therapy (Table 3). Patients treated with tigecycline were excluded from the analysis because of limited sample size. In this population of 190 patients, mechanical ventilation (OR 3·4, 95% CI 1·5–7·7, P=0·003) and renal insufficiency (OR 4·1, 95% CI 1·9–9·1, P<0·001) were predictors of increased mortality. Receipt of a solid organ or stem cell transplant was associated with improved survival (OR 0·2, 95% CI 0·06–0·8, P=0·027). Delayed administration of antibiotics was associated with decreased mortality (OR 0·8, 95% CI 0·55–1·00, P=0·051), but this was not significant. In addition, there was an association of daptomycin therapy and increased mortality (OR 2·1, 95% CI 0·99–4·7, P=0·052), but this did not reach statistical significance. In a model with age, sex, race, timing of antibiotics, transplant and CCI (instead of individual comorbidities), only CCI was an independent predictor of mortality (OR 1·13, 95% CI 1·00–1·29, P=0·03).
Table 3. Multivariable logistic regression analysis of factors related to 30-day mortality in patients who received vancomycin-resistant Enterococcus-active therapy (n=190)Footnote * Footnote †
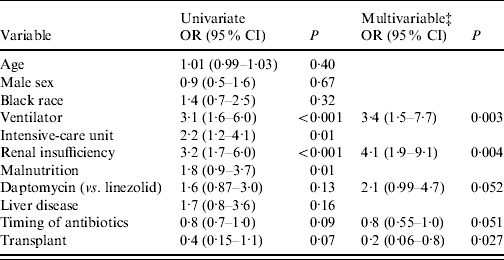
OR, Odds ratio; CI, confidence interval.
* Logistic regression analysis. Variables with P<0·20 on univariate analysis were included in a multivariable stepwise regression model in addition to variables for age, sex and race. Results are listed for variables with P<0·10. P values obtained are two-tailed.
† Due to limited sample size two patients treated with tigecycline were not included in this analysis.
‡ Each of the multivariable odds ratios are adjusted for all of the other variables remaining in the final model.
Characteristics of patients receiving either linezolid or daptomycin were compared in a sub-analysis (Table 4). Neutropenia was more common in patients who received daptomycin (29% vs. 7·7%, P<0·001); and ID consultation was more common in patients who received linezolid (24·0% vs. 8·1%, P=0·003). Overall duration of therapy was a median of 11 days (range 3–47 days), and was not significantly different between the two groups.
Table 4. Characteristics of patients treated with linezolid (n=104) or daptomycin (n=86)
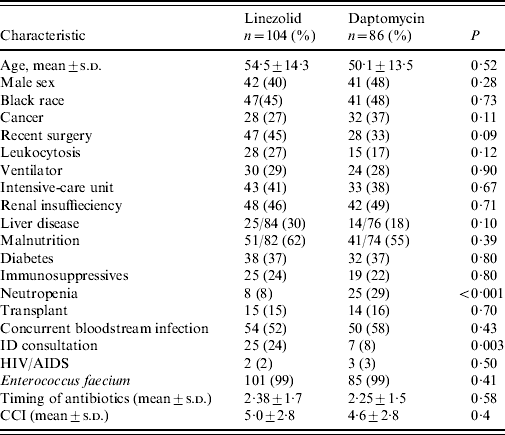
CCI, Charlson comorbidity index, HIV/AIDS, human immunodeficiency virus/acquired immunodeficiency syndrome; ID, infectious diseases; s.d., standard deviation.
DISCUSSION
This retrospective cohort study of 235 patients represents one of the largest published investigations of nosocomial VRE-BSI. Our findings provide important insights into the epidemiology and management of this growing health problem. For example, we observed an almost threefold increase in VRE-BSI incidence during the 2·5-year study period. We also report a 30-day all-cause mortality of 35%, consistent with previous observations [Reference Deshpande2–Reference DiazGranados12]. These data provide ‘real world’ evidence that VRE-BSI is a growing problem for hospitalized patients in the USA [Reference Deshpande2, 3, Reference Boucher21, Reference Reik22].
A crucial component of the epidemiology of VRE-BSI is the identification of factors that relate to patient outcomes. Previous studies found CCI, number of positive blood cultures, parenteral nutrition, severity of illness, decreased renal function, liver disease, malignancy, and prior VRE infection to be associated with poor outcomes in cases of VRE infection [Reference Vergis4, Reference Erlandson5, Reference Gearhart7, Reference Mave16–Reference Bhavnani18]. Most of these studies were conducted when there were relatively limited treatment options for VRE. Our study confirmed several previous findings. In both the overall population and those receiving antibiotics, renal insufficiency and mechanical ventilation were independent predictors of increased mortality [Reference Erlandson5, Reference Gearhart7, Reference Camins17, Reference Ghanem23]. Similarly to the recent study from Camins and colleagues [Reference Camins17], we also observed that CCI was a significant predictor of mortality. Previous studies have not specifically addressed lack of antibiotics and mortality. In our cohort, treatment with a VRE-active antimicrobial was associated with improved survival in univariate analysis (P=0·03). Although these findings did not reach statistical significance in multivariable analysis (P=0·13), we favour treatment for patients with VRE-BSI.
Providers at our institution prescribed daptomycin and linezolid frequently; prescribed tigecycline infrequently; and did not use quinupristin-dalfopristin. Antimicrobial stewardship guidelines during the study period required ID consultation or specific approval prior to use of quinupristin-dalfopristin and tigecycline, which probably led to decreased use of these agents. However, the lack of consensus on treatment strategy is also probably related to lack of published data to guide therapy. To date, there are few retrospective observational investigations comparing treatment options for VRE–BSI [Reference Erlandson5, Reference Mave16]. Furthermore, most data supporting the use of newer agents with in vitro activity against VRE, i.e. daptomycin, linezolid, quinupristin-dalfopristin, and tigecycline, are based on case series and case reports [Reference Erlandson5, Reference Warren13–Reference Mave16, Reference Poutsiaka24–Reference Chien, Kucia and Salata31]. Additional research, preferably an adequately powered randomized controlled trial, is needed to determine optimal selection of antimicrobial therapy in this context.
When limiting our analysis to patients who received VRE-active antimicrobial therapy, use of daptomycin showed a trend towards an association with mortality, but was not significant in multivariable analysis (P=0·052). Our observations that daptomycin therapy may be associated with poorer outcomes when compared to linezolid were unexpected; however, ours is the second investigation to suggest a potential difference. In a retrospective study of 98 patients with VRE bacteraemia, Mave and colleagues [Reference Mave16] demonstrated a trend towards higher mortality with daptomycin when compared to linezolid. Our observations, while similar, should not be considered conclusive, as they are possibly influenced by several important factors. First, when we forced the CCI into the multivariable model (data not shown), the association of daptomycin and mortality was less pronounced (OR 1·7), suggesting that ‘sicker’ people received daptomycin therapy. Second, there were important differences in patients who received daptomycin or linezolid therapy, including neutropenia and number of ID consultations. Differences in ID consultation may be relevant, as published reports demonstrate decreased mortality in patients with candidaemia and staphylococcal bacteraemia in patients who received ID consultation [Reference Fowler32, Reference Patel33]. Last, our finding that transplant patients had a lower mortality is probably spurious and suggests confounding in this investigation. Although our data are limited by being observational in nature, further research into the comparative effectiveness of these two agents is warranted.
Our study has several other limitations. A high number of concurrent bloodstream infections (n=120) was observed. The high frequency of bloodstream infection (51%) is probably the result of our generous time-frame for concurrent infection (±14 days). Although the majority of patients in our cohort received effective therapy for concurrent bloodstream infection (>90%), the bloodstream isolates were heterogeneous in grade of bacteraemia (number of positive cultures) and causative organism, e.g. Klebsiella, Candida, Staphylococcus, and other species. Future studies should consider a different time interval (+7/−2 days) and make specific adjustments for type and grade of bloodstream infection. Although this represents a large cohort of VRE-BSI patients, some analyses were limited by small variable frequencies. Our study did not evaluate treatment of concurrent pneumonias. As daptomycin has limited efficacy in lung parenchyma, there may have been increased mortality due to pulmonary infections not captured. We were unable to determine appropriate dosing or levels of daptomycin in patients on haemodialysis. As this was a retrospective study, we were unable to collect robust severity-of-illness data such as APACHE scores.
In conclusion, this investigation confirms that VRE-BSI continue to be a challenging problem and are associated with increased mortality. Our observations on ‘real-world’ management of VRE-BSI indicate that limited clinical trial data has lead to treatment equipoise. Given the growing burden of disease and the serious consequences of infection, there is a pressing need to understand optimal management of VRE-BSI, especially the impact of newer antimicrobial therapy. Identifying predictors of mortality in VRE-BSI patients treated with newer antimicrobial agents may aid in future study design.
ACKNOWLEDGEMENTS
We thank Darlene Green, Aaron Jones, Steve Duncan, and Marga Jones for assistance in data collection and organization. We thank Dr Arnold Bayer and Dr Loren Miller for their review of the manuscript. We recognize the logistic support of the M01 RR 00425 grant to the GCRC at Harbor–UCLA Medical Center.
DECLARATION OF INTEREST
M.P. is a member of the speakers' bureaus for Cubist Pharmaceuticals and AstraZeneca Pharmaceuticals. J.W.B. has received research support from Pfizer.







