Introduction
Following controlled ovarian hyperstimulation (COH), approximately 20% of oocytes are immature (Ashourzadeh et al., Reference Ashourzadeh, Khalili, Omidi, Mahani, Kalantar, Aflatoonian and Habibzadeh2015). These oocytes are usually discarded in the majority of the IVF programmes (Lee et al., Reference Lee, Barad, Kushnir, Shohat-Tal, Lazzaroni-Tealdi, Wu and Gleicher2016). However, rescue in vitro maturation (IVM) of the immature oocytes can increase embryo quantity, particularly in poor responder women (Omidi et al., Reference Omidi, Khalili, Ashourzadeh and Rahimipour2014). In these patients, immature oocytes can be a source of urgently required extra embryos, although their implantation and pregnancy outcomes are considered low (Shin et al., Reference Shin, Cho, Lee, Yang, Lim and Lee2013). IVM is a well known technique in ART, especially in women with polycystic ovary syndrome (PCOS) or who are at high risk of ovarian hyperstimulation syndrome (OHSS) during COH (Omidi et al., Reference Omidi, Khalili, Ashourzadeh and Rahimipour2013). Nevertheless, IVM is carried out in only a few ART centres, as it entails intensive work with low clinical outcomes. Over recent years there have been extensive studies undertaken to improve outcomes (Guzman et al., Reference Guzman, Ortega-Hrepich, Polyzos, Anckaert, Verheyen, Coucke, Devroey, Tournaye, Smitz and De Vos2013). As rescue IVM has only limited expectation for success, this type of procedure has not been widely used in common clinical practice (Lee et al., Reference Lee, Barad, Kushnir, Shohat-Tal, Lazzaroni-Tealdi, Wu and Gleicher2016).
The developmental potential of in vitro matured oocytes is controversial. Possible detrimental developmental competence in IVM oocytes may be affected by IVM conditions, which do not exactly mimic in vivo conditions. Chromosomal segregation fidelity or essential cytoplasmic changes occur during germinal vesicle (GV) to MII stage oocyte transition (Coticchio et al., Reference Coticchio, Dal-Canto, Guglielmo, Mignini-Renzini and Fadini2013) (Smitz et al., Reference Smitz, Thompson and Gilchrist2011). Full imaging of the developmental competence of the embryos derived from IVM oocytes is an important task to ascertain feasible ways to improve the IVM technique as a clinical approach. It is clear that the final proof of oocyte developmental competence is its ability to develop a viable pregnancy (Dal Canto et al., Reference Dal Canto, Novara, Coticchio, Renzini, Brambillasca, Brigante, De Ponti and Fadini2016).
Over recent decades, the competence of embryo development has been commonly assessed by static conventional morphological criteria including blastomere number and symmetry, anucleated fragmentation rate, multinucleation, etc. These morphological evaluations of embryos are limited at daily discrete time points (Faramarzi et al., Reference Faramarzi, Khalili, Micara and Agha-Rahimi2017c). Conventional morphological assessments have provided limited information about how embryos are generated from conventional IVM and rescue IVM oocytes (Son et al., Reference Son, Chung, Demirtas, Holzer, Sylvestre, Buckett, Chian and Tan2008, Lee et al., Reference Lee, Barad, Kushnir, Shohat-Tal, Lazzaroni-Tealdi, Wu and Gleicher2016). However, non-invasive time-lapse monitoring (TLM) using timing of embryo developmental events and imaging of dynamic morphology has provided another dimension to complement conventional morphological assessment (Meseguer et al., Reference Meseguer, Herrero, Tejera, Hilligsøe, Ramsing and Remohí2011; Faramarzi et al., Reference Faramarzi, Khalili and Soleimani2015).
To the best of our knowledge, there are only limited amounts of literature that report morphokinetics of embryos generated following rescue IVM in a clinical setting. The assessment of embryo developmental competency derived from rescue IVM may be important for poor responder women. It is possible that MII oocytes derived from rescue IVM lead to embryos with different developmental potential, which can be assessed by the TLM system. Therefore, the aim of this study was to compare the cleavage kinetics and abnormal phenotypic events of embryos derived from in vivo-matured with rescue IVM from the same patients using TLM.
Materials and methods
Study design
In this retrospective study, 620 GV oocytes and 315 MII oocytes were included from 310 infertile patients (29±5 years) undergoing oocyte retrieval for ICSI at the Yazd institute for reproductive sciences. The couples were referred to the ICSI programme due to male factor aetiology. Only women less than 38 years old with basal follicle-stimulating hormone (FSH) <10 IU/ml and at least six mature oocytes were included. Exclusion criteria were women with endometriosis and polycystic ovaries as well as couples with sperm concentrations of less than 1 × 106/ml, frozen or surgically retrieved spermatozoa. The study population included patients who had both mature (MII) and immature (GV) oocytes. In addition, the same numbers of embryos derived from in vivo (group I) and in vitro matured (group II) oocytes from each patient were included in this study to diminish possible couple specific biases. According to the normal fertilized in vitro matured GV oocytes, the numbers of normal fertilized in vivo matured oocytes were selected from each patient. Also, normal fertilized in vivo matured oocytes were selected randomly.
Ovarian stimulation
The patients were stimulated with the standard GnRH antagonist protocols. In the antagonist protocol, 150 IU/day of follicle-stimulating hormone (Gonal F, Serono, Geneva, Switzerland) was administered on day 2 of the menstrual cycle. When at least one follicle reached 14 mm, 0.25 mg of a GnRH antagonist (Cetrotide, Merck Serono, Darmstadt, Germany) was administered and continued until the day of human chorionic gonadotropin (hCG) injection. When at least two 17-mm follicles were seen on transvaginal ultrasound, recombinant hCG (rhCG; Merck Serono, Darmstadt, Germany) was injected to trigger final maturation and ovulation. 36 h later, ultrasound-guided oocyte collection was done using a single lumen aspiration needle (Wallace; Smiths Medical International, UK).
Oocyte preparation, rescue IVM and injection
Approximately 2–3 h after retrieval, the cumulus–oocyte complexes (COCs) were denuded of cumulus cells by exposure to HEPES-buffered medium containing 80 IU/ml hyaluronidase (Irvine Scientific, CA, USA), and by pipetting the COCs with a Pasteur pipette. Nuclear maturity of the denuded oocytes was checked. MII oocytes were injected and GV oocytes were cultured in blastocyst medium (G2; Vitrolife Co., Sweden) supplemented with 75 IU/l of human menopausal gonadotropin (Ferring) in a triple gas incubator at 5% O2 and 6% CO2 and 89% N2. The GV oocytes were assessed for maturity after 24 h of IVM programme. Only 24 h matured oocytes were included in the study and injected using the partner’s spermatozoa.
Embryo culture
After injection, the oocytes were individually placed in a culture slide containing pre-equilibrated G-1 medium (Vitrolife, Sweden) covered with sterile mineral oil and cultured in the time-lapse microscope (Primo Vision Time-lapse Embryo Monitoring System, Goteborg, Sweden) in a triple gas incubator. Injected oocytes were cultured under a time-lapse microscope immediately after ICSI for 3 days.
Time-lapse analysis
The images of each embryo were taken in seven focal planes every 10 min. Only embryos with normal fertilization (presence of two pronuclei) were included in the study. All annotations were done by one embryologist. Embryo morphokinetics, including time of second PB extrusion (tPB2), time of pronuclei appearance (tPNa), time of pronuclei fading (tPNf), time of two to eight discrete cells (t2–t8) were assessed. In addition, abnormal cleavage patterns such as: (a) uneven blastomeres embryos at two cells stage (more than one third different from sibling blastomeres in size was described uneven) (Ciray et al., Reference Ciray, Campbell, Agerholm, Aguilar, Chamayou, Esbert and Sayed2014); (b) cell fusion (Fu), in which fusion or merging of blastomeres entails reduction in embryo cells number, which gives the reverse cleavage appearance to embryos or a blastomere reabsorbed after cleavage; or (c) trichotomous mitoses (TM), in which a single blastomere divided directly from 1±3 cells. Also, the rate of embryo arrest was monitored.
Statistics
Results are expressed as mean±standard deviation (SD) for normal numeric variables, median±interquartile range and percentage for categorical variables. The means of samples with normal distribution and of sufficient size were compared by independent samples parametric test (independent samples t-Test). For non-normal distribution, the medians were compared by Mann–Whitney U-test non-parametric test. Categorical variables were compared using chi-squared test. P-values <0.05 was considered as significant. Statistical analysis was performed using the Statistical Package for the Social Sciences 20 (SPSS Inc., Chicago, IL, USA).
Results
Out of 620 GV oocytes, 411 (66.29%) were matured during 24 h, and underwent ICSI. Table 1 shows the characteristics of oocytes and derived embryos. In total, 218 (53.04%) oocytes were fertilized normally (group II). Also, of the 315 MII oocytes that were injected, 218 (69.20%) were fertilized normally (group I). Immature oocyte selection was based on GV oocytes that were matured up to 24 h, as well as normally fertilized ones. Some of the normally fertilized oocytes were excluded from our analysis due to the early developmental arrest or ample fragmentation at different stages of development. The embryos derived from in vivo matured oocytes according to other embryos and patients conditions were either transferred or cryopreserved. All embryos derived from IVM oocytes were cryopreserved.
Table 1 Summary of the characteristics of GV and MII oocytes and derived embryos
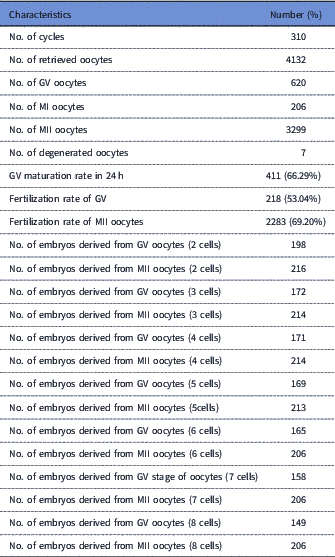
GV, germinal vesicle; MII, metaphase II.
Time-lapse analysis showed that some early developmental events including tPB2, tPNa, tPNF, t2, t3 and t4 occurred significantly later in group II than in group I (P<0.05; Table 2). But, no differences were observed between timings of t5, t6, t7 and t8 (P>0.05).
Table 2 Comparisons of cleavage timings in groups I and II
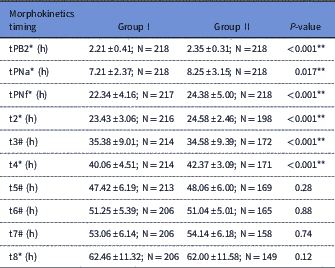
Results are expressed as mean±standard deviation (SD) for normal numeric variables, median±Interquartile range and percentage for categorical variables. 2nd PB extrusion (tPB2), pronuclei (PN) appearance (PNA), PN fading (PNF), t2=first cleavage (2-cell stage); t3=second cleavage (3-cell stage); t4=4-cell stage; t5=5-cell stage; t6=6-cell stage; t7=7-cell stage t8=8- cell stage.
Data were presented as *: median±IQ.
Data were presented as #: mean±SD.
There was significant difference regarding uneven blastomeres between group II and group I. As shown in Table 3, group I had a lower rate of uneven blastomeres when compared with group II (5.5% vs 16.1%, respectively) (P=0.001).
Table 3 Comparisons of uneven blastomeres between groups I and II

CI, confidence interval; OR, odds ratio.
There was significant difference regarding cell fusion (Fu) between groups I and II. As stated in Table 4, embryos within group II had higher rate of Fu when compared with group I (51.6% vs 27.8%, respectively) (P<0.001).
Table 4 Comparisons of cell fusion (Fu) between group I and group II
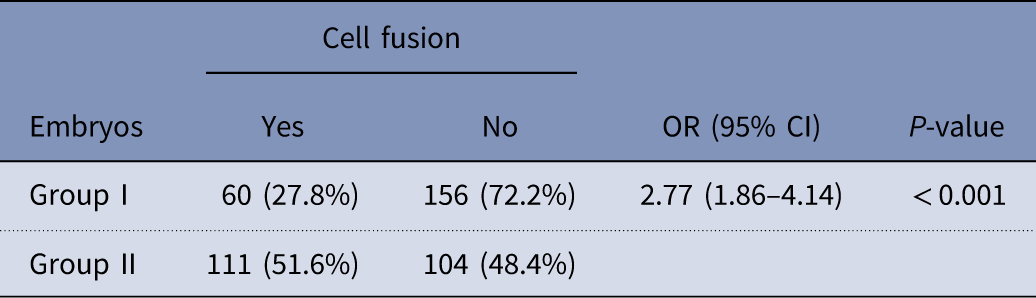
OR, odds ratio; CI, confidence interval.
There was significant difference regarding trichotomous mitosis (TM) embryos between groups I and II. As mentioned in Table 5, group I had lower rate of TM compared with embryos derived from rescue IVM (14.0% vs 25.7% respectively) (P=0.003).
Table 5 Comparison of rate of trichotomous mitosis (TM) embryos between groups I and II
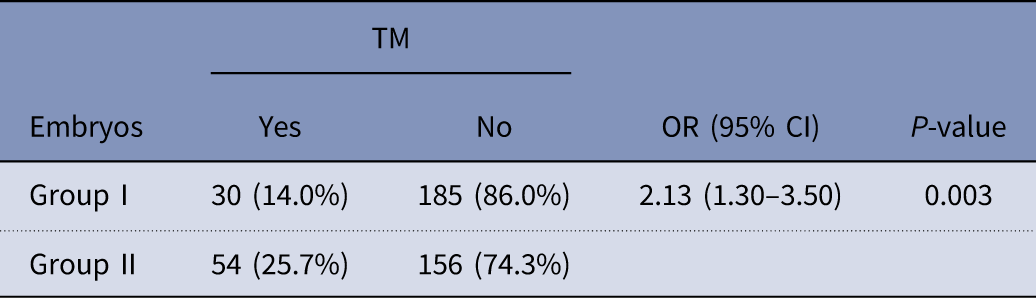
CI, confidence interval; OR, odds ratio.
There were significant differences in the rates of arrest between embryos derived from group I and group II (Table 6). Group II had higher rates of arrest when compared with group I (31.7%% vs 5.5% respectively) (P<0.001).
Table 6 Comparisons of the rates of arrest between embryos group I and group II
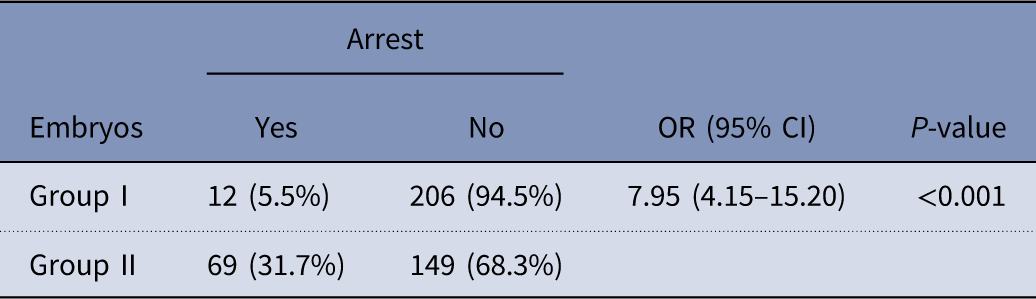
CI, confidence interval, OR, odds ratio.
Discussion
Despite optimizing the COH protocols, nearly 20% of the retrieved oocytes remain immature at the GV or MI stages (Ashourzadeh et al., Reference Ashourzadeh, Khalili, Omidi, Mahani, Kalantar, Aflatoonian and Habibzadeh2015). Such immature eggs are considered unsuitable for clinical use and they are commonly discarded from the IVF programme (Escrich et al., Reference Escrich, Grau, Mercader, Rubio, Pellicer and Escribá2011). A good source of embryos can originate from immature oocytes that undergo IVM procedure for poor responders. However, these embryos are generally involved with poor clinical outcomes (Shin et al., Reference Shin, Cho, Lee, Yang, Lim and Lee2013). To our knowledge, this is the first study to compare the morphokinetics of the embryos derived from rescue IVM and in vivo matured oocytes in a ICSI setting. We found that morphokinetics timings, including tPB2, tPNa, tPNf, t2, t3 and t4, occurred later in embryos generated from rescue IVM oocytes.
Our data are in contrast with the previous studies reported by Walls et al. (Reference Walls, Ryan, Keelan and Hart2015). Dal Canto et al. (Reference Dal Canto, Novara, Coticchio, Renzini, Brambillasca, Brigante, De Ponti and Fadini2016) and Roesner et al. (Reference Roesner, Dietrich, Weigert, Montag, Toth and Strowitzki2017). Both the Wall and Dal Canto groups did not report any differences in morphokinetics time points between embryos derived from in vitro or in vivo matured oocytes (Walls et al., Reference Walls, Ryan, Keelan and Hart2015; Dal Canto et al., Reference Dal Canto, Novara, Coticchio, Renzini, Brambillasca, Brigante, De Ponti and Fadini2016). Also, Roesner and colleagues showed that there were no differences in the tPB2, tPNf, t2, t3, t4 and t5 time points. However, they reported that tPNA was shorter and t6, t7 and t8 were longer in the embryos derived from in vitro matured oocytes compared with the in vivo matured oocytes (Roesner et al., Reference Roesner, Dietrich, Weigert, Montag, Toth and Strowitzki2017). These results do not concur with our findings and were mainly due to differences in the sources of the oocytes, e.g. unstimulated vs. stimulated cycles. Also, Dal Canto and colleagues used IVM with the surrounding cumulus cells, but we used denuded oocytes. However, our data are somewhat in accordance with Kim and colleagues (2016), who observed longer morphokinetic timing (time point) from tPB2 to t8 in the embryos developed from MI oocytes after COH (Kim et al., Reference Kim, Jang, Lee, Yoon, Shin, Shin, Jung and Lim2016). Several have studies reported that cleavage kinetic timings may predict the outcome of embryo development (Meseguer et al., Reference Meseguer, Herrero, Tejera, Hilligsøe, Ramsing and Remohí2011; Hashimoto et al., Reference Hashimoto, Kato, Saeki and Morimoto2012). Also, Dal Canto’s group showed that embryos with high competence were related to the earlier time point of cleavage during the first three rounds of mitosis divisions (Dal Canto et al., Reference Dal Canto, Coticchio, Renzini, De Ponti, Novara, Brambillasca, Comi and Fadini2012).
Oocyte maturation can be one of the determining factors for cleavage timing (Gardner et al., Reference Gardner, Weissman, Howles and Shoham2012). The reasons for these delays in cleavage timings may be related to the inadequate maturation of IVM oocytes or poor quality immature oocytes from COH cycles (Kim et al., Reference Kim, Lee, Kim, Han and Kim2000). Oocytes undergoing IVM protocols may not synchronize orchestrated nuclear and cytoplasmic maturations as probably in vitro conditions may not be able to completely mimic intrafollicular conditions. Conventional assessment shows no sign of cytoplasmic maturation. Unlike nuclear maturation, this is determined by first PB extrusion. Poor outcomes of IVM were mostly related to poor cytoplasmic maturity (Liu et al., Reference Liu, Li, Gao, Yan and Chen2010). However, diminished developmental potential can be associated with post-meiotic chromosomal errors that have been induced by oocyte cytoplasmic immaturity (Álvarez et al., Reference Álvarez, García-Garrido, Taronger and De Merlo2013; Shin et al., Reference Shin, Cho, Lee, Yang, Lim and Lee2013). In addition, rescue IVM changes the mitochondria distribution (Liu et al., Reference Liu, Li, Gao, Yan and Chen2010) and morphology of oocytes (Shahedi et al., Reference Shahedi, Khalili, Soleimani and Morshedizad2013) that may be potential factors for diminished development.
In addition, this study demonstrated no differences between t5, t6, t7 and t8 in in vivo or in vitro matured oocytes that were normally fertilized. However, the maternal genome controls the early stages of embryo development. Embryonic genome activation (EGA) is vital for late embryo development. Furthermore, decay of maternal RNAs starts after fertilization. EGA starts at the end of the second division that dominates during third cytokinesis (Wong et al., Reference Wong, Loewke, Bossert, Behr, De Jonge, Baer and Pera2010). Therefore, rescue IVM treatment may influence embryo development during this transition and before EGA completion. The genome of embryos after activation at the end of the third cleavage could affect timing of cleavage, so reverting these to normal status.
Moreover, we found that the rates of uneven blastomeres, Fu and TM embryos were significantly increased in embryos derived from rescue IVM oocytes, compared with in vivo matured oocytes. Also, the rate of arrest increased in the embryos derived from rescue IVM oocytes when compared with in vivo matured oocytes. Concurred with our data, Walls and teammates showed that PCOS-IVM group showed significantly more uneven two cells embryos, when compared with the control-ICSI group (Walls et al., Reference Walls, Ryan, Keelan and Hart2015). Aneuploidies and genetic disorders are possible causes for embryos to fail from normal cleavage pattern (Faramarzi et al., Reference Faramarzi, Khalili, Omidi, Agha-Rahimi and Taheri2018). So, it could be a strategy to properly not selecting the poor rescue IVM embryos. Further, it has been indicated that multiple aneuploidies are in the majority of embryos that do not develop normal cell divisions timing (Meseguer et al., Reference Meseguer, Herrero, Tejera, Hilligsøe, Ramsing and Remohí2011). Several previous studies showed that cleavage pattern, such as embryos with uneven blastomere size at 2-cell stage, direct cleavage, reverse cleavage that resulted in the lower developmental competence as well as implantation potential (Rubio et al., Reference Rubio, Kuhlmann, Agerholm, Kirk, Herrero, Escribá, Bellver and Meseguer2012) (Meseguer et al., Reference Meseguer, Herrero, Tejera, Hilligsøe, Ramsing and Remohí2011) (Liu et al., Reference Liu, Chapple, Roberts and Matson2014). The molecular mechanisms that entail the abnormal cleavage pattern are not obvious. Yet, it is assumed that mitotic fault may play a major role in these events (Almagor et al., Reference Almagor, Or, Fieldust and Shoham2015). In our recent works, it was shown that oocyte quality can affect the embryo cleavage patterns in clinical programme (Faramarzi et al., Reference Faramarzi, Khalili and Ashourzadeh2017b) (Faramarzi et al., Reference Faramarzi, Khalili, Agha-Rahimi and Omidi2017a, Faramarzi et al., Reference Faramarzi, Khalili and Omidi2017d). As, quality of the rescue IVM oocytes was lower when compared with in vivo matured oocytes, it may be one of the reasons for increased abnormal cleavage patterns and arrest.
This is the first study that evaluated the development of embryos derived from in vitro matured GV oocytes obtained from COH by TLM. The limitations of this study were the small number of data sets, and lack of information on blastocyst development and clinical outcomes. However, future larger studies are needed to validate the present findings. Based on the exact annotation of timing parameters and cleavage patterns made possible by TLM, the present data agree with the concept that rescue IVM GV stage oocytes negatively influence embryo morphokinetics as well as increase embryo arrest. Therefore, cautious use of embryos derived from rescue IVM of GV oocytes should be made. Additional studies for improvement of the IVM medium in order to enhance overall embryo development and implantation rates are needed. It is suggested that IVM of MI stage oocytes may improve the generated embryo development.
Acknowledgements
The authors would like to thank the colleagues who helped with data collection.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflicts of interest
All authors declare no conflict of interest.
Ethical standards
This study was approved by Ethics Committee of our institution.








