New Zealand’s population is ageing, and those aged over 80 years are the fastest growing population segment predicted to increase 6-fold by 2050( 1 ). Māori are the indigenous people of New Zealand. At present, <0·2 % of Māori reach 85 years of age( 1 ). However, the population of Māori aged over 80 years is expanding faster than the non-Māori octogenarian population( 2 ). As older people are at increased risk of nutritional deficiencies, which closely predict morbidity and mortality( Reference Chapman 3 ), social and healthcare systems will be impacted.
To date, there is very little information available about the nutritional status of those aged 80 years and over in New Zealand as the New Zealand Adult Nutrition Survey (NZANS) data are aggregated over age 55 years for Māori and over 70 years for non-Māori populations( 4 ). Typically, BMR declines with age because of age-related body composition changes. This physiological change along with social, psychological and medical factors predisposes older adults to weight loss( Reference Morley 5 ). Consequently, older people are at increased risk of nutrition-related health problems such as increased functional difficulties, co-morbidities and cognitive decline( Reference Cereda, Pedrolli and Zagami 6 ).
In New Zealand, the adult recommendations for dietary energy are prescribed according to age and sex with the goal of maintaining a BMI of 22 kg/m2, consistent with the midpoint of the healthy weight range for all adults( 7 ). In older people, BMI in the overweight range are associated with optimal survival( Reference Stevens, Cai and Pamuk 8 – Reference Diehr, O’Meara and Fitzpatrick 10 ). Accordingly, it has been suggested that the desirable healthy weight range be set higher for improved health outcomes in older people( Reference Rejeski, Marsh and Chmelo 11 ). Estimates of total energy requirements are based on predictive equations that have not been validated in the older age group( 7 ).
An adequate protein intake is especially important for older adults to maintain a healthy functional status and decrease the risk of prolonged infections that lead to hospitalisation( Reference Volpi, Campbell and Dwyer 12 ). The current estimated average requirement (EAR) and RDI for protein were developed for adults aged above 70 years and may not reflect the protein needs of people aged 80 years and over, for whom little data exist on dietary intake. Thus, more detailed nutrition information is needed for those aged 80+ years.
The applicability of these dietary intake recommendations for Māori is uncertain. Older Māori are more likely to have a higher BMI than non-Māori( Reference Wham, Teh and Moyes 13 ), and this may be due to differences in body composition. In younger age groups, Māori are known to have a higher proportion of lean body mass compared with non-Māori( Reference Swinburn, Ley and Carmichael 14 ). Thus, more detailed nutrition information is needed, particularly for Māori.
Energy and macronutrient intake can be influenced by the nutrient density of the food, frequency of consumption and the quantity consumed. Knowledge of food sources in conjunction with how well older people meet current recommended nutrient intakes is important to inform future recommendations. To date, no comprehensive analysis has been undertaken to identify macronutrient and foods sources in people of advanced age. Te Puāwaitanga o Ngā Tapuwae Kia ora Tonu, Life and Living in Advanced Age: A Cohort Study in New Zealand (LiLACS NZ) is a population-based cohort study of Māori aged 80–90 years and non-Māori aged 85 years at inception in 2010( Reference Dyall, Kepa and Hayman 15 , Reference Hayman, Kerse and Dyall 16 ). The aim of this study was to examine energy and macronutrient intakes and the contribution of food groups to that intake in Māori and non-Māori participating in LiLACS NZ.
Methods
Subjects
LiLACS NZ recruited 937 participants from the Bay of Plenty and Rotorua regions of New Zealand in 2010 – 421 Māori born in 1920–1930 (aged 80–90 years, 56 % of those eligible) and 516 non-Māori born in 1925 (aged 85 years, 59 % of those eligible). The details of LiLACS NZ recruitment and baseline assessments have been described elsewhere( Reference Dyall, Kepa and Hayman 15 , Reference Hayman, Kerse and Dyall 16 ). For the Māori cohort, participant sex and age distributions were roughly equivalent to the underlying same-age population, and for the non-Māori cohort women were slightly under-represented( Reference Dyall, Kepa and Hayman 15 ). Interviewers for Māori were fluent users of te reo Māori me ngā tikanga (Māori language and culture). Trained interviewers completed multidimensional interviews using standardised techniques, and trained research nurses completed standardised physical assessments.
A 12-month follow-up visit was completed in 2011, and a detailed dietary assessment using the 24-h multiple pass recall on two separate days (2×MPR) was offered as part of that stage of the study. A total of 660 participants took part in any data collection at this 12-month follow-up (Fig. 1).
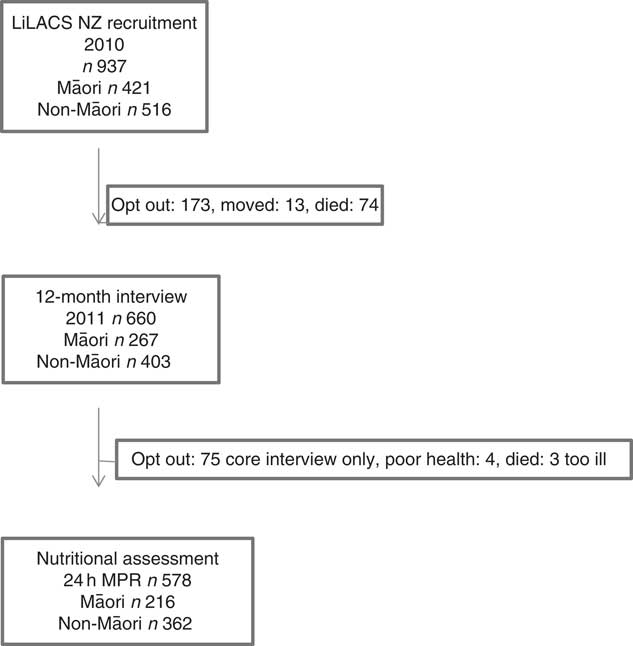
Fig.1 Flow chart of dietary assessment, 24-h multiple pass recall (24 h MPR), at the 12-month follow-up interview for Life and Living in Advanced Age: A Cohort Study in New Zealand (LiLACS NZ) participants.
Of the 267 Māori engaged in the 12-month interviews, 216 (81 %) completed the dietary assessment (four participants completed the nutrition interview without completing the main questionnaire). Results of these assessments are reported in this article. There were no differences between Māori who completed the dietary assessments and Māori who did not with regard to living arrangement, sex, age or depression status. Of the 403 non-Māori who took part in the 12-month interviews, 362 (90 %) completed the dietary assessment. There were no differences between non-Māori who completed the dietary assessment and non-Māori who did not complete the assessment with regard to living arrangement, sex, age and depression status.
This study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human participants were approved by the Northern X Regional Ethical Committee (NXT 09/09/088) in 2009. Written informed consent was obtained from all the participants( Reference Hayman, Kerse and Dyall 16 ).
Measures
Demographic information including age and sex was ascertained during baseline interviews; current living arrangement was categorised as living alone, with only the spouse or with others, which could also include the spouse. Smoking status and alcohol intake were ascertained by self-report. The New Zealand Deprivation index, an index constructed on the basis of access to resources from small area census data, based on the address at enrolment, was obtained from the Ministry of Health and was used as an indication of socio-economic deprivation( Reference Salmond, Crampton and Atkinson 17 ). Depression was assessed by the fifteen-item Geriatric Depression Scale (GDS-15)( Reference Montorio and Izal 18 ), a reliable and valid self-rating depression screening scale developed specifically for older people( Reference Yesavage, Brink and Rose 19 ). A higher score indicates more depressive symptoms, with a cut-off of 5 or more considered to indicate significant depressive symptoms( Reference Yesavage and Sheikh 20 ).
The Short Form Health Survey (SF-12)( Reference Fleishman, Selim and Kazis 21 ) was used to measure health-related quality of life. The maximum score is 100; any score lower than 40 is indicative of perception of poor health and above 60 indicative of perception of reasonable and better health.
A Physical Activity Scale for the Elderly (PASE), validated in community-living, older adults( Reference Washburn, McAuley and Katula 22 ), was used to assess physical activity. Weight and height measurements were completed by trained research nurses following the protocol used in the NZANS( 23 ). Weight was ascertained using a Tanita digital measuring scale (BC-541; Tanita Corporation) and height using a portable stadiometer (SECA 213). For participants who were unable to stand, height was estimated from the demispan, a measure closely related to height( Reference Hirani and Mindell 24 ), incorporating the distance from the mid sternum to the webspace between the third finger and the ring finger of the horizontally outstretched arm; two readings were obtained, and a third measurement was obtained if the difference of the first two readings was >0·5 cm. Height and weight were used to calculate BMI, and height, weight, age and sex were used to calculate the BMR using the Fredrix technique( Reference Fredrix, Soeters and Deerenberg 25 ).
Dietary assessment: 24-h multiple pass recall
LiLACS NZ participants completed a 24-h multiple pass recall (24 h MPR) on two different days of the week. The mean interval between food intake interviews was 23 (sd 36·7) d for Māori and 17 (sd 33·2) d for non-Māori. Interviewers were trained in the conduct of the 24-h MPR and subject to monitoring and quality controls. The protocol established for the 24 h MPR has been reported, and the conduct of the method has been reported elsewhere( Reference Adamson, Collerton and Davies 26 ).
Where possible, actual food weights were recorded from food packages or food labels or were estimated using household measuring spoons or a jug. Where this was not possible, a ‘Photographic Atlas of Food Portion Sizes’( Reference Nelson, Atkinson and Meyers 27 ) used in Newcastle 85+ assessments( Reference Adamson, Collerton and Davies 26 ) was adapted for use in the LiLACS NZ study. Nutrient intakes were calculated by coding all food and drinks recorded by participants using the New Zealand Food Composition Database (NZFCDB). All coding was completed by nutritionists experienced in dietary data coding. FOODfiles (2010), an electronic subset of data from the NZFCDB, was used as the main source of food composition data( 28 ) and contained information on fifty-eight components of 2739 foods.
Food groups
Food items reported in the 24 h MPR were allocated to food groups in order to calculate sources of nutrients by the type of food. The individual ingredients were assigned to separate food groups from the detailed description of the food and recipes. The thirty-three food groups used in the 2008/09 NZANS were used.
Misreporting
Misreporting of energy intake (EI) comprising both under- and over-reporting is a common problem in dietary assessments and has been described in the NZANS( Reference Gemming, Jiang and Swinburn 29 , Reference Pikholz, Swinburn and Metcalf 30 ). Misreporting is most commonly measured by comparing reported EI with an individual’s estimated BMR (BMRest), calculated using the Fredrix equation( Reference Fredrix, Soeters and Deerenberg 31 ), and by applying cut-off values as described by Goldberg, identified as the optimal method in a review( Reference Poslusna, Ruprich and de Vries 32 ) based on the ratio between reported EI:BMRest (EI:BMRest) for a specified energy expenditure (physical activity level)( Reference Black 33 ). In this analysis, under-reporting was defined as EI:BMRest<0·9( Reference Black 33 ), and EI:BMRest>2·0 was used to define over-reporters( Reference Black 33 , Reference Bazelmans, Matthys and De Henauw 34 ).
Statistical analysis
Differences between those completing and not completing the dietary assessment were assessed. Living arrangement and sex were tested using Cochran–Mantel–Haenszel tests; depression and age were tested using Student’s t test. Descriptive statistics were used to report macronutrient intake and the food groups contributing to these groups. Distribution of daily EI, carbohydrate, total sugars, sucrose, protein, fat, dietary fibre, water and alcohol intakes were examined, and mean (standard deviation) values were reported for Gaussian distribution and median (interquartile range, IQR) values for non-Gaussian distribution (Table 2). Energy-adjusted carbohydrate, protein, fat, fibre and protein per kg body weight were calculated. Comparisons used Mann–Whitney–Wilcoxon ranked sum test to check for differences between ethnic groups and sex singly. As sex varied in the ethnic groups, further multivariate generalised linear regression analyses estimated differences in intake as a continuous measure between the sexes, adjusting for age and ethnic group and between ethnic groups adjusting for age and sex. Age was necessary to adjust for as the age varied for Māori (Table 2). Table 3 shows differences in intakes according to different living arrangements, adjusted for sex in non-Māori analyses and age and sex in Māori analyses (as all non-Māori were born in the same year) using multivariate generalised linear regression models. Comparisons were also made between intake and depression, quality of life and physical activity (data not shown).
Table 4 compares nutrient reference values (NRV) achievement between sex and ethnic groups. Macronutrient levels were coded into the dichotomous, achieved NRV or outside the NRV range, and logistic regression models controlled for age (Table 4). NRV for Australia and New Zealand were used( 7 ) to report the proportion of participants meeting the NRV.
Sensitivity analyses were performed on macronutrient intakes excluding those with EI:BMRest<0·9 and EI:BMRest>2·0 and are presented in the online Supplementary Tables S5 and S6.
The data analyses for this study were generated using SAS/STAT (software 12·1, version 9.3; SAS Institute Inc.) A P value of <0·05 was considered statistically significant.
Results
Attrition between Wave 1 (baseline) and Wave 2 (12-month follow-up) was higher among Maori (66 % retained) than non-Māori (78 % retained) (Fig. 1). Of the 660 participants in Wave 2, 216 (81 %) Māori and 362 (90 %) non-Māori consented to food records, and 201 Māori (75 %) and 354 non-Māori (88 %) completed 2×24-h MPR with fifteen Māori (6 %) and eight non-Māori (2 %) completing only 1×24-h MPR. Comparing Wave 1 characteristics of those completing the dietary assessments with those who did not, Māori who lived with others (compared with those living alone or with spouse), those with severe depression and those with low physical activity were less likely to participate in the Wave 2 interview and dietary assessment (P<0·05 for all). In total, complete data sets for 216 Māori and 362 non-Māori participants were included in the analysis. Table 1 shows the characteristics of participants.
All participants
Overall, non-Māori consumed more alcohol, total sugars, dietary fibre and less energy-adjusted protein compared with Māori. Dietary fibre intake was generally low (Table 2).
Sex
For both Māori and non-Māori, indicative of higher median EI, men consumed higher levels of total carbohydrate, protein and fat than women (Table 2). When adjusted for energy intake, men across both groups had lower energy-adjusted carbohydrate, compared with women at both univariate and multivariate analyses. Alcohol intake was higher in men than women.
Māori participants
In all, 51 % of women lived alone, and depressive symptoms were evident in 24 % of Māori – 31 % of men and 19 % of women (Table 1). The median BMI for Māori was 28·3 kg/m2, and the median EI:BMRest was 1·1 (IQR 0·9–1·3), similar for both sexes (1·1 (IQR 0·8–1·3) for men and 1·1 (IQR 0·9–1·3 for women)). Using the cut-off of EI:BMRest<0·9 and EI:BMRest>2·0, 20 (36 %) men and 23 (27 %) women were found to be potential misreporters.
Table 1 Social, physical and health characteristics of Māori and non-Māori participants by sex (Numbers and percentages; medians and interquartile ranges (IQR))

NZDep, New Zealand Deprivation Index; GDS, Geriatric Depression Scale, higher score, worse depressive symptoms; SF-12, Short Form Health Survey; PASE, Physical Activity Scale for the Elderly, higher score means more activity.
* Percentage of ethnic group. The proportion of women in the non-Māori group is lower than the Māori group, although this is not statistically significant (P=0·25).
† With others includes living with extended family or in residential care (eight in residential care).
Energy intakes and food contribution to macronutrients
For Māori, 43·3 % of EI was from carbohydrate, 16·3 % from protein and 38·5 % from fat (Table 2). There was no variation in macronutrient intake by living situation (Table 3). Controlling for age and sex, EI was not associated with depression symptoms (GDS-15), quality of life (SF-12) and physical activity level (PASE) (data not shown).
Table 2 Daily energy and macronutrient intakes for all Māori and non-Māori participants by sex (Medians and interquartile ranges (IQR))Footnote *
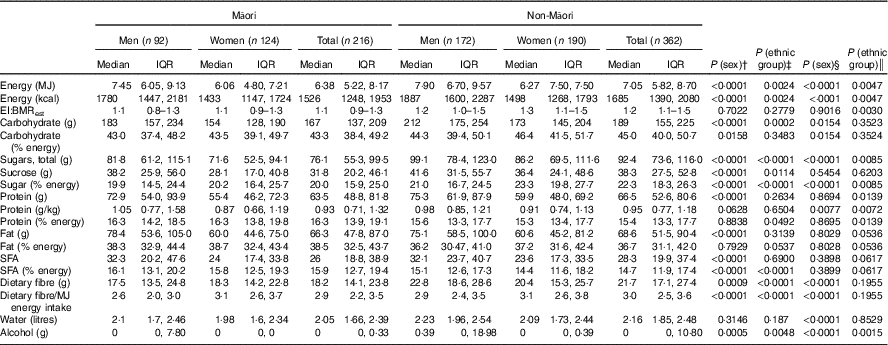
EI:BMR, ratio of energy intake to estimated BMR (BMR estimated using the Fredrix formula); % energy, percent of energy intake; g/kg, grams per kilogram body weight.
* Median IQR is presented unless specified as % energy.
† Comparing all men and all women, Wilcoxon’s univariate non-parametric test.
‡ Comparing all Māori with all non-Māori, Wilcoxon’s univariate non-parametric test.
§ Comparing all men and all women, multivariate generalised linear model controlling for age and ethnicity.
║ Comparing all Māori with all non-Māori, multivariate generalised linear model controlling for age and sex.
Table 3 Daily energy and macronutrient intakes of Life and Living in Advanced Age: A Cohort Study in New Zealand participants by living situation
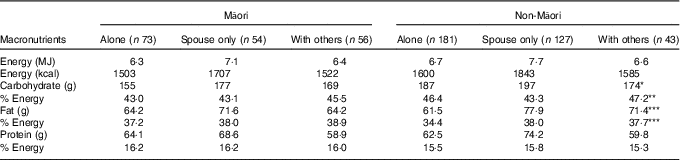
* P<0·05, ** P<0·01, *** P<0·001, difference across living arrangement, adjusted for age (only in Māori as all non-Māori were aged 86 years by design) and sex comparing with non-Māori living alone and living with spouse only. No significant differences in intake detected among Māori related to living arrangement.
Food groups contributing to macronutrient intakes are shown in Fig. 4. Bread followed by butter and margarine, potatoes, kumara and taro (sweet potato and starchy tuber), fruit, milk and cereals were the top contributors to total energy and carbohydrate intake with the order differing by sex. Protein intake came from beef and veal (both sexes 11 %), fish and seafood (men 11 %, women 9 %), bread (men 10 %, women 13 %), milk (men 9 %, women 11 %) and poultry (men 9 %, women 11 %) with differing order of frequency by sex. Fat mainly came from butter and margarine (men 17 %, women 19 %) (Fig. 4(m) and (n)).
Meeting recommendations
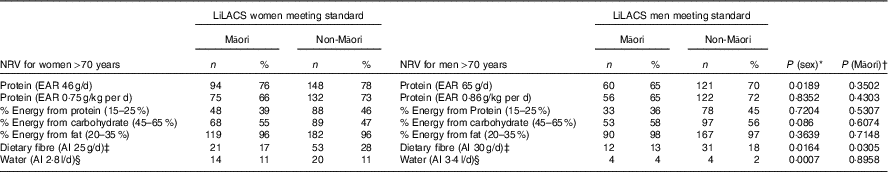
Table 4 and Fig. 2 and 3 show the proportion of participants meeting the NRV. For Māori, the acceptable macronutrient distribution range (AMDR)( 7 ) for protein was met by 39 % of women and 36 % of men, for carbohydrate by 55 % of women and 58 % of men and for fat by most men and women. The adequate intake (AI)( 7 ) for dietary fibre was met by 17 % of women and 13 % of men and for water intake by 11 % of women and 4 % of men.
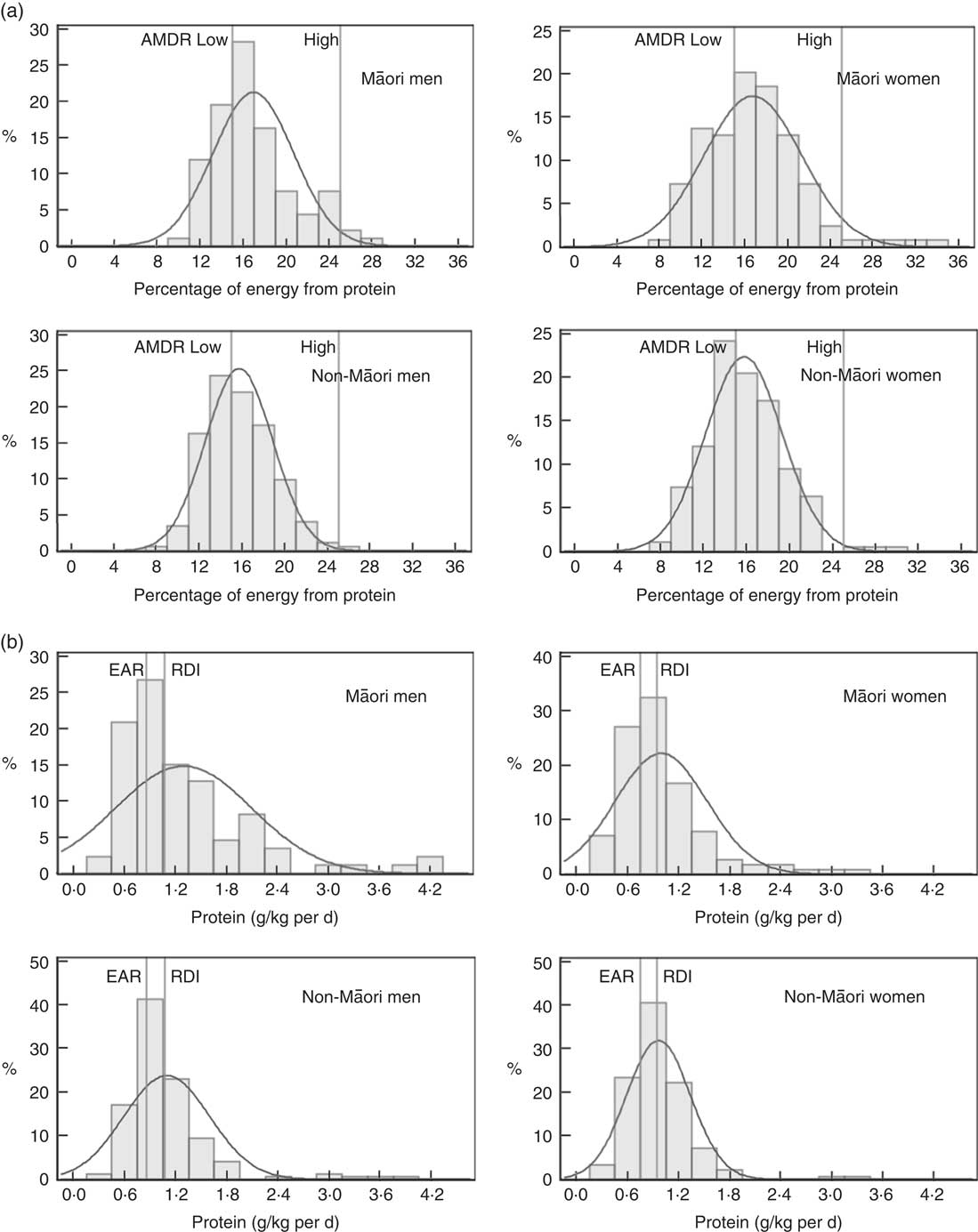
Fig. 2 Intake of protein of Life and Living in Advanced Age: A Cohort Study in New Zealand participants by sex and ethnic group: (a) percent energy from protein, (b) protein g/kg body weight per d. AMDR, acceptable macronutrient distribution range; EAR, estimated average requirement.
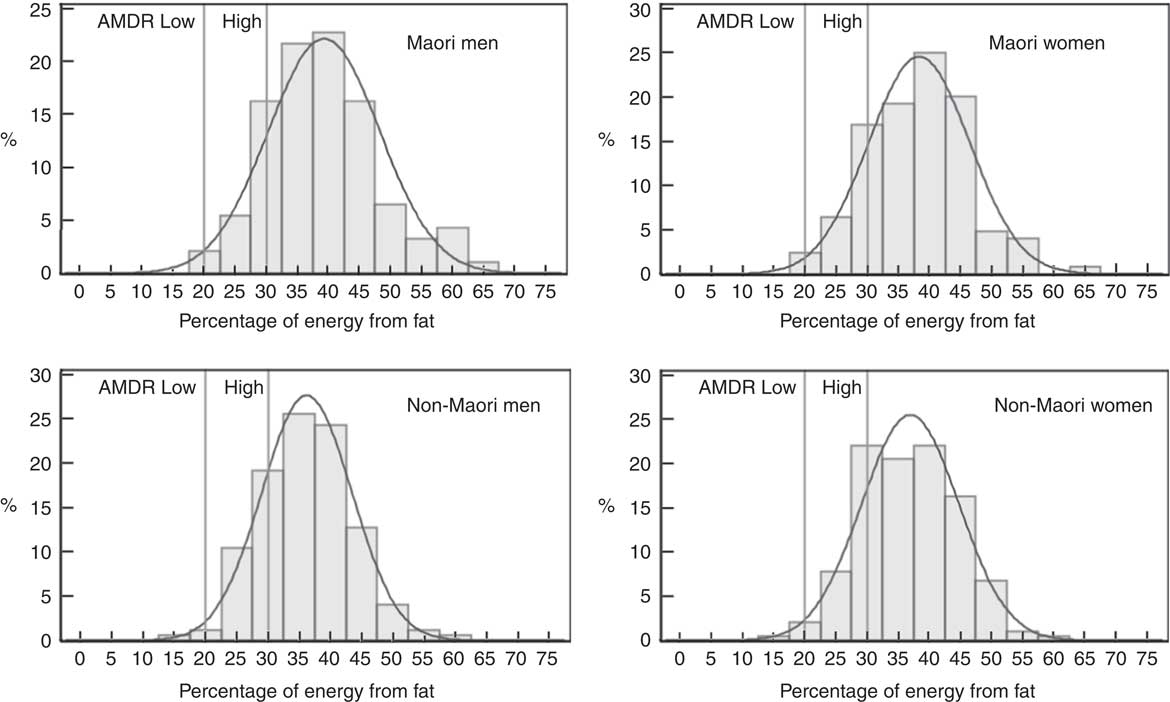
Fig. 3 Intake distribution of percentage of energy from fat for Māori and non-Māori Life and Living in Advanced Age: A Cohort Study in New Zealand participants by sex. AMDR, acceptable macronutrient distribution range.
Table 4 Proportion of Māori and non-Māori participants who met the nutrient reference values (NRV) for Australia and New Zealand for intake of macronutrients, dietary fibre and water by sex
LiLACS, Life and Living in Advanced Age: A Cohort Study; EAR, estimated average requirement; AI, adequate intake; NZFCDB, New Zealand Food Composition Database.
* Comparing all men with all women adjusting for age.
† Comparing all Māori with all non-Māori adjusting for age.
‡ The NZFCDB FOODfiles uses Prosky’s method of analysis for total dietary fibre (AOAC 991.43)( Reference Prosky, Asp and Furda 35 ) because the Joint Australia New Zealand Food Standards Code prescribes the Prosky’s method of analyses for the purpose of food labelling.
§ Total water includes water from foods as well as fluids.
Māori participants
In all, 65 % of non-Māori women lived alone. Depressive symptoms were evident in 16 % among both men and women. The median BMI of 26·2 kg/m2 and the median EI:BMRest of 1·2 (IQR 1·1–1·5) were similar for both sexes (1·2 (IQR 1·0–1·5) for men and 1·3 (IQR 1·1–1·5 for women)). Using the cut-off of EI:BMRest<0·9 and EI:BMRest>2·0, 27 (19 %) men and 21 (14 %) women were found to be potentially misreporters (Table 1).
Energy intakes and food contribution to macronutrients
For non-Māori, 15·4 % of EI was from protein, 45 % from carbohydrate and 36·7 % from fat (Table 2). Macronutrient intakes varied by living arrangement, with those living alone having lower total fat and energy-adjusted fat intake, those living with others having lower absolute amounts of carbohydrate intake, and those living with spouse only having a lower energy-adjusted carbohydrate intake (Table 3). Controlling for sex, higher EI was associated with better mental health-related quality of life (β 0·13 (95 % CI 0·002, 0·025), P=0·026) but not physical health-related quality of life, depression symptoms (GDS-15) or physical activity level (PASE) (data not shown).
Fig. 4 shows that bread was the main food group contributor to EI for both men and women (11 % respectively), followed by butter and margarine, cereals, milk, fruits, and potato, kumara and taro differing in order by sex. Bread (men 19 %, women 17 %), fruits (men 12 %, women 17 %), cereals (men 10 %, women 8 %), and potatoes, kumara and taro (men 10 %, women 7 %) were the key contributors to carbohydrate. Protein intake for both men and women was mainly from beef and veal (men 13 %, women 10 %), bread (both sexes 12 %) and milk (men 11 %, women 12 %) with differences in the order by sex. Butter and margarine (men 18 %, women 17 %) were the main contributors of fat intake (Fig. 4(o) and (p)).
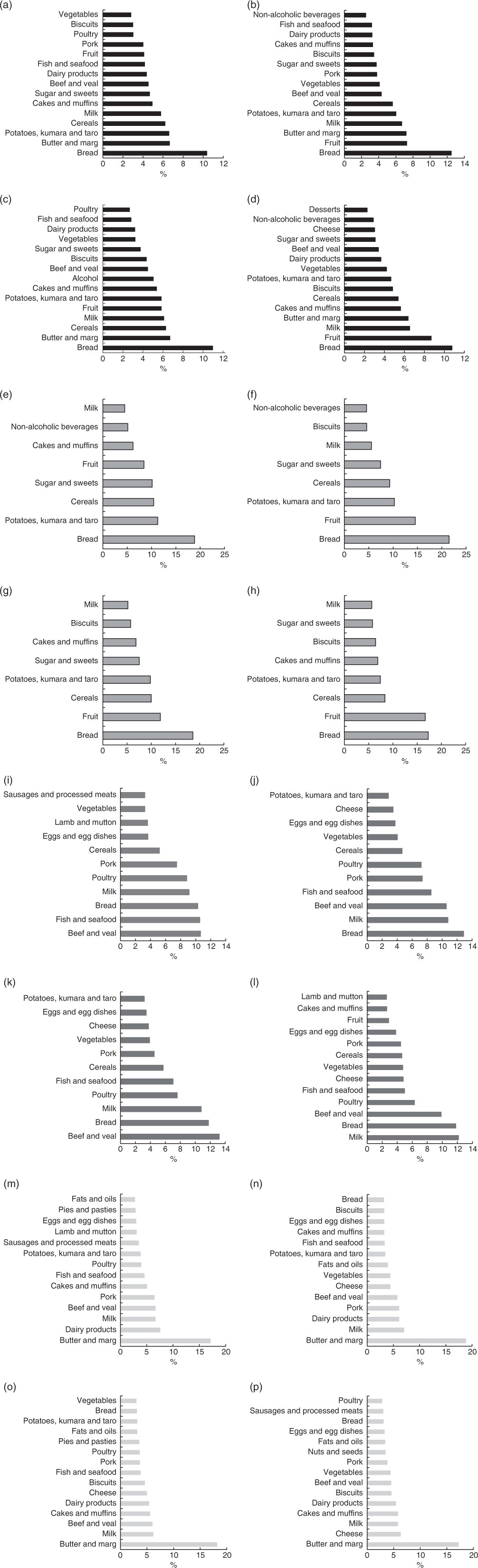
Fig. 4 Percentage of contribution from food groups to energy and macronutrient intake for Māori and non-Māori by sex. Food sources contributing to ≥75 % of total energy: (a) Māori men, (b) Māori women, (c) non-Māori men, (d) non-Māori women; food sources contributing to ≥75 % of carbhohydrate: (e) Māori men, (f) Māori women, (g) non-Māori men, (h) non-Māori women; food sources contributing to ≥75 % of protein: (i) Māori men, (j) Māori women, (k) non-Māori men, (l) non-Māori women; food sources contributing to ≥75 % fat: (m) Māori men, (n) Māori women, (o) non-Māori men, (p) non-Māori women.
Meeting recommendations
Table 4 and Fig. 2 and 3 show that for non-Māori, 46 % of women and 45 % of men met the AMDR for protein, 47 % of women and 56 % of men for carbohydrate and the majority for fat; 28 % of women and 18 % of men met the AI for dietary fibre, and 11 % of women and 2 % of men met the AI for water intake.
Sensitivity analyses
The online Supplementary Tables S5 and S6 show results for the sample restricted to those with a EI:BMR of between 0·9 and 2·0. Sensitivity analyses (excluding those whose average EI suggested potential misreporting EI:BMRest <0·9 and >2·0) for Māori made 10 % difference to the intake of protein, 6 % difference to the intake of carbohydrate and 4 % difference to the intake of fat. For non-Māori, excluding misreporters made 1 % difference to the intake of protein, 1 % difference to the intake of carbohydrate and 3 % difference to the intake of fat. For these sensitivity analyses, excluded Māori had significantly lower absolute intake of protein, fat and carbohydrate, but these were not significant as a percentage of EI. Non-Māori who were excluded had significantly lower intakes of fat and carbohydrate, but not protein or in the percentage of EI from fat, carbohydrate or protein. This indicates that, although the majority of misreporters may be under-reporting, the relative proportions of macronutrients are plausible.
Excluding misreporters, the EAR for daily protein intake was met by more women (88 % of Māori and 84 % of non-Māori women) than men (80 % Māori and 74 % non-Māori men) (P=0·024). Approximately two-thirds of Māori (66 % women and 65 % men) and three-quarters of non-Māori (73 % women and 72 % men) met the EAR for protein intake adjusted for body weight. In addition, more women (11 %) than men (3 %) met the AI for water (P<0·001) (online Supplementary Table S6).
Discussion
This is the first study to provide a detailed examination of energy, macronutrient intake and the contribution of food groups in Māori and non-Māori octogenarians.
The median EI for Māori men and women was 7·45 and 6·06 MJ, respectively; this is lower than that estimated in the 2008/09 NZANS( 4 ) (8·95 and 6·59 MJ for men and women aged 51+ years, respectively). In non-Māori, the median EI for men (7·90 MJ) was similar to the NZANS 2008/09 results (men aged 71+ years, 7·93 MJ) but slightly higher in women (6·27 MJ v. women aged 71+ years, 6·01 MJ). The 2008/09 NZANS aggregated data for older non-Māori aged 71+ years and for Māori aged 51+ years due to the small numbers above these ages included in the study. Data on the energy requirements of people over 80 years are scarce( Reference Rothenberg, Bosaeus and Westerterp 36 ). The energy requirements of eighty-seven octogenarians (mean age 82 (sd 3·1) years) participating in the Health, Aging and Body Composition study based on doubly labelled water measures of total energy expenditure were 9·24 (sd 1·57) MJ/d for men and 7·59 (sd 1·41) MJ/d for women( Reference Cooper, Manini and Paton 37 ) and comparable with the EI of participants in the current study. Independent of age, non-Māori men and women had a higher EI than Māori. Considering Māori had a similar or higher BMR and potentially higher physical activity level, this indicates that older Māori may have a negative energy balance – that is, inadequate daily EI to match energy expenditure. This finding agrees with the higher nutrition risk observed among Māori LiLACS NZ participants.
Māori dietary intake differs from non-Māori with a trend for higher fat intake contributing to total EI. This is not surprising as culturally related food patterns, living situation, BMI and BMR all differ. Kai (food) with cultural significance for Māori include fish and seafood( Reference Wham, Maxted and Dyall 38 ), and these were primary contributors to protein intake, especially for Māori men. Older Māori who are able to access important traditional foods on a regular basis have been found to have a better nutritional status than those without access( Reference Wham, Maxted and Dyall 38 ). Similarly, the nutritional quality of food intake has been shown to be improved on days when traditional foods are consumed among the indigenous peoples in Canada( Reference Chan, Receveur and Sharp 39 ). Maintaining access to desired traditional foods may benefit nutritional status for older Māori.
Nutrient intake differs for older people in different living arrangements, with those living alone being at increased nutrition risk in other studies( Reference Keller, Roy and Kane 40 , Reference Visvanathan, Macintosh and Callary 41 ). Māori in LiLACS NZ were more likely to live with their spouse or others such as extended family compared with non-Māori, and living alone was not associated with a difference in macronutrient intake for Māori. Culturally driven food preparation and sharing may systematically differ between ethnic groups facilitated by whānaungatanga, described as relationships through shared experiences and working together, providing a sense of belonging( Reference Locher, Robinson and Roth 42 ). Frequent contact with whānau (family group) may enhance food intake as well as strengthen cultural identity. For older Māori, their roles in the Māori world connected to whānau and upholding tikanga (correct cultural procedures) are positive contributors to health and well-being( Reference Durie 43 ). In LiLACS NZ participants, we have found that more frequent participation in cultural activities is associated with higher health-related quality of life( Reference Dyall, Kepa and Teh 44 ). Food intake may contribute to this association as Māori macronutrient intake differs from non-Māori and may be resilient to the potential influence of living arrangement.
For non-Māori, those who lived alone had a significantly lower fat intake, and those who lived with their spouse took less carbohydrate. Other studies have shown that those who live alone are at increased risk of malnutrition( Reference Keller 45 ), and for the non-Māori cohort our data support this.
Overall median EI and EI:BMRest of the participants in this study were as expected( Reference Gemming, Jiang and Swinburn 29 ). The significance of the protein intakes described here is unknown. In the current study, men had a higher median protein intake than women, and Māori had a marginally higher energy-adjusted protein intake than non-Māori. The median weight-adjusted protein intake for Māori and non-Māori men was 1·05 and 0·98 g/kg per d, respectively and for Māori and non-Māori women 0·87 and 0·91 g/kg per d, respectively. Protein intakes observed here appear to meet the current EAR for people over age 70 years but may be low, especially for women, when compared with newer recommendations (1·0–1·2 g/kg per d to preserve and regain lean body mass and function) made by the The PRevention in Older people-Assessment in Generalists’ practices (PROT-AGe) Study Group( Reference Bauer, Biolo and Cederholm 46 ).
The sources of protein varied between sex and ethnic groups with a balanced intake of animal and vegetable protein. Bread is an important source of protein, particularly for women, as is seafood for Māori. Ensuring access to low-cost, high-protein foods that are desirable and familiar will be important to maintaining protein intake as this cohort ages. Further studies need to examine the variation in protein intake, the nature of that protein (animal or vegetable) and timing (through the day) of protein intake in relation to nutrition-related health outcomes over time.
Bread was the main contributor of energy and carbohydrate for men and women of both ethnicities in keeping with findings from the NZANS for adults over 70 years( 4 ). Participants consumed percent energy from carbohydrate at the lower end of the AMDR of 45–65 % for people over 70 years.
Butter and/or margarine was the largest single contributor of fat for all participants (>15 %). Fat intake, as median percent energy, was above the maximum AMDR range (20–35 %), the lowest being 36 % for non-Māori men, and was greater than that for adults aged 71+ years in the 2008/09 NZANS (33 %), in which butter and margarine were also the main contributors to total fat – 16 and 15 % for men and women, respectively. On the basis of the AMDR, these participants meet and exceed the minimum of 15 % energy from fat proposed to ensure adequate consumption of total energy, essential fatty acids and fat-soluble vitamins( 7 , 47 ).
Dietary fibre intake among all participants was low compared with the AI for men (30 g) and women (25 g) aged 71+ years, especially for Māori men (17·5 g), and were lower than the median dietary fibre intake for those aged 71+ years recorded in the 2008/09 NZANS. Water is considered an essential nutrient and is important in older age because of declining kidney function and use of medications such as diuretics, which predisposes older adults to the consequences of dehydration such as acute infection( Reference Warren, Bacon and Harris 48 ). The median intake of water from food and fluids ranged from 2 to 2·2 litres lower than the AI for men (3·4 l/d) and women (2·8 l/d) over 70 years. However, the AI for water has been set at the highest level of median intake for people aged 71+ years of each sex in the National Nutrition Survey of Australia, 1995( 7 ), and may not reflect the requirements of older adults living in a more temperate climate.
Dietary intakes reported here could be interpreted as less than ideal; however, this group has successfully survived into advanced age. Potentially, these food and fluid intakes may represent good nutrition for those ageing relatively successfully, and the balance of intakes will be examined longitudinally to establish prediction of maintenance of ongoing strength, high function and avoidance of adverse nutrition-related health outcomes in advanced age.
Limitations
Limitations should be considered when interpreting these results. Comparisons between Māori and non-Māori may be limited because Māori participants were younger with a higher BMI than non-Māori. Culturally different food habits were evident. Generalisability of these data may be questioned because of the response rate of <60 % overall and further attrition over the 12 months follow-up. Any dietary assessment must be treated with some caution; however, inaccuracy may be less likely in this study as the 24-h MPR is potentially the best method available for this age group( Reference Adamson, Collerton and Davies 26 ), and the training and attention given to adherence to the protocol attempted to ensure a quality dietary assessment. An accurate assessment of nutrient status, nevertheless, requires a combination of dietary, anthropometric, biochemical and clinical measurements( Reference Gibson 49 ).
We chose not to restrict our analyses to only participants with an EI:BMR between 0·9 and 2·0 as the reporting may be an accurate reflection of actual intake and some participants may be at increased nutrition risk( Reference Wham, Teh and Moyes 50 ). This potentially introduces a bias that is quantified in the sensitivity analysis (online Supplementary Tables S5 and S6).
The 2008/09 NZANS used a 24-h diet recall programme to collect dietary data, and was used as the reference population to compare macronutrient and food group intakes. As the data for people aged over 70 years were aggregated, it may not reflect the food habits of the oldest old. Similarly, with limited data available, the NRV for Australia and New Zealand are based on extrapolation from younger age groups. The EAR for protein for adults aged over 70 years was increased by 25 % over that of younger adults without robust data on which to base that estimate. The AI for dietary fibre was set at the median dietary fibre intake for this age group based on the 1997 National Nutrition Survey of New Zealand, plus an allowance for resistant starch not included in the food database.
Detailed comparisons between studies may be hindered by the differences in dietary assessment methods, forms of data presentation as well as participant characteristics such as geographical location, age, body composition, health and nutritional status, and level of physical activity.
Conclusion
This study provides the first detailed examination of food intake in Māori and non-Māori octogenarians. Clear sex and ethnic group differences have been reported for energy, macronutrients and dietary fibre intakes. The optimal levels of macronutrient intakes remain to be determined for the oldest old. Future guidelines may need to identify the nutritional needs of older people in relation to their functional ability and illnesses( Reference Suominen, Jyvakorpi and Pitkala 51 ). For Māori, actions that facilitate cultural-based food practices may help improve nutrition-related outcomes. Health outcomes related to dietary intake will be examined longitudinally.
Acknowledgements
Betty McPherson advised nutrition assessment for Māori, and Hone and Florence Kameta assisted with the translation of the interview. The authors thank the organisations contracted to conduct the LiLACS NZ study in the communities of origin: Western Bay of Plenty PHO, Ngā Matāpuna Oranga Kaupapa Māori PHO, Rotorua Area Primary Health Services, Te Korowai Aroha Trust and Te Rūnunga o Ngati Pikiao, Te Rūnunga o Ngati Awa Research and Archives Trust, Te Rūnunga o Ngati Iripuaia and Te Whānau a Apanui Community Health Centre. The authors acknowledge the support of the Ministry of Health for manuscript production and the authors thank all participants and their whānau for participation.
Funders were the Health Research Council of New Zealand programme grant (HRC 06/068B, 09/068B; main funding body) and Ngā Pae o te Māramatanga (the New Zealand National Centre for Research Excellence for Māori; funded Māori engagement and project management). A. A. is funded by the National Institute of Health Research, UK, as a Research Professor in translational research. Funders had no role in the design, analysis or writing of this article.
N. K. and A. A. conceived the study and led its design; A. R. and M. M.-L. provided Māori leadership for the study; N. K., R. T., C. W. and K. H. were involved in formulating the research question; A. A. provided specialist training for MPR; K. H. provided project management oversight; C. W., R. T. and K. H. participated in data collection; S. A. M. and R. T. performed statistical analyses; and C. W., R. T., N. K., A. A. and A. R. participated in manuscript preparation.
The authors declare that there are no conflicts of interest.
Supplementary material
For supplementary material/s referred to in this article, please visit http://dx.doi.org/doi:10.1017/S0007114516003020











