The microbial colonisation of the gastrointestinal tract at birth, predominantly by lactic acid bacteria, including Lactobacillus, Bifidobacteria and Streptococcus, is of paramount importance to health and performance through the stimulation of gut function, immunity and maturation( Reference Hooper, Wong and Thelin 1 – Reference Bauer, Williams and Smidt 4 ). After weaning, the gut microbiota becomes more complex and is capable of fermenting indigestible dietary components to monocarboxylates, mainly acetate, propionate, butyrate and lactate. The absorption of acetate, propionate and butyrate by colonic epithelial cells provides a valuable energy source, with butyrate also regulating homeostasis of the colonic epithelium by controlling the expression of genes associated with the proliferation, differentiation and apoptosis of colonic epithelial cells( Reference Bergman 5 – Reference Putaala, Barrangou and Leyer 9 ). Furthermore, the production of lactic acid reduces colonic pH, thereby inhibiting pathogenic organisms( Reference Lähteinen, Malinen and Koort 10 ).
In today's commercial pig industry, piglets are weaned between 21 and 35 d of age( Reference Lalles, Bosi and Smidt 11 ). Although early weaning increases the number of piglets born per annum, the sudden and major change in the diet greatly increases the risk of enteric disease, diarrhoea and malnutrition( Reference Lalles, Bosi and Smidt 11 ). It has been suggested that the high susceptibility of early-weaned piglets to enteric disorders is due to disruption in the establishment of a stable intestinal microbiota, thereby allowing pathogenic bacteria to flourish and cause disease( Reference Hopwood, Hampson, Pluske, Le Dividich and Verstegen 12 – Reference Konstantinov, Awati and Williams 14 ).
Nutritional strategies, designed to prevent enteric disorders and improve the health and growth of piglets, may have the potential to influence the gastrointestinal microbiota. These strategies commonly entail the inclusion of dietary supplements such as dairy products( Reference Vente-Spreeuwenberg, Verdonk and Bakker 15 ), natural sugars( Reference Pierce, Sweeney and Brophy 16 , Reference Pierce, Callan and McCarthy 17 ), artificial sweeteners( Reference Sterk, Schlegel and Mul 18 ), fermentable carbohydrates( Reference Konstantinov, Awati and Smidt 19 , Reference Loh, Eberhard and Brunner 20 ) and even probiotic micro-organisms( Reference Huang, Qiao and Lifa 21 ). However, the precise effects of these dietary supplements on the gastrointestinal microbiota have not been fully characterised. We employed 16S rDNA-based 454 pyrosequencing technology( Reference Meyer, Stenzel and Hofreiter 22 ) to assess the composition of caecal microbiota in piglets. The present brief study focuses on the effect of dietary supplementation of the piglets' feed with either lactose or an artificial sweetener (saccharin/NHDC) specifically on the population abundance of gut Lactobacillus.
Dietary lactose has previously been shown to act as a prebiotic, promoting the growth of beneficial commensal bacteria, such as Bifidobacteria and Lactobacillus, and improving gut health and growth performance in weaning piglets( Reference Vente-Spreeuwenberg, Verdonk and Bakker 15 , Reference Pierce, Sweeney and Brophy 16 ). Dietary supplementation with saccharin/NHDC (SUCRAM®), the only artificial sweetener that has approval for use as a feed additive in the European Union( 23 ), has also been shown to dramatically reduce enteric disease and to enhance growth performance in early-weaned piglets( Reference Sterk, Schlegel and Mul 18 ). However, very few studies( Reference Beards, Tuohy and Gibson 24 , Reference Thabuis, Herbomez and Desailly 25 ) on the prebiotic-like effects of sweeteners on the gastrointestinal microbiota have been published.
In the present study, we observed significant increases in the caecal population abundance of Lactobacillus in response to the inclusion of artificial sweetener consisting of saccharine and NHDC in the piglets' feed. The mechanism(s) underlying the increased abundance of caecal Lactobacillus in response to dietary supplementation with saccharin/NHDC are presently unknown. Processes, such as the influence of host-derived factors upon the microbiota( Reference Schluter and Foster 26 , Reference Benson, Kelly and Legge 27 ) and/or the involvement of a Lactobacillus cell membrane-associated sensor for recognising artificial sweetener, are proposed to explain this observation.
Methods
Animals and collection of samples
Male and female suckling Landrace × Large White piglets aged 28 d were placed in pairs and housed in standard pens (1·5 m2, 12 h light–dark cycle and 26·7°C). A total of three groups, each consisting of eight animals, were weaned to and maintained on the following isoenergetic (16·76–16·82 kJ/g) diets for 2 weeks: group 1, a commercial wheat- and soya-based swine basal diet (Target Feeds Limited) containing 42 % (w/w) hydrolysable carbohydrates (HC); group 2, the same basal diet but containing 5 % (w/w) lactose (in the form of dairy crest whey) (HC+L); group 3, the same basal diet but supplemented with 0.015 % (w/w) SUCRAM® (an artificial sweetener consisting of saccharin and neohesperidin dihydrochalcone) (HC+S). All the animals had free access to food and water at all times and consumed the same amount of feed. They all remained healthy throughout the course of the feeding trial, and had no signs of enteric disturbances. After 2 weeks, the piglets were killed with an intravenous injection of pentobarbitone (200 mg Pentoject/ml; AnimalCare Limited) into the cranial vena cava (according to UK Home Office Schedule 1 regulations). National/institutional guidelines for the care and use of animals were followed, and all experiments were approved by the University of Liverpool Ethics Committee. Immediately post-mortem, caecal and rectal contents were removed, wrapped in aluminium foil and frozen in liquid N2. All samples were subsequently stored at − 80°C until used for microbial DNA extraction or capillary GC analysis.
Extraction of bacterial DNA from caecal content samples
Nucleic acid was extracted from the samples of caecal contents using the method outlined by Lin & Stahl( Reference Lin and Stahl 28 ) and described previously( Reference Daly and Shirazi-Beechey 29 ). Approximately 1 g aliquots of frozen samples were transferred to screw-cap tubes containing SDS, Tris-buffered phenol (pH 8·0) (Sigma-Aldrich Company Limited) and sterile acid-washed glass beads. The samples were immediately homogenised using a mini beadbeater (Biospec Corporation, Stratech Scientific). The aqueous supernatant was then extracted with phenol–chloroform–isoamylalcohol and treated with DNase-free RNase A to remove contaminating RNA. Total DNA (primarily bacterial) was precipitated by the addition of sodium acetate and isopropanol. Purified DNA was resuspended in sterile Tris buffer and stored at − 80°C. DNA integrity was assessed by agarose gel electrophoresis. We observed that rapid freezing of samples in liquid N2, followed by homogenisation in a buffer containing phenol, is an effective method for inactivating nuclease activity. This approach also avoids repeated freeze–thawing of samples that may be deleterious to the efficient isolation of DNA from Gram-negative microbes.
PCR amplification of bacterial 16S rRNA genes (rDNA)
Purified DNA was used as a template for PCR amplification of bacterial 16S rRNA genes using GS FLX+ Titanium fusion primers, targeted to flanking regions of the V1–V3 loop of bacterial 16S rDNA, producing amplicons of approximately 500 bp (sense 5′-CAGGCCTAACACATGCAAGTC-3′; antisense 5′-ATTACCGCGGCTGCTGG-3′). PCR cycling was kept to a maximum of eighteen cycles to avoid chimera production. Amplicons from each group of piglets, labelled by the inclusion of a Multiplex Identifier sequence tag, were pooled in equimolar amounts, and sequenced at the Centre for Genomics Research, University of Liverpool, on a 454 GS FLX+ Titanium sequencing platform (Roche).
Analysis of 454 GS FLX+sequence reads
Post-sequencing, raw reads were de-multiplexed using the Multiplex Identifier sequence tags and corrected for PCR and sequencing artifacts using AmpliconNoise( Reference Quince, Lanzén and Curtis 30 ) and ChimeraSlayer( Reference Haas, Gevers and Earl 31 ). The reads were aligned using PyNAST and Greengenes( Reference Caporaso, Bittinger and Bushman 32 , Reference DeSantis, Hugenholtz and Larsen 33 ) and phylogenies calculated with FASTTREE( Reference Price, Dehal and Arkin 34 ). Operational taxonomic units were defined, using a similarity threshold of 97 %, with UCLUST( Reference Edgar 35 ) and taxonomy was assigned via RDP Classifier 2.2( Reference Wang, Garrity and Tiedje 36 ) using Qiime version 1.5( Reference Caporaso, Kuczynski and Stombaugh 37 ) to implement analysis workflow.
Measurement of caecal lactic acid concentrations
The concentration of lactic acid in the caecal contents of piglets was measured as described previously( Reference Daly, Proudman and Duncan 38 ). Briefly, thawed caecal contents were centrifuged to remove particulates, and lactic acid was extracted from supernatants by the addition of concentrated HCl and diethyl ether. The diethyl ether layer extracts were then derivatised and lactic acid concentrations determined by capillary GC, quantified in relation to an internal standard.
Statistical analysis
Data are presented as means with their standard errors. Significance of differences was determined using one-way ANOVA with Bonferroni's multiple comparison test (GraphPad Prism 5; GraphPad Software, Inc.). Results were considered significant if P< 0·05.
Results
Effect of lactose on the population abundance of swine gut Lactobacillus
Supplementation with the HC+L diet resulted in a significant enhancement of the caecal Lactobacillus population (expressed as a percentage of the total number of sequences) from 8·7 (sem 1·7) to 24·5 (sem 4·1) %, a 2·8-fold increase (P< 0·01; Fig. 1). Furthermore, this increase was observed to be almost entirely due to one particular phylotype, designated Lactobacillus OTU4228, which increased from 7·4 (sem 1·5) % of the total microbiota in piglets weaned to the basal HC diet to 21·4 (sem 4·0) % of the total microbiota in those weaned to the HC+L diet (P< 0·01; Fig. 1).
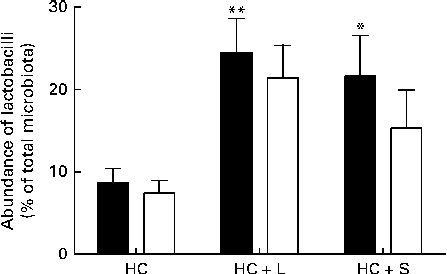
Fig. 1 Population abundance of caecal total lactobacilli (■) and Lactobacillus OTU4228 (□) (expressed as a percentage of the total microbiota) in piglets weaned to a basal hydrolysable carbohydrate (HC) diet and a HC diet supplemented with 5 % (w/w) lactose (HC+L) or 0·015 % (w/w) saccharin/NHDC (HC+S). Values are means, with their standard errors represented by vertical bars. Mean values were significantly different from those of the HC diet: * P< 0·05, ** P< 0·01.
Moreover, the measurements of lactic acid concentrations in the caecal contents of piglets weaned to the HC+L diet showed lactic acid to be present at a concentration of 15·2 (sem 1·8) mm; a 10-fold increase compared with caecal lactic acid concentrations in piglets weaned to the basal HC diet (1·5 (sem 0·2) mm) (P< 0·001; Fig. 2).
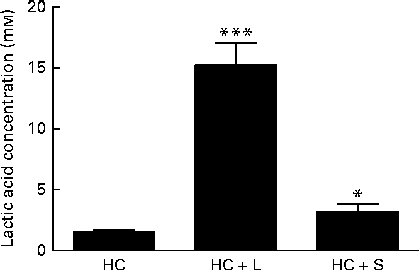
Fig. 2 Concentration of lactic acid in the caecal contents of piglets fed a basal hydrolysable carbohydrate diet (HC) and a HC diet supplemented with 5 % (w/w) lactose (HC+L) or 0·015 % (w/w) saccharin/NHDC (HC+S). Values are means, with their standard errors represented by vertical bars. Mean values were significantly different from those of the HC diet: * P< 0·05, *** P< 0·001.
Effect of saccharin/NHDC on the population abundance of swine gut Lactobacillus
A significant enhancement of the relative population size of caecal Lactobacillus was similarly observed in piglets weaned to the HC+S diet. In these piglets, Lactobacillus accounted for 21·6 (sem 4·9) % of the total microbiota (expressed as a percentage of the total number of sequences), compared with 8·7 (sem 1·7) % in piglets weaned to the basal HC diet. This represents an increase of 2·5-fold (P< 0·05; Fig. 1). Again, this increase was observed to be almost solely due to an increase in the population of Lactobacillus OTU4228, which comprised 15·3 (sem 4·6)% of the total microbiota in piglets weaned to the HC+S diet (P< 0·05; Fig. 1).
Furthermore, caecal lactic acid concentrations in response to the inclusion of saccharin/NHDC were increased by 2·1-fold over concentrations of caecal lactic acid in piglets weaned to the basal HC diet (3·2 (sem 0·6) v. 1·5 (sem 0·2) mm, P< 0·05; Fig. 2).
Discussion
Lactobacilli are the predominant lactic acid bacteria found in the pig intestine and constitute a major proportion of the entire intestinal microbiota. As such, they are of particular importance to the maintenance of gut health. The presence and activity of lactobacilli have a stimulatory effect on both gut immunity and maturation, enhancing immune protection and reducing gastrointestinal inflammatory responses( Reference Kimura, McCartney and McConnell 39 , Reference Blum and Schiffrin 40 ). They also display antimicrobial activities that participate in host epithelial defence, such as reduction of colonic pH (through the production of lactic acid), protection against mucosal pathogen invasion and production of bacteriocins( Reference Putaala, Barrangou and Leyer 9 , Reference Casla, Requena and Gómez 41 , Reference Varcoe, Krejcarek and Busta 42 ).
We used PCR amplification of bacterial 16S rRNA gene sequences and subsequent 454 pyrosequencing to identify changes in swine gut microbiota in response to dietary supplementation. The average number of sequence reads per sample was in excess of 15 000 and using a similarity threshold of 97 % allowed classification of over 1000 phylotypes. Taxonomic assignment of these phylotypes showed that, in these piglets, the caecal microbiota is dominated by three major bacterial classes: Bacteroidia, Bacilli and Clostridia, which together contribute over 90 % of all sequences. Major bacterial groups represented include Porphyromonas and Prevotella (Bacteroidia), Lactobacillus (Bacilli) and Ruminococcus, Lachnospira and Veillonella (Clostridia).
In the present study, which focuses exclusively on changes within the Lactobacillus populations, we report that there were significant enhancements in the relative population abundance of lactobacilli in the caecal contents of piglets in response to dietary supplementation with either a natural sugar, lactose or an artificial sweetener (saccharin/NHDC). The addition of lactose (5 %, w/w) to the basal diet resulted in caecal Lactobacillus populations increasing from approximately 9 % to over 24 % of the total microbiota. Notably, supplementation of the basal diet with saccharin/NHDC (0·015 %, w/w; a 330-fold lower concentration than lactose) also increased caecal Lactobacillus populations to almost the same level (approximately 22 % of the total microbiota) (Fig. 1). In parallel, caecal lactic acid concentrations (1·5 mm in piglets weaned to the basal HC diet) increased 10-fold to over 15 mm in piglets weaned to the same diet containing lactose and 2-fold to over 3 mm in piglets weaned to the diet containing saccharin/NHDC (Fig. 2).
Interestingly, the increase in Lactobacillus abundance observed in the caecal contents of piglets weaned to diets containing either lactose or artificial sweetener is not a general enhancement in all Lactobacillus populations present in the pig caecum. In fact, one particular phylotype, designated Lactobacillus OTU4228, is almost solely responsible for the observed increase. Lactobacillus OTU4228 constitutes approximately 7 % of the total microbiota in piglets weaned to the basal HC diet (over 85 % of the total Lactobacillus community). This increases to over 21 and 15 % of the total microbiota in piglets weaned to the HC+L and HC+S diets, respectively (Fig. 1). Although the response of Lactobacillus OTU4228, in terms of increased population abundance, is similar in piglets weaned to diets supplemented with either lactose or saccharin/NHDC, the disparity between caecal lactic acid concentrations suggests that the underlying mechanisms are quite different.
It has been shown that there is a rapid and significant decrease in pig intestinal lactase activity with both age and weaning( Reference Kelly, Smyth and McCracken 43 – Reference Marion, Petersen and Romé 45 ), indicating that a substantial amount of ingested lactose may not be digested by the host( Reference Kim, Benevenga and Grummer 46 ). Lactose is then available as a highly metabolisable substrate readily utilised by bacteria (particularly lactobacilli), initially in the distal regions of the small intestine as well as in the caecum( Reference Kim, Benevenga and Grummer 46 ). Increases in the population abundance of Lactobacillus have previously been demonstrated in piglets fed diets supplemented with lactose( Reference Vente-Spreeuwenberg, Verdonk and Bakker 15 , Reference Pierce, Sweeney and Brophy 16 ), primarily due to the metabolism of lactose by lactobacilli.
The highly fermentable nature of lactose is reflected in the large increase in lactic acid concentrations seen here in the caecal contents of piglets weaned to the diet containing lactose (population abundance of lactobacilli increases 2·8-fold; lactic acid increases 10-fold). In contrast, the increase in lactic acid concentrations measured in the caecal contents of piglets weaned to the same diet containing saccharin/NHDC is in proportion to the increase in the population abundance of Lactobacillus (2·1- and 2·5-fold, respectively). This suggests that, unlike lactose which provides an additional substrate for the growth of lactobacilli and subsequent lactic acid production, this artificial sweetener is not a metabolisable energy source that can be fermented by Lactobacillus populations to produce lactic acid.
The effects of artificial sweeteners on gut microbiota have previously been studied in human subjects. It has been shown that the addition of maltitol, a sugar alcohol, to confectionery significantly enhanced the population abundance of both Bifidobacteria and Lactobacillus ( Reference Thabuis, Herbomez and Desailly 25 ). However, it is notable that maltitol is a fermentable substrate for these gut microbes( Reference Thabuis, Herbomez and Desailly 25 , Reference Oku, Akiba and Lee 47 ).
In the mammalian intestine, the sweet taste receptor, T1R2–T1R3, expressed in enteroendocrine cells, can detect the presence of sugars and artificial sweeteners. This initiates an intracellular signalling pathway leading to the up-regulation of the intestinal glucose transporter, Na+/glucose co-transporter 1 (SGLT1), and an increased capacity of the gut to absorb glucose( Reference Dyer, Daly and Salmon 48 – Reference Shirazi-Beechey, Moran and Batchelor 50 ). Likewise, yeasts, such as Saccharomyces cerevisiae, possess mutated glucose transporters (Snf3 and Rgt2) that act as transmembrane sweet sensors controlling the expression of hexose transporter proteins in the presence of glucose and other sugars( Reference Ozcan and Johnston 51 , Reference Forsberg and Ljungdahl 52 ).
Lactobacilli, and many other enteric bacteria, express multiple sugar transport and metabolic systems that allow them to utilise a variety of carbohydrate substrates and adapt quickly to changes in nutrient availability( Reference Barrangou, Azcarate-Peril and Duong 53 ). This versatility is of particular importance in an environment such as the gastrointestinal tract. The predominant sugar transport mechanism in these bacteria is the phosphoenolpyruvate: carbohydrate phosphotransferase system (PTS); with over twenty different PTS systems being identified, each is specific for only one or a few sugars( Reference Kotrba, Inui and Yukawa 54 ). There are also multiple non-PTS sugar transport systems such as non-PTS permeases and ABC transporters for various poly- and oligosaccharides( Reference Kotrba, Inui and Yukawa 54 ). The vast majority of these systems are regulated in the presence of a specific substrate( Reference Barrangou, Azcarate-Peril and Duong 53 , Reference Kotrba, Inui and Yukawa 54 ).
Extracellular sensing is a key method employed by bacteria in order to respond to changes in their environment such as alterations in pH, chemical composition or nutrient availability. Many of these sensory responses are independent of transport or metabolism, but involve the binding of chemical ligands to membrane-spanning sensory receptors in order to initiate intracellular signalling pathways( Reference Armitage and Sockett 55 , Reference Xu, Chiang and Bjursell 56 ). Notably, recent evidence has shown that transcription of genes responsible for utilisation of diverse polysaccharides by enteric Bacteroides species can be directly activated by the recognition of signature oligosaccharide ligands by specific receptors( Reference Sonnenburg, Sonnenburg and Manchester 57 , Reference Martens, Lowe and Chiang 58 ), demonstrating that these systems play a key role in bacterial ability to sense and utilise polysaccharides in gut ecosystems. In the light of results presented here, we propose that lactobacilli may possess a plasma membrane-associated sweet sensor capable of sensing artificial sweeteners and initiating pathways controlling carbohydrate transport and metabolism.
However, the influence of the host on the composition and activity of the gut microbiota is becoming increasingly evident and should not be underestimated. Epithelial factors, such as the secretion of growth-promoting mucosal glycans or toxic inhibitory compounds, have been highly implicated in regulating the composition of the intestinal microbiota( Reference Schluter and Foster 26 ). Furthermore, differences in microbial community structure between different host species have been proposed to arise from distinct selective pressures imposed from within the gut habitat of the respective host( Reference Rawls, Mahowald and Ley 59 ). Moreover, host genetic factors have also been shown to contribute in part to gut microbiota composition( Reference Benson, Kelly and Legge 27 , Reference Spor, Koren and Ley 60 ).
Whatever the underlying mechanism(s), the data presented here show that dietary supplementation with saccharin/NHDC artificial sweetener can alter the gastrointestinal microbiota by positively influencing the population abundance of lactobacilli, commensal bacteria that are able to exert a beneficial effect on gut health, immunity and maturation( Reference Kimura, McCartney and McConnell 39 , Reference Blum and Schiffrin 40 ).
The identification and characterisation of the underlying mechanism(s) will assist in the design of nutritional strategies aimed at manipulating the pig commensal microbiota, promoting the health of the gut particularly during the critical post-weaning period.
Acknowledgements
The authors gratefully acknowledge the financial support provided by Pancosma SA.
The authors' contributions are as follows: K. D. and S. P. S.-B. designed the research, analysed the data and wrote the paper; K. D. performed the research; A. D. and N. H. performed the bioinformatic analyses of pyrosequencing data; A. N. and D. B. provided scientific and nutritional advice.
K. D., A. D., N. H. and S. P. S.-B. declare no conflicts of interest. A. N. and D. B. are employees of Pancosma SA.




