Introduction
The standard of practice in post-mortem examination of the brain has been refined over time in the hands of experts dedicated to understanding disease pathogenesis. Generations of neuropathologists have received specialised training in this setting – that is, examining the nervous system, the body’s most complex and least well-understood component, as precisely as possible. With rare exceptions, neuropathologists practice in academic centres where diagnostic accuracy, discovery and education are not only mutually valued but interdependent.
A thorough examination requires removal and inspection of the nervous system at the time of autopsy, in the “fresh” state, followed by a more extensive examination after complete fixation. Reference Simpson and Berson1–Reference Katelaris, Kencian, Duflou and Hilton3 Slicing of unfixed brain tissue can introduce macroscopic and microscopic artefacts. Furthermore, the need to return to the fixed specimen for additional sampling to confirm, clarify and extend the examination on the basis of unexpected findings or additional information that arrives after the post-mortem examination is a common occurrence in a neuropathologist’s practice. New neuroanatomic findings in the brains of dementia subjects, for example, often mandate that a previously unsuspected region of interest must be examined in archival specimens. Without the availability of the whole fixed brain (and at times the spinal cord), the neuropathologist and the completeness of the examination are compromised before the examination has begun. Beyond the importance of diagnostic accuracy and thoroughness of examination lie other critical dimensions to training programmes and the practice of neuropathology and medicine in general: biosafety and academic deliverables.
To add further expertise, objectivity and perspective, neuropathologists in Canada were surveyed for their opinion on how the fresh and fully fixed states compare with respect to a number of factors including diagnostics, biosafety, teaching and research.
Methods
A brief, voluntary, anonymised survey was conducted in 2012 to derive opinion on the relative utility of various aspects of examination of the nervous system, comparing the fresh and fixed states. The survey was distributed by email to 46 Canadian neuropathologists with one reminder email approximately 2 weeks later. Participation in the survey explicitly indicated consent. Formal REB review was waived. The factors examined were those that directly impact patient care, diagnostic accuracy, education, research and/or safety:
-
Speed and efficiency;
-
Technical ease;
-
Symmetry and anatomical preservation;
-
Reliability and Consistency;
-
Sensitivity, thoroughness and resolution;
-
Teaching (individual and group);
-
Scheduling and convenience;
-
Biosafety/infection control.
Each factor offered a perspective by which to compare brain cutting in the fresh state versus the fully fixed state on a scale of 1 (inferior) to 7 (superior) with a score of 4 representing no difference (equivocal).
Means and standard deviations of factor ratings were then compared to an equivocal score Reference Wiebe and Mackay4 to determine whether the fresh state examination was inferior, no different from or superior to the fixed examination. Following a repeated measures analysis of variance of the entire data set, each factor was analysed by t-tests (SPSS), with significance set at p < 0.01.
Results
A total of 14 surveys were returned (out of 46), representing a 30% response rate, assuming that all email invitations and responses were received.
Brain cutting in the fresh state was rated as “inferior” (significantly different from equivocal) for all factors (p < 0.01) except for “speed/efficiency”, where no significant difference was found (Figure 1). In brief, factor means and standard deviations were as follows: speed/efficiency (4.2 ± 2.2), technical ease (2.1 ± 0.9), symmetry/anatomy (1.9 ± 0.9), reliability/consistency (1.8 ± 0.8), sensitivity/thoroughness/resolution (2.0 ± 1.1), teaching/education (1.8 ± 1.1), scheduling/convenience (2.5 ± 1.3), biosafety/infection control (1.7 ± 1.1). These findings are summarised in Figure 1.

Figure 1: This bar graph plots the relative merit of brain cutting in the fresh state versus the fixed state. The factors assessed include speed and efficiency (“speed”), technical ease (“technical”), symmetry and anatomical preservation (“anatomy”), reliability and consistency (“reliability”), sensitivity, thoroughness and resolution (“sensitivity”), teaching, scheduling and convenience (“scheduling”) and biosafety/infection control (“biosafety”). The relative merits of brain cutting in the fresh state are expressed as mean scores (±SD) on a scale ranging from 1 (inferior) to 7 (superior) with a score of 4 representing no difference (equivocal). Examination of the brain in the fresh state was judged to be inferior for all criteria with the exception of speed.
Discussion
Neuropathologists who responded to the survey indicated clear diagnostic, teaching, research and biosafety advantages for examination of the nervous system in the fixed state. The only factor where the fresh and fixed states were judged to be equivocal was that of speed. Cutting and examination of the brain in the fresh state is inferior in several dimensions and irreparably so.
Several limitations to the study exist. First, our survey response rate was modest, but within the reported range for Canadian physician surveys. Reference Wiebe and Mackay4 Non-response bias is reported to be lower in a relatively homogeneous professional population (such as a group of specialists) in comparison to the general public. Reference Kellerman and Herold5 However, we cannot be certain that non-responders were simply indifferent to the question and might have opined “no difference”. Although the data presented are those from a survey of expert opinions that collectively represents hundreds of years of hands-on practical experience examining the nervous system in the fresh and fixed states, a prospective double-blinded study would be superior.
Several jurisdictions in recent years have enacted policies to discourage the temporary retention of whole brains in favour of minimal tissue retention. Whole-brain retention in Ontario, for example, has become relatively uncommon, in both forensic and non-forensic examinations. It remains possible but encumbered by the requirement to seek administrative permission via multiple phone calls and additional paperwork, which present a collective disincentive. In this manner, despite decades of a mutually beneficial coexistence, sociopolitical priorities were suddenly at odds with the basic principles of diagnostic accuracy, research, teaching, biosafety and other academic deliverables. The choice to be intentionally less thorough runs contrary to fundamental professional principles in neuropathology, medicine and science. Similarly, imprecision is a strange bedfellow for justice.
Recent inquiries in Canada Reference Goudge6 and the UK Reference Hunter7–Reference Kennedy9 cast an important, revealing and critical light on the aberrant practices of a few pathologists. Although their practices of indefinite tissue retention and inadequate consent were the exception, these revelations were highly unsettling in medical, social and political circles. Some jurisdictions curtailed the temporary retention of whole organs, forcing minimal sampling in place of a thorough examination. While this may have little impact in the examination of some tissues, it is problematic in the context of the nervous system. Reference Squier and Cowan10 This is compounded by the fact that subtle changes and those not visible to the naked eye in the fresh or incompletely-fixed state can have far-reaching consequences in diagnostic, teaching and research domains. For example, in the early days of the AIDS epidemic, brains were often examined grossly and not even sampled for histology if they looked normal. By the mid-1980s, one realised this was extremely shortsighted and wrong, and delayed important discoveries about HIV and the brain. Reference Anders, Guerra, Tomiyasu, Verity and Vinters11 Maria Thom and colleagues demonstrated a significant loss of diagnostic proficiency in the detection of epileptogenic lesions in the setting of sudden unexpected death in epilepsy when comparing fresh brain examinations by forensic pathologists to fixed brain examinations by neuropathologists. Reference Thom, Michalak and Wright12 Are we doomed to repeat?
Minimal or random sampling in the hopes of accounting for a complex disease process, a novel or unexpected finding, or explaining a patient’s demise is misguided and potentially negligent. To be thorough, the nervous system examination requires optimal visual resolution in its gross and microscopic forms. Abnormalities that are near the limit of visual acuity, even in the hands of an expert, can be easily missed. Reference Simpson and Berson1–Reference Katelaris, Kencian, Duflou and Hilton3 The only defense is to sample more extensively than might be necessary under optimal conditions, inviting additional costs of time and resources for technical and professional staff.
In the absence of a complete examination (or the opportunity to revisit the specimen), the potential for misleading clinical-pathological correlations, misdiagnosis, missed diagnosis, erroneous conclusions and even miscarriage of justice increases. Once fresh tissue has been “returned to the body”, all missed or incomplete findings in that tissue are irretrievable. Again, the clinical analogy would be absurd – a single, rushed opportunity to examine an ill patient. This is in contrast to a simple, efficient and traditional alternative: having the fixed brain available for sampling for a short period of time until the examination is complete, at which time it is cremated and/or returned to the family or estate for respectful burial as per the family’s expressed wishes. Fortunately, in Canada, the majority of jurisdictions support routine retention of the brain for complete examination at the discretion of the consultant neuropathologist.
Examining the nervous system at the time of autopsy (fresh) is important for a limited set of reasons and is a common practice in many Medical Examiner’s and Coroner’s offices. There are occasional personal, cultural and religious circumstances that may preclude tissue retention for fixation and require sampling in the fresh state. In this setting, fresh sampling under informed consent becomes a rationalised compromise. The fresh state affords the opportunity to examine the relationship of the nervous system to other structures and findings and to carry out additional special procedures that are best performed at this time (e.g. dissecting the Circle of Willis). Similarly, sampling of tissues in the fresh state may be necessary for some molecular, microbiological and toxicological examinations. Examination in the fresh state is an important component of the post-mortem examination. What it is not is definitive or complete.
For those unfamiliar with examining the nervous system post-mortem, it may be helpful to provide a brief comparison between the fresh and fixed states (Figure 2). In the fresh state, the brain is very soft, pliable and easily fragmented, particularly if there has been any significant post-mortem delay. The infant’s brain is even more susceptible to distortion and loss of visual detail. Prior to cutting, the brain’s own weight is more than adequate in many cases to disrupt anatomical landmarks and distort subtle findings beyond recognition. While cutting, despite the sharpest blades, further artefactual disruption is unavoidable; it is also not possible to cut the brain as thin or precisely in the fresh state, thereby decreasing the resolution of the examination. In the fresh state, individual brain slices also have irregular, fragile and glistening surfaces that impair the ability to detect subtle abnormalities (adding insult to injury, fixing such slices introduces additional deformations). Perinatal brains often feature marked congestion of the cerebral hemispheric white matter, a phenomenon known as “ribbon effect”, wherein the cerebral hemispheric white matter appears relatively darker than the neocortical ribbon. In the fresh state, “ribbon effect” manifests as a pinkish discoloration in the white matter that could potentially obscure true pathology (Figure 3). Preparation of blocks (samples for microscopic examination) is also hampered by the highly deformable and adhesive nature of the tissue in the unfixed state. At each of these stages, visual resolution and technical limitations impose compounding constraints on diagnostic accuracy. In addition to the potential problems evaluating the brain macroscopically, slicing the brain fresh invariably introduces microscopic artefacts (Figure 4). Reference Cammermeyer13 Dark neuron change is the best-described artefact; this can be especially problematic when trying to evaluate early hypoxic-ischemic neuronal changes. Reference Rahaman and Del Bigio14 Ultimately, to specialists trained in the examination of fixed tissue, limiting the examination to the fresh state is no different than placing limitations on the accuracy of any specialist’s examination: a radiologist intentionally reducing the resolution of their images, a cardiologist auscultating the heart or a surgeon examining the abdomen through the patient’s clothing. The examinations remain possible and may still detect major abnormalities, but the identification and management of subtle findings are placed unnecessarily at risk.
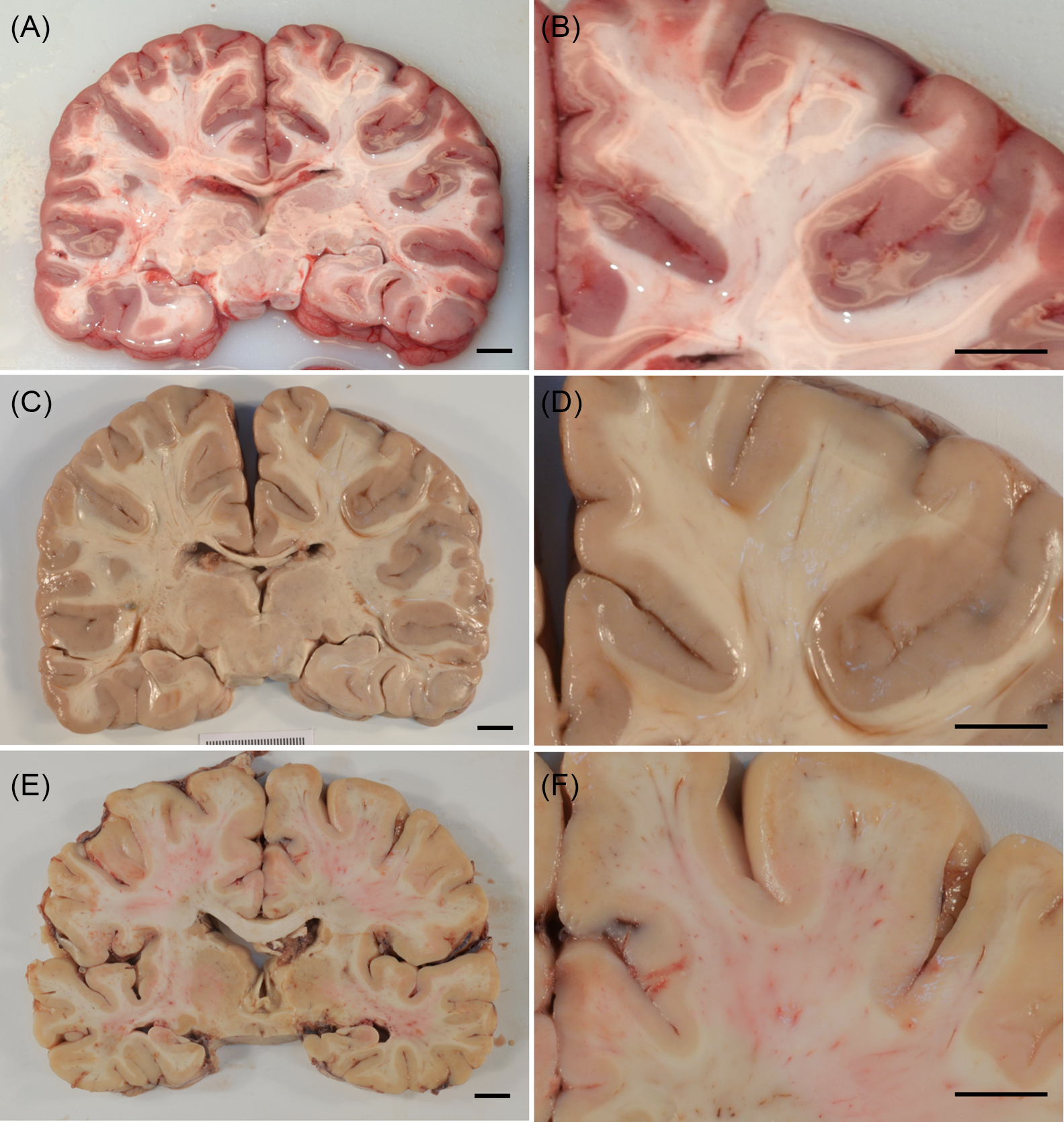
Figure 2: Comparing the effect of fixation in coronal brain slices. (A and B) Fresh brain cut, demonstrating a soft, irregular and glistening cut surface. (C and D) Fresh brain cut subsequently fixed, demonstrating further artefactual puckering and other surface irregularities that can imitate or obscure true pathology (same specimen as depicted in A and B). (E and F) Fixed brain cut, demonstrating optimal tissue integrity and superior visual resolution.

Figure 3: “Ribbon effect” in the fresh and fixed perinatal brain. (A) Coronal section from a 37-week gestational-aged brain that has undergone only 1 day of fixation (i.e. “fresh”). There is a pinkish discoloration present that is most notable in the cerebral hemispheric white matter due to its differential congestion (i.e. “ribbon effect”). This discoloration could obscure true pathology in the cerebral hemispheric white matter. (B and C) Coronal sections from a 30-week gestational-aged brain that has undergone 2 weeks of fixation (i.e. “fixed”). This brain also demonstrates evidence of “ribbon effect”, but the pinkish discoloration of the white matter is absent due to prolonged fixation; as such, a focus of subacute periventricular leukomalacia (PVL; see arrow in Figure 2C) is clearly visible.

Figure 4: Comparison of putamen from fresh cut (A) and post-fixation cut (B) samples of the brain of an adult. The eosinophilic properties are the same, but neuronal cytologic features differ. In brains cut fresh, nuclei of the medium-sized neurons and glial cells are often dark and pyknotic and the cell bodies of large neurons are shrunken (arrow) with enlarged pericellular spaces (hematoxylin and eosin stain, bar = 50 um).
Some, forced by legislation, have experimented with a partial fixation for 1–2 days with fixative penetration of approximately 1 cm per day, Reference Scott and MacDonald15 resulting in a “compromise” in the authors’ words (Figure 5). However, for typical specimens, more than 10 cm in thickness, producing a thin “rind” of semi-fixed tissue around a large core of unfixed tissue is of little advantage in comparison to the tissue integrity and resolution gained with full fixation. Indeed, full fixation of a brain may take several weeks, but typically 2 weeks are adequate if the quantity of formalin is sufficient. Reference Yong-Hing, Obenaus, Stryker, Tong and Sarty16–Reference Birkl, Langkammer and Golob-Schwarzl18

Figure 5: Overnight fixation of the adult brain produces a thin “rind” or “ribbon” of fixed tissue around an unfixed core.
In contrast, the fully fixed brain presents the examiner with optimal tissue integrity and definition. Cutting the fixed brain produces surfaces with superior visual resolution. Similarly, sampling of fine anatomical structures and systems is technically easier and more accurate. It is the state of the nervous system that is most familiar to the neuropathologist, taking advantage of their training and experience and fostering greater diagnostic accuracy. In addition to diagnostic limitations, there are equal concerns with respect to safety (greater biohazard exposure to autopsy room personnel, students, trainees and pathologists when cutting an unfixed brain), health care education and research. Individually and collectively, this is a steep price for society, medicine and science to pay.
Why then might some jurisdictions choose to impose or favour a suboptimal approach to the examination of the nervous system? This is partly the fault of Pathologists themselves. Inquiries in Canada and abroad uncovered examples of tissue storage and specimen tracking that can only be described as unprofessional. Reference Goudge6,Reference Hunter7,Reference Kennedy9,Reference Batty19–Reference Smith23 While the vast majority of pathologists and pathology departments have consistently followed and enforced professional standards for decades the carelessness and incompetence of a few have led to policies that are corrective for a vanishingly small minority and punitive for everyone else.
In our experience, it is uncommon for next of kin (when their opinion is honoured by asking) to not consent to a complete examination (retention of the brain for full fixation, cutting and sampling), expressing consternation at the prospect of anything less for their loved one. Regardless, in cases where personal, cultural or religious practices are at odds with a complete examination, each perspective deserves and receives respectful consideration. However, it must be stressed that part of a respectful and fully informed conversation includes disclosure of the significant limitations of examining the nervous system that is not fully fixed.
Not surprisingly, there is relatively little in the literature on this topic. The advantages of fixation to the examination of the nervous system are elementary to practicing neuropathologists. In addition to the present survey, a few authors have specifically commented on the importance of examining the brain after fixation before the examination could be considered reliable, thorough or complete. Reference Simpson and Berson1–Reference Katelaris, Kencian, Duflou and Hilton3 As noted by Kalimo et al, “Neuropathologists favour fixation of the brain in toto, which allows better handling and more exact sampling and localization of lesions. If the findings in the brain are pivotal for the legal assessment, for example, in trauma cases, this is the optimal way to process the brain for obtaining reliable results”. Reference Kalimo, Saukko and Graham2 Katelaris adds that “the major advantages associated with this method of examination include the ability to perform a more precise and useful topographic study of the brain, and detection of small but important lesions. Appropriate blocks for histological examination can be more readily selected, without which the definitive diagnosis of such conditions as multiple sclerosis, Huntington’s chorea, the differential diagnosis of the later onset dementias, diffuse axonal injury, etc, may not be made”. Reference Katelaris, Kencian, Duflou and Hilton3 Without fixation, gross (and microscopic) abnormalities are more likely to be missed in virtually all categories of disease whether the examination has been undertaken in a clinical and/or forensic context. Reference Simpson and Berson1–Reference Katelaris, Kencian, Duflou and Hilton3 In the setting of a post-mortem examination where the nervous system is relevant, Simpson and Berson offered a simple axiom: “The greater the importance of the case, the greater is the need to fix the brain” (Simpson and Berson, SAMJ, 1987, quote on page 14). They do not suggest that all cases require complete examination of the nervous system by a neuropathologist. However, when a consultation is deemed worthwhile, so is the optimal format for that consultant’s examination.
Acknowledgements
The authors would like to thank their colleagues in the Canadian Association of Neuropathologists (CANP) for their wisdom, expertise and willingness to participate in the survey.
Conflicts of Interest
MDB has received payment for expert testimony (Province of British Columbia). The remaining authors have no conflicts of interest to declare.
Statement of Authorship
RH, CD, DM: concept, initial drafts and edits
MDB, MP-R, HVV: critical review and edits







