Introduction
Epistasis is an integral feature of the genetic architecture of quantitative traits (Anholt & Mackay, Reference Anholt and Mackay2004; Flint & Mackay, Reference Flint and Mackay2009; Mackay et al., Reference Mackay, Stone and Ayroles2009). Epistasis occurs when the effect of variation at one locus is suppressed or enhanced by the genotype at another locus. Epistatic interactions can bias estimates of the effects of quantitative trait loci (QTLs) in mapping populations when present but not accounted for (Carlborg et al., Reference Carlborg, Jacobsson, Ahgren, Siegel and Andersson2006); enable inferences of genetic networks affecting complex traits (Phillips, Reference Phillips2008); and affect predictions of long-term response to artificial and natural selection (Carlborg et al., Reference Carlborg, Jacobsson, Ahgren, Siegel and Andersson2006; Phillips, Reference Phillips2008). Epistasis is difficult to detect in classical quantitative genetic analyses based on resemblance between relatives in outbred populations (Falconer & Mackay, Reference Falconer and Mackay1996), and epistatic interactions contribute largely additive genetic variation in outbred populations when the contributing alleles are rare (Hill et al., Reference Hill, Goddard and Visscher2008). However, epistatic interactions are common in experiments designed to examine their effects on trait means in QTL mapping populations. For example, in Drosophila epistatic interactions have been reported between QTLs affecting bristle number (Long et al., Reference Long, Mullaney, Reid, Fry, Langley and Mackay1995; Gurganus et al., Reference Gurganus, Nuzhdin, Leips and Mackay1999; Dilda & Mackay, Reference Dilda and Mackay2002), wing morphology (Weber et al., Reference Weber, Eisman, Morey, Patty, Sparks, Tausek and Zeng1999), lifespan (Leips & Mackay, Reference Leips and Mackay2000, Reference Leips and Mackay2002) and startle-induced locomotor behaviour (Jordan et al., Reference Jordan, Morgan and Mackay2006). In mice, epistasis has been reported between QTLs affecting growth, body weight and morphometry (Brockmann et al., Reference Brockmann, Kratzsch, Haley, Renne, Schwerin and Karle2000; Cheverud et al., Reference Cheverud, Vaughn, Pletscher, Peripato, Adams, Erikson and King-Ellison2001; Workman et al., Reference Workman, Leamy, Routman and Cheverud2002; Klingenberg et al., Reference Klingenberg, Leamy and Cheverud2004; Yi et al., Reference Yi, Zinniel, Kim, Eisen, Bartolucci, Allison and Pomp2006). Epistasis is also a prominent feature of the genetic architecture of growth rate in Arabidopsis (Kroymann & Mitchell-Olds, Reference Kroymann and Mitchell-Olds2005), chickens (Carlborg et al., Reference Carlborg, Jacobsson, Ahgren, Siegel and Andersson2006) and yeast (Steinmetz et al., Reference Steinmetz, Sinha, Richards, Spiegelman, Oefner, McCusker and Davis2002; Sinha et al., Reference Sinha, David, Pascon, Clauder-Munster, Krishnakumar, Nguyen, Shi, Dean, Davis, Oefner, McCusker and Steinmetz2008).
Although epistatic interactions have been detected in QTL mapping experiments, it is easier to study epistasis in crosses among lines with reduced genetic heterogeneity in largely homozygous genetic backgrounds (Eshed & Zamir, Reference Eshed and Zamir1996; Clark & Wang, Reference Clark and Wang1997; Sambandan et al., Reference Sambandan, Yamamoto, Fanara, Mackay and Anholt2006). Drosophila melanogaster is an excellent model system to study epistasis affecting quantitative traits due to the ease of constructing chromosome substitution and introgression lines, and generating mutations in a common homozygous genotype. Epistasis has been documented for aggressive behaviour by constructing chromosome substitution lines in which small segments of one genotype were introgressed into a different genetic background (Edwards & Mackay, Reference Edwards and Mackay2009). Epistasis for aggression was also evident from behavioural and whole genome transcriptional analyses of an ensemble of co-isogenic hyper-aggressive P-element insertion lines (Zwarts et al., Reference Zwarts, Magwire, Carbone, Versteven, Herteleer, Anholt, Callaerts and Mackay2011). Epistasis for metabolic activity was revealed by constructing all possible two-locus genotypes for several pairs of P-element insertion mutations (Clark & Wang, Reference Clark and Wang1997). Diallel cross analysis of co-isogenic P-element insertion lines enabled identification of epistatic networks of genes affecting negative geotaxis (Van Swinderen & Greenspan, Reference van Swinderen and Greenspan2005), olfactory avoidance behaviour (Fedorowicz et al., Reference Fedorowicz, Fry, Anholt and Mackay1998; Sambandan et al., Reference Sambandan, Yamamoto, Fanara, Mackay and Anholt2006), aggression (Zwarts et al., Reference Zwarts, Magwire, Carbone, Versteven, Herteleer, Anholt, Callaerts and Mackay2011) and startle behaviour in Drosophila (Yamamoto et al., Reference Yamamoto, Anholt and Mackay2009).
Previously, Yamamoto et al. (Reference Yamamoto, Anholt and Mackay2009) created pairs of chromosome substitution lines in which isogenic Canton-S B (CSB) chromosomes with P-element insertions in genes affecting startle behaviour and their P-element free co-isogenic control chromosomes were substituted into different homozygous wild-derived D. melanogaster genotypes. This design enables the quantification of the extent to which naturally segregating variants modify the effects of single mutations, as well as the magnitude of variation of epistasis among the different lines. This study reported widespread suppressing epistasis of naturally segregating modifiers on startle behaviour. Since the magnitude of suppressing epistasis was proportional to the magnitude of the mutational effect of the P-element insertion on startle behaviour, it was concluded that suppressing epistasis buffers the effects of new mutations in natural populations.
P-element insertions at genes previously implicated in startle behaviour, Semaphorin-5C (Sema-5c) and Calreticulin (Crc) also affect olfactory behaviour (Sambandan et al., Reference Sambandan, Yamamoto, Fanara, Mackay and Anholt2006) and in the case of Crc, sleep phenotypes (Harbison & Sehgal, Reference Harbison and Sehgal2008). The objective of the present study was to ask whether suppressing epistasis by naturally segregating modifiers on behavioural traits is a general principle or unique to the startle response, and, moreover, to assess whether the effects of the same P-element insertion on different phenotypes is modulated by the same or different epistatic modifiers.
Materials and methods
Drosophila stocks
P-element insertion lines for Crc and Sema-5c, which were generated as part of the Berkeley Drosophila Gene Disruption Project (Bellen et al., Reference Bellen, Levis, Liao, He, Carlson, Tsang, Evans-Holm, Hiesinger, Schulze, Rubin, Hoskins and Spradling2004), contain single P[GT1] insertions generated in the isogenic w1118 , CSB background. Crc and Sema-5c have pleiotropic effects on olfactory avoidance of benzaldehyde (Sambandan et al., Reference Sambandan, Yamamoto, Fanara, Mackay and Anholt2006; Rollmann et al., Reference Rollmann, Yamamoto, Goossens, Zwarts, Callaerts-Vegh, Callaerts, Norga, Mackay and Anholt2007), bristle number (Norga et al., Reference Norga, Gurganus, Dilda, Yamamoto, Lyman, Patel, Rubin, Hoskins, Mackay and Bellen2003) and startle response (Yamamoto et al., Reference Yamamoto, Zwarts, Callaerts, Norga, Mackay and Anholt2008, Reference Yamamoto, Anholt and Mackay2009), and Crc also has pleiotropic effects on sleep traits (Harbison & Sehgal, Reference Ho and Sehgal2008). Both Crc and Sema-5c are located on chromosome 3 (C3). The construction of chromosome substitution lines carrying either a CSB C3 or the Crc and Sema-5c P[GT1] mutations on the same CSB C3 in inbred lines of the Drosophila melanogaster Genetic Reference Panel (DGRP; Mackay et al., Reference Mackay, Richards, Stone, Barbadilla, Ayroles, Zhu, Casillas, Magwire, Cridland, Richardson, Anholt, Barrón, Bess, Blankenburg, Carbone, Castellano, Chaboub, Duncan, Han, Harris, Javaid, Jayaseelan, Jhangiani, Jordan, Lara, Lawrence, Lee, Librado, Linheiro, Lyman, Mackey, Munidasa, Muzny, Nazareth, Newsham, Perales, Pu, Qu, Ràmia, Reid, Rollmann, Rozas, Turlapati, Worley, Wu, Yamamoto, Zhu, Bergman, Thornton, Mittleman and Gibbs2012), derived from a Raleigh (North Carolina) population of wild D. melanogaster, has been reported previously (Yamamoto et al., Reference Yamamoto, Anholt and Mackay2009). Thirteen chromosome substitution lines with P-element insertions at Sema-5c and 14 chromosome substitution lines with P-element insertions at Crc and the corresponding controls were used in this study (Fig. 1). All flies were reared in large mass cultures on cornmeal/molasses/agar medium at 25°C and a 12 h light/12 h dark cycle (lights on at 6:00 am; lights off at 6:00 pm).

Fig. 1. Generation of co-isogenic CSB C3 substitution lines in inbred DGRP genetic backgrounds. The left side of the diagram illustrates the three major D. melanogaster chromosomes in co-isogenic CSB lines, with arrows indicating the locations of P-element insertions in Sema-5c and Crc. The right side of the diagram illustrates the introduction of CSB C3 with or without P-element insertions into different DGRP lines, indicated with different colours (Yamamoto et al., Reference Yamamoto, Anholt and Mackay2009).
Behavioural assays
We measured olfactory behaviour for C3 substitution lines with Crc and Sema-5c mutations, and the corresponding CSB C3 substitution lines contemporaneously using a modification of the ‘dipstick’ assay (Anholt et al., Reference Anholt, Lyman and Mackay1996), as described previously (Swarup et al., Reference Swarup, Williams and Anholt2011). We measured olfactory behaviour of single-sex groups of 50 flies/replicate and three replicates/sex for each line. Assays were conducted between 2:00 and 4:00 pm using 0·3% (v/v) benzaldehyde (Sigma-Aldrich, St. Louis, MO). Replicate measurements on individual lines were collected over multiple days to account for environmental variation. Flies between 4 and 7 days old were collected a day prior to the assay and food deprived for 2 h in a 50 ml conical tube containing a cotton wool swab tip (referred to as ‘odour tube’). The measurement is initiated by depositing 0·1 ml of odorant solution on the cotton wool swab tip in the odour tube. The odour tube is then connected to a collection tube and flies are given 2 min to partition between the tubes. At the end of the assay, a response index (RI) is calculated as follows: RI=number of flies in the collection tube/total number of flies. An RI of 1 indicates the highest avoidance response to benzaldehyde, while 0 indicates indifference (or attraction) to the odorant.
We measured sleep and waking activity of the Crc chromosome substitution lines and their respective controls by recording locomotion of virgin male and female flies for seven continuous days using the Drosophila Activity Monitoring (DAM) System (Trikinetics, Waltham, MA). Each fly was housed separately in an activity monitor tube. The DAM system uses an infrared beam to detect movement in the monitor tube; the movement is recorded as activity counts in 1-min intervals. We eliminated flies that died after 7 days of recording from the analysis. We used a custom C++ program to compute day and night sleep duration in minutes, and waking activity as counts per waking minute. Sleep is defined as inactivity lasting 5 min or longer (Hendricks et al., Reference Hendricks, Finn, Panckeri, Chavkin, Williams, Sehgal and Pack2000; Shaw et al., Reference Shaw, Cirelli, Greenspan and Tononi2000; Huber et al., Reference Huber, Hill, Holladay, Biesiadecki, Tononi and Cirelli2004, Ho & Sehgal, Reference Ho and Sehgal2005).
Mutational effects and epistatic interactions
We estimated the effects (2a) of each mutation on olfactory behaviour and sleep phenotypes in the CSB background as the deviation of the mean phenotypic value of the homozygous mutant from that of the CSB control (Falconer & Mackay, Reference Falconer and Mackay1996). We used Student's t tests to assess the significance of the difference in phenotypic values between mutant and control.
We estimated the epistatic interaction for each DGRP line as the difference between the expected and observed phenotypic values. There are two chromosome substitution lines for each DGRP line, one with a mutant C3 and the other with a wild-type C3. The observed phenotypic value of each DGRP line is the mean of the line with the mutant C3. The expected phenotypic value of each line is the difference between the mean of the line with the wild-type C3 and the estimate of 2a for the appropriate mutation obtained in the pure CSB background. We assessed the significance of epistatic interactions in each DGRP line background by performing three-way fixed effect analyses of variance (ANOVAs) using the model: Y=μ+G+L+S+G×L+G×S+L×S+G×L×S+ε, where Y is the observed value, μ is the overall mean, G is the effect of the presence or absence of the P-element, S is the effect of sex, L is the random effect of the DGRP line versus CSB genetic backgrounds, G×L, G×S, L×S and G×L×S are the interaction terms, and ε is the environmental variance between replicates. A significant interaction term (G×L and/or G×L×S) indicates epistasis. To assess variation in epistatic effects among DGRP lines, we performed similar mixed model ANOVAs across all genotypes, treating the DGRP genotypes and interactions with them as random effects. Finally, to determine the significance of epistatic interactions among different wild-derived genetic backgrounds, we estimated individual epistatic effects for each background and tested for significance using ANOVA.
Results
Effects of Crc and Sema-5c mutants on olfactory behaviour
To assess the effects of naturally segregating epistatic modifiers on P-element insertional mutations that affect olfactory behaviour, we selected P-element insertions in the Sema-5c and Crc genes that have large effects on olfactory avoidance behaviour towards benzaldehyde (Sambandan et al., Reference Sambandan, Yamamoto, Fanara, Mackay and Anholt2006; Rollmann et al., Reference Rollmann, Yamamoto, Goossens, Zwarts, Callaerts-Vegh, Callaerts, Norga, Mackay and Anholt2007). We verified the previously reported effects on responsiveness to benzaldehyde using our modified behavioural assay. To analyse the data, we used a two-way ANOVA model, Y=μ+L+S+L×S+E, where μ is the overall mean, L is the fixed effect of line, S is the fixed effect of sex, L×S is the line×sex interaction term and E is the environmental variance.
As we observed a significant line effect (P<0·0001), but no significant sex (P=0·99) or line×sex effect (P=0·90), measurements of sexes separately were pooled for analyses. The RI at 0·3% (v/v) benzaldehyde for the CSB control was 0·98±0·01 (n=3 replicates/sex/genotype, 50 individuals per replicate), showing strong avoidance behaviour. RIs for the Sema-5c and Crc mutants were 0·68±0·03 and 0·56±0·04, respectively (n=3 replicates/sex/genotype, 50 individuals per replicate for each mutant), significantly lower than the CSB control (P<0·0001; Fig. 2 a).

Fig. 2. Effects of Crc and Sema-5c mutations on olfactory behaviour and sleep phenotypes compared with the co-isogenic CSB control. (a) Olfactory behaviour. Bars represent mean response indices for pooled sexes; error bars are standard errors of the mean. (b) Night sleep. (c) Day sleep. (d) Waking activity. Bars represent mean day and night sleep and waking activity for males and females, separately, for the CSB control (open bars) or the Crc mutant (black bars); error bars are standard errors of the mean.
Effects of Crc mutations on sleep
Like Sema-5c, the Crc locus is a hotspot for P-element insertions (Spradling et al., Reference Spradling, Bellen and Hoskins2011). Previously a P-element insertion allele at Crc (Crc BG02566) was found to affect sleep phenotypes (Harbison & Sehgal, Reference Harbison and Sehgal2008), but this insertion was at a different site than the P-element insertion allele (Crc BG01724) previously implicated in startle behaviour (Yamamoto et al., Reference Yamamoto, Anholt and Mackay2009). Although both insertions are in the first exon, it is possible that different insertion locations may have distinct phenotypic effects (Rollmann et al., Reference Rollmann, Magwire, Morgan, Özsoy, Yamamoto, Mackay and Anholt2006, Reference Rollmann, Edwards, Yamamoto, Zwarts, Callaerts, Norga, Mackay and Anholt2008). Therefore, we assessed the effects of the Crc BG01724 allele on day and night sleep and waking activity. There were significant differences between Crc BG01724 and the co-isogenic control for night sleep in both sexes (P<0·0001), for day sleep for males (P<0·0001), and for waking activity in females (P=0·0024) (Fig. 2 b–d). There was a significant sex effect for day sleep and waking activity (P<0·0001), and a significant sex×line interaction for day sleep (P<0·0001, the mutation increases day sleep only in males).
Epistasis between Crc and Sema-5c mutations and wild-derived DGRP backgrounds
We used inbred DGRP lines in which C3 has been replaced by a P-element free CSB wild-type C3 or a co-isogenic C3 carrying a P-element insertion in Crc or Sema-5c (Fig. 1; Yamamoto et al., Reference Yamamoto, Anholt and Mackay2009) to assess the effects of naturally segregating epistatic modifiers of the mutations on olfactory behaviour and sleep phenotypes. We measured olfactory behaviour for 13 DGRP lines in which C3 was replaced by either an isogenic CSB C3 or a co-isogenic CSB chromosome with a Sema-5c mutation. We also assessed olfactory behaviour and sleep phenotypes for 14 pairs of DGRP lines with wild-type CSB and co-isogenic Crc C3. Since there were no significant effects of sex or sex×line interaction in the analyses of olfactory behaviour among the chromosome substitution lines, whereas these terms were significant for night sleep, day sleep and waking activity, we report the results for olfactory behaviour pooled across sexes, and the sleep and activity data for males and females separately.
In the absence of epistasis, the expected phenotype of a DGRP line bearing a Sema-5c or Crc mutation is the difference between the effect (2a) of the mutation in the CSB background and the observed mean phenotype of the DGRP line with the CSB C3. Epistasis is implicated by a significant difference between this expected value and the observed mean phenotype of the DGRP line with a mutant C3. The significance of the estimate of the epistatic effect is given by the P-value of the genotype by line interaction in an ANOVA comparing the effect of the mutation in CSB and the DGRP line. Epistatic interactions that amplify the effect of the mutation are considered enhancer effects, whereas those that counteract the effect of the mutation are defined as suppresser effects.
We found significant epistasis for olfactory behaviour in all but one instance (Sema-5c in RAL_437) (Fig. 3, Table 1). In all cases where significant epistatic interactions were observed for olfactory behaviour, the epistatic effects were negative; i.e. the observed responses of the substitution lines to benzaldehyde were greater than predicted based on the estimate of 2a in the CSB background (Table 1, Fig. 3). Since the effect of the mutations is to reduce the response to benzaldehyde in the CSB background, the negative difference between observed and expected olfactory behaviour in the DGRP lines indicates suppression of the mutant effect in wild-type backgrounds. We also found substantial and sex-specific epistasis between DGRP lines and the Crc mutation for night sleep, day sleep and waking activity (Fig. 4, Table 2). For night sleep, epistatic interactions were mostly suppressing, as for olfactory behaviour, with few exceptions (e.g. RAL_358 and RAL_852 for females and RAL_365 for males; Fig. 4 a and b). The Crc mutation increases day sleep in males (Fig. 2 b); thus, suppressing epistasis would counteract the Crc mutation by reducing day sleep duration. Interestingly, three genetic backgrounds showed epistatic interactions for day sleep for females, two of which were enhancer effects (Fig. 4 c), indicating that mutations with no effects on a phenotype in one genetic background can have significant effects in other backgrounds (i.e. the effect of the mutation was suppressed in the CSB background). There were extensive epistatic effects for male day sleep (Fig. 4 d). These effects were exclusively suppresser effects; that is, epistasis caused day sleep duration to be diminished in mutants that gave rise to prolonged day sleep. Few epistatic effects were observed for waking activity, with suppresser effects for both sexes in the RAL_365 background and enhancer effects for females in RAS_391 and males in RAL_517 (Fig. 4 e and f).

Fig. 3. Observed (closed bars) and expected (open bars) mean response indices for olfactory behaviour of (a) 13 DGRP C3 substitution lines with a P-element insertion at Sema-5c and (b) 14 DGRP C3 substitution lines with a P-element insertion at Crc. The error bars indicate standard errors of the mean for pooled sexes. ns, not significant, *P<0·05, **P<0·01, ***P<0·001.

Fig. 4. Observed (closed bars) and expected (open bars) sleep phenotypes in DGRP C3 substitution lines with a P-element insertion at Crc. (a) Night sleep in females. (b) Night sleep in males. (c) Day sleep in females. (d) Day sleep in males. (e) Waking activity in females. (f) Waking activity in males. The error bars indicate standard errors of the mean for sexes separately. ns, not significant, *P<0·05, **P<0·01, ***P<0·001.
Table 1. Epistatic interactions for olfactory behaviour in DGRP chromosome substitution lines with Sema-5c or Crc mutations
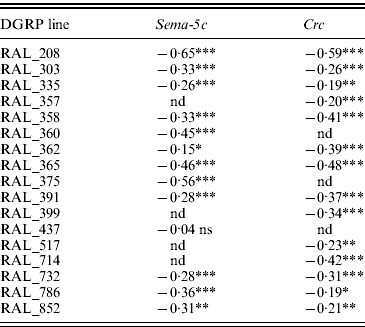
The values indicate estimated epistatic effects for olfactory RI of individual chromosome substitution lines with Sema-5c or Crc mutations. ***P<0·0001; **0·0001<P<0·01; *0·01<P<0·05; ns, P>0·05; nd, not determined.
Table 2. Epistatic interactions for sleep phenotypes in DGRP chromosome substitution lines with a Crc mutation

The values indicate estimated epistatic effects of individual chromosome substitution lines with a Crc mutation. m, males; f, females; *** P<0·0001; **0·0001<P<0·01; *0·01<P<0·05; ns, P>0·05.
We assessed whether there was significant variation in epistasis among the wild-type and mutant C3 substitution lines for each trait, as indicated by a significant genotype (wild-type versus mutant) by line (DGRP line) interaction in the ANOVA. This term was significant for all traits (Tables 3 and 4). Thus, there is variation in the extent to which natural variants modify mutational effects.
Table 3. ANOVAs of olfactory behaviour among DGRP lines with CSB and Sema-5c or Crc mutant third chromosomes
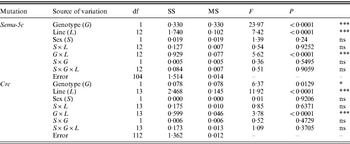
df, degrees of freedom; SS: sums of squares (type III); MS, mean squares; ***P<0·0001; *0·01<P<0·05; ns, not significant.
Table 4. ANOVAs of sleep phenotypes and waking activity among DGRP lines with CSB and Crc mutant third chromosomes

df, degrees of freedom; SS, sums of squares (type III); MS, mean squares; ***P<0·0001; **0·0001<P<0·01; *0·01<P<0·05; ns, not significant.
Pleiotropic epistatic effects
In addition to their effects on olfactory behaviour (Sambandan et al., Reference Sambandan, Yamamoto, Fanara, Mackay and Anholt2006; Rollmann et al., Reference Rollmann, Yamamoto, Goossens, Zwarts, Callaerts-Vegh, Callaerts, Norga, Mackay and Anholt2007), the Sema-5c and Crc mutations also show reduced startle behaviour (Yamamoto et al., Reference Yamamoto, Zwarts, Callaerts, Norga, Mackay and Anholt2008) and a mutation at Crc has been associated with reduced night and day sleep, and increased waking activity (Harbison & Sehgal, Reference Harbison and Sehgal2008). To assess whether the same epistatic modifiers affect the effects of the Sema-5c and Crc mutations on multiple traits, we first asked whether there was a correlation between the estimates of epistatic effects for olfactory behaviour and those of startle-induced locomotion, measured previously on the same lines (Yamamoto et al., Reference Yamamoto, Anholt and Mackay2009). We did not observe a significant correlation for either Sema-5c (Fig. 5 a) or Crc (Fig. 5 b). Similarly, epistasis of olfactory behaviour was not significantly correlated with epistasis for day sleep and night sleep for Crc, and the correlation with waking activity in males was only nominally significant (P=0·04; Fig. 6). Epistasis of day time sleep, night time sleep or waking activity was also not correlated with epistasis of startle behaviour (Supplementary Fig. S1 available at http://journals.cambridge.org/grh). These results show that different naturally segregating epistatic modifiers modulate different phenotypes affected by mutations at the same pleiotropic gene.

Fig. 5. Relationship between the estimates of epistatic interactions for olfactory behaviour (I olf) and startle induced locomotion (I startle) in DGRP C3 substitution lines. (a) Sema-5c: r 2=0, P>0·05. (b) Crc: r 2=0·186, P>0·05.
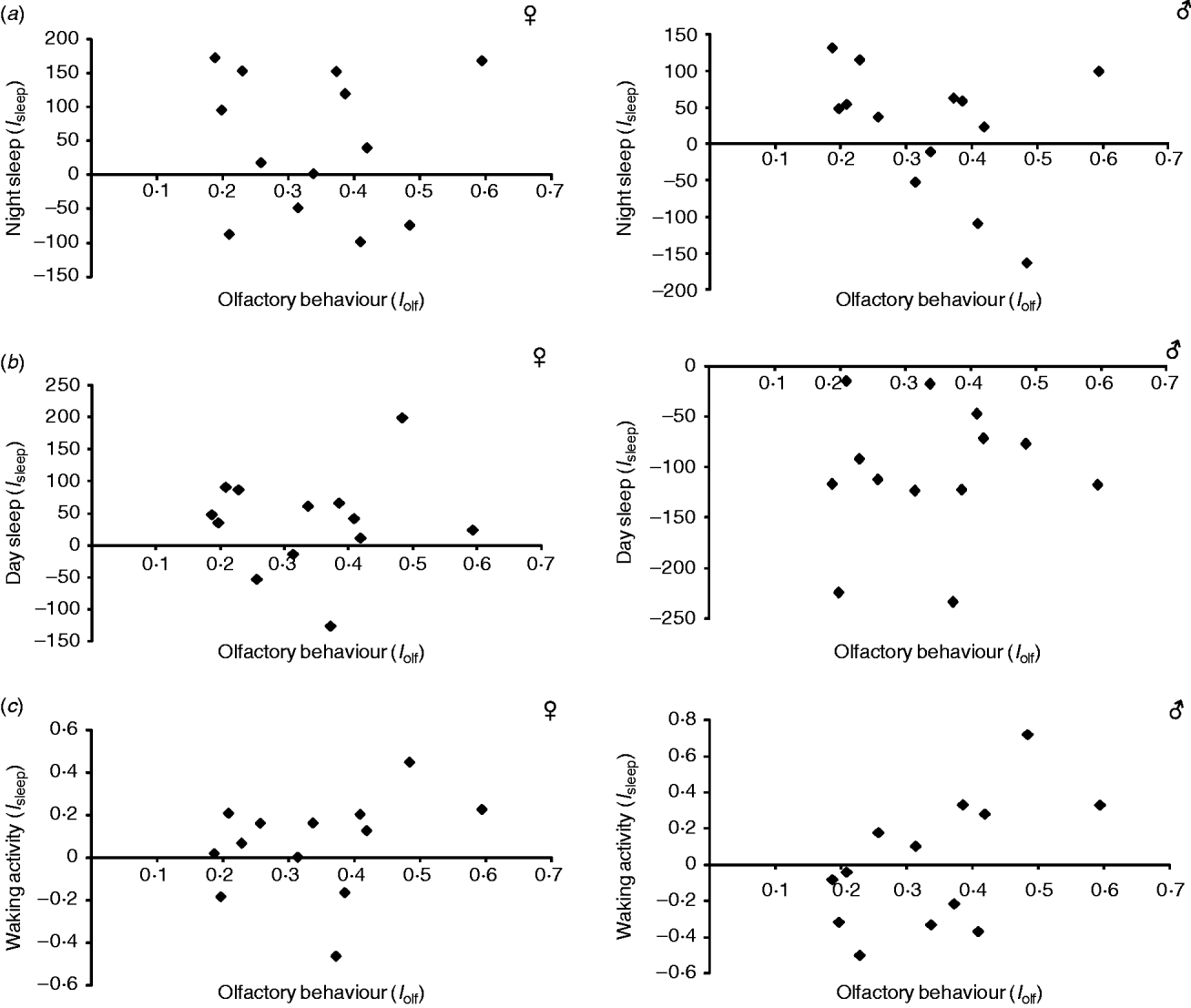
Fig. 6. Relationship between the estimates of epistatic interactions for olfactory behaviour (I olf) and sleep phenotypes (I sleep) in DGRP C3 substitution lines with a P-element insertion at Crc (a) Night sleep. Females: r 2=0·001, P>0·05. Males: r 2=0·137, P>0·05. (b) Day sleep. Females: r 2=0, P>0·05. Males: r 2=0·008, P>0·05. (c) Waking activity. Females: r 2=0·086, P>0·05. Males: r 2=0·319, P=0·044.
Discussion
Previously, olfactory behaviour in D. melanogaster has been used as a model trait to dissect the genetic architecture of behaviour (Anholt, Reference Anholt2010) and dynamic epistatic networks of pleiotropic genes have been implicated as a major feature of the genetic ensembles that underlie the manifestation of this behavioural phenotype (Fedorowicz et al., Reference Fedorowicz, Fry, Anholt and Mackay1998; Sambandan et al., Reference Sambandan, Yamamoto, Fanara, Mackay and Anholt2006). D. melanogaster can also serve as a genetic model to study sleep (Hendricks et al., Reference Hendricks, Finn, Panckeri, Chavkin, Williams, Sehgal and Pack2000; Shaw et al., Reference Shaw, Cirelli, Greenspan and Tononi2000). While epistasis can be hypothesized from co-regulated gene expression networks (Harbison et al., Reference Harbison, Carbone, Ayroles, Stone, Lyman and Mackay2009), no previous study has quantified the impact of epistasis on sleep in flies. In addition to modulation of behavioural phenotypes, suppressing epistasis may explain the paradox between developmental robustness in the face of genetic variation, as illustrated by the effects of genetic background modifiers on mutations in Sevenless and Drosophila Epidermal Growth Factor Receptor that affect development of photoreceptors (Polaczyk et al., Reference Polaczyk, Gasperini and Gibson1998).
The recent generation of a panel of diverse homozygous wild-derived chromosome substitution lines that carry the same homozygous CSB C3 with or without a P-element insertion (Yamamoto et al., Reference Yamamoto, Anholt and Mackay2009) enables analyses of the effects of naturally segregating epistatic modifiers. We used chromosome substitution lines with Sema-5c and Crc mutations to analyse epistatic modulation of mutations that affect olfactory behaviour, sleep and waking activity in Drosophila in wild-derived genetic backgrounds. Sema-5c has been implicated in early development (Khare et al., Reference Khare, Fascetti, DaRocha, Chiquet-Ehrismann and Baumgartner2000) and Crc, a calcium-binding chaperone, is involved in intracellular protein transport, exocytosis and development of the nervous system in Drosophila (Prokopenko et al., Reference Prokopenko, He, Lu and Bellen2000). Mutations in Sema-5c reduce olfactory avoidance behaviour (Sambandan et al., Reference Sambandan, Yamamoto, Fanara, Mackay and Anholt2006; Rollmann et al., Reference Rollmann, Yamamoto, Goossens, Zwarts, Callaerts-Vegh, Callaerts, Norga, Mackay and Anholt2007) and startle behaviour (Yamamoto et al., Reference Yamamoto, Zwarts, Callaerts, Norga, Mackay and Anholt2008). Mutations in Crc result not only in aberrant chemosensory responses (Stoltzfus et al., Reference Stoltzfus, Horton and Grotewiel2003; Sambandan et al., Reference Sambandan, Yamamoto, Fanara, Mackay and Anholt2006) and reduced startle behaviour (Yamamoto et al., Reference Yamamoto, Zwarts, Callaerts, Norga, Mackay and Anholt2008), but also reduce day and night sleep duration and increase waking activity (Harbison & Sehgal, Reference Harbison and Sehgal2008). We confirmed the effects of these P-element insertions in the CSB background on olfactory behaviour using a recently developed modified high throughput olfactory behavioural assay (Swarup et al., Reference Swarup, Williams and Anholt2011; Fig. 2 a) and confirmed the effects on sleep, using the same P-element insertion line in Crc previously implicated in startle behaviour (Yamamoto et al., Reference Yamamoto, Anholt and Mackay2009) and olfaction (Sambandan et al., Reference Sambandan, Yamamoto, Fanara, Mackay and Anholt2006) (Fig. 2 b).
We found that mutational effects were generally reduced in the chromosome substitution lines compared with the original effect observed in the CSB background. The presence of variation in epistatic effects for each phenotype for each P-element insertion indicates that different wild-derived genetic backgrounds harbour different segregating epistatic modifiers that alter the effect of the P-element mutation. Although phenotypic measurements of a larger number of chromosome substitution lines might reveal correlations in epistatic measures among olfactory behaviour, startle behaviour and sleep, the lack of correlation of epistatic effects across these phenotypes among the 27 lines that were available for our study (Figs 5 and 6) suggests that different epistatic modifiers are likely to interact with the same pleiotropic gene to modulate different phenotypes (Fig. 7). This complex genetic architecture is in line with previous conclusions that the manifestation of complex behavioural phenotypes can be altered by ensembles of epistatic genes (Sambandan et al., Reference Sambandan, Yamamoto, Fanara, Mackay and Anholt2006; Anholt, Reference Anholt2010; Zwarts et al., Reference Zwarts, Magwire, Carbone, Versteven, Herteleer, Anholt, Callaerts and Mackay2011). Independent segregation of components of these ensembles in a natural population will result in variation in epistatic effects and these effects may express themselves differently for different pleiotropic phenotypes associated with the same causal variant.

Fig. 7. Epistasis and pleiotropy. The diagram illustrates a focal P-element-tagged gene (red circle) that forms part of three genetic networks affecting different phenotypes, indicated by green, blue and orange colours, respectively. Gene ensembles that generate phenotype-specific epistatic interactions with the focal gene, indicated by the dotted arrows, are shown in corresponding muted colours.
In conclusion, we have shown that epistasis appears to be a pervasive general feature of natural populations and our results suggest that epistatic interactions may protect against adverse effects of new mutations. Furthermore, different epistatic interactions modulate different phenotypes affected by mutations at the same pleiotropic gene. The prevalence of epistasis in the genetic architecture of complex traits is relevant to the design and interpretation of genetic studies in human populations. Widespread suppressing epistasis may account for the ‘missing heritability’ for human traits, such as height (Manolio et al., Reference Manolio, Collins, Cox, Goldstein, Hindorff, Hunter, McCarthy, Ramos, Cardon, Chakravarti, Cho, Guttmacher, Kong, Kruglyak, Mardis, Rotimi, Slatkin, Valle, Whittemore, Boehnke, Clark, Eichler, Gibson, Haines, Mackay, McCarroll and Visscher2009). Our study underscores the importance of D. melanogaster as a model system for the analysis of quantitative traits, as a similar detailed analysis of epistasis under conditions in which we can introduce a mutation in a range of tightly controlled genetic backgrounds would not be possible in human populations. Substitution of chromosomes with P-element insertions in DGRP backgrounds will enable future mapping of epistatic modifiers and, ultimately, genome-wide characterization of epistatic interactions between defined alleles and transposon-tagged sites that affect organismal phenotypes.
This work was supported by grants from the National Institutes of Health (GM45146, GM59469) to TFCM and RRHA.













