Emotional and behavioral disorders result from a series of complex relationships between factors at multiple levels through the course of development. The etiology of psychopathology is multifactorial and warrants consideration of individual differences in biology and experience, as well as their evolving influences on each other, through the life course (Bush & Boyce, Reference Bush, Boyce and Cicchetti2016; Cicchetti & Dawson, Reference Cicchetti and Dawson2002; Doom & Gunnar, Reference Doom and Gunnar2013). In particular, exposures to social adversity and resultant stress responses have been identified as key risk factors underlying the development of psychopathology and its intermediate phenotypic precursors. Appreciation of the critical importance of developmental processes during the intrauterine period of life has grown in recent decades in the examination of life course exposures to adversity. Although the precise mechanisms for such inter- and transgenerational effects are not yet well understood, a substantial body of animal and human research suggests that maternal prenatal stress predicts offspring behavioral and biological regulation (Dunkel Schetter, Reference Dunkel Schetter2011; Entringer, Buss, & Wadhwa, Reference Entringer, Buss and Wadhwa2015; Sandman, Davis, Buss, & Glynn, Reference Sandman, Davis, Buss and Glynn2011), and a smaller body of evidence reveals direct effects on offspring brain structure and function (e.g., Buss, Davis, Muftuler, Head, & Sandman, Reference Buss, Davis, Muftuler, Head and Sandman2010; Buss et al., Reference Buss, Davis, Shahbaba, Pruessner, Head and Sandman2012).
Despite strong theory around prenatal programming of offspring stress physiology across stress-responsive systems (Wadhwa, Entringer, Buss, & Lu, Reference Wadhwa, Entringer, Buss and Lu2011), there are very few studies of prenatal programming of the infant autonomic nervous system (ANS), a key system in stress and behavior regulation that underlies mental and physical health (Beauchaine, Reference Beauchaine2015). There is also limited examination of prenatal programming effects on offspring behavioral and ANS functioning within diverse or low-income populations. This is a critical gap given the higher likelihood of excess and more severe exposure to prenatal stress among low-income, racial/ethnic minority mothers due to higher risk of financial hardship, limited resources, and lower education (Knight et al., Reference Knight, Craig, Theda, Bækvad-Hansen, Bybjerg-Grauholm, Hansen and Smith2016). A concerted effort to focus on high-risk populations will address the challenges and complexities in generalizing extant stress research findings to these communities and the importance of understanding the impact of adversity within populations more chronically exposed to severe stressors. Moreover, although maternal mood is commonly included in models, few extant studies simultaneously compare multiple levels of maternal stress, which limits understanding of the potential unique and combined contributions of these exposures during this sensitive developmental period. Accordingly, the primary objective of this study was to identify the extent to which objective and subjective measures of maternal stress during pregnancy predict infant temperament and ANS reactivity in a cohort of ethnically diverse, low socioeconomic status mother–infant dyads.
Biological Embedding of Early Adversity
Social disparities are well documented for many forms of developmental psychopathology, with more socially and economically disadvantaged children demonstrating increased risk for cognitive, social, emotional, and behavioral problems (Bradley & Corwyn, Reference Bradley and Corwyn2002; Duncan & Brooks-Gunn, Reference Duncan and Brooks-Gunn1997). Hertzman (Reference Hertzman1999) and Hertzman and Boyce (Reference Hertzman and Boyce2010) described the process of “biological embedding,” whereby differential human experiences systematically affect health across the life cycle. In particular, they proposed that differences in quality of the early environment affect the neurochemistry and shaping of the central nervous system, and that such effects impact the individual's interpretation of her or his environment and consequent relationships with the endocrine, immune, and vascular systems. Therefore, systematic differences in stress exposures could affect an organism's subsequent physiological patterns of response, the “experience” of the stressfulness of circumstances, and the biological cascade following interpretation of events and circumstances. Such differences have the potential to alter the long-term structure and function of biological pathways at varying levels of scale and complexity (i.e., synaptic strength, epigenetic marks, gene expression, neuroendocrine and immune function, etc.), creating stress-related differentials in psychopathology and a wide variety of other disease processes (Belsky & Pluess, Reference Belsky and Pluess2009; Bush, Lane, & McLaughlin, Reference Bush, Lane and McLaughlin2016; Cicchetti, Reference Cicchetti2011; Ellis & Del Giudice, Reference Ellis and Del Giudice2014; Hertzman & Boyce, Reference Hertzman and Boyce2010; Pluess & Belsky, Reference Pluess and Belsky2011).
Converging epidemiological, clinical, and experimental evidence in animals and humans suggests that this process of biological embedding begins as early as during the intrauterine period of life (i.e., the concept of fetal programming of health and disease risk; cf. Barker, Reference Barker1998, Reference Barker2007; Wadhwa, Buss, Entringer, & Swanson, Reference Wadhwa, Buss, Entringer and Swanson2009). The phenomenon of fetal programming describes the journey across the multicontoured landscape from genotype to phenotype, whereby the embryo/fetus seeks, receives, and responds to the intrauterine environment during sensitive periods of proliferation, differentiation, and maturation, resulting in structural and functional changes in cells, tissues, organ systems, and homeostatic set points. The changes resulting from this developmental plasticity, independently or through interactions with subsequent processes and environments, confer immediate consequences for fetal health and birth outcomes (Bates, Mächler, Bolker, & Walker, Reference Bates, Mächler, Bolker and Walker2015) as well as critical long-term consequences for health and disease susceptibility (Entringer et al., Reference Entringer, Buss, Swanson, Cooper, Wing, Waffarn and Wadhwa2012; Gluckman & Hanson, Reference Gluckman and Hanson2004; Gluckman, Low, Buklijas, Hanson, & Beedle, Reference Gluckman, Low, Buklijas, Hanson and Beedle2011; Glynn & Sandman, Reference Glynn and Sandman2011; Hanson, Godfrey, Lillycrop, Burdge, & Gluckman, Reference Hanson, Godfrey, Lillycrop, Burdge and Gluckman2011; Wadhwa et al., Reference Wadhwa, Buss, Entringer and Swanson2009). Even when exposure to prenatal adversity may not directly cause disease, it may alter susceptibility for a broad range of morbidities and mortality in later life by shaping an individual's phenotypic responsivity to exposures related to health and disease risk. The embryonic and fetal period represents one of the most sensitive windows of development during which the effects of stress may be transmitted across generations, and prenatal programming models are useful for understanding and predicting psychopathology-relevant outcomes.
Maternal Stress During Pregnancy and Offspring Reactivity and Regulation
The notion that maternal experience during pregnancy may affect the development of her child that has yet to be born has existed throughout recorded human history, appearing in the writings of the ancient Hindu scriptures of the Vedas (500 BCE), the fourth-century BCE Greek physician Hippocrates (Ferreira, Reference Ferreira1965), and in the advice passed down through generations of women and their care providers. Although empirical study of the impact of maternal experience on the fetus dates back to nearly a century ago (Sontag & Richards, Reference Sontag and Richards1938), over the last few decades there has been a sharp increase in research examining the role of prenatal maternal stress (and related factors such as depression an anxiety) in offspring neurodevelopment. This work draws on concepts in evolutionary biology and developmental plasticity (Ellis & Del Giudice, Reference Ellis and Del Giudice2014; Gluckman et al., Reference Gluckman, Low, Buklijas, Hanson and Beedle2011; Hanson et al., Reference Hanson, Godfrey, Lillycrop, Burdge and Gluckman2011; Pluess & Belsky, Reference Pluess and Belsky2011). Key environmental conditions that are understood to have shaped evolutionary selection and developmental plasticity include variation in the availability of energy substrate (nutritious food) and other challenges that have the potential to impact an organism's structural or functional integrity and reproductive fitness (shelter, safety, social structures, etc.). Considering the role of stress biology as the primary mediator of these conditions, it is plausible that maternal prenatal stress represents an important aspect of the intrauterine environment that would be expected to influence many developmental outcomes (Wadhwa et al., Reference Wadhwa, Entringer, Buss and Lu2011). An empirically robust body of evidence now suggests that such prenatal stress exposures play a fundamental role in organizing infant stress responses across multiple levels, including physiologic and behavioral functioning (for reviews, see DiPietro, Reference DiPietro2004; Dunkel Schetter, Reference Dunkel Schetter2011; Entringer et al., Reference Entringer, Buss and Wadhwa2015; Moisiadis & Matthews, Reference Moisiadis and Matthews2014a; Monk, Spicer, & Champagne, Reference Monk, Spicer and Champagne2012). Animal and human research demonstrates that stress “signals,” predominantly in the form of maternal glucocorticoids, are transmitted from the mother to the fetus during gestation (Moisiadis & Matthews, Reference Moisiadis and Matthews2014b; Wadhwa, Dunkel-Schetter, Chicz-DeMet, & Sandman, Reference Wadhwa, Dunkel-Schetter, Chicz-DeMet and Sandman1999), and epigenetic mechanisms for impacts on fetal development are an exciting new area of research (Moisiadis & Matthews, Reference Moisiadis and Matthews2014b; Monk et al., Reference Monk, Spicer and Champagne2012).
Infant temperament
Temperament, broadly defined, refers to stable individual differences in basic dispositions of emotionality, attention, activity, and self-regulation that emerge early in life and result from the complex interplay of genetics, biology, and environmental exposures across development (Shiner et al., Reference Shiner, Buss, McClowry, Putnam, Saudino and Zentner2012). Temperament is widely documented as an important predictor of developmental psychopathology (for a recent review, see Stifter & Dollar, Reference Stifter, Dollar and Cicchetti2016). For example, higher levels of infant negativity, characterized by sadness, anger/frustration, fear, and poor soothability, predict greater levels of both internalizing and externalizing problems later in life (Eisenberg et al., Reference Eisenberg, Sadovsky, Spinrad, Fabes, Losoya, Valiente and Shepard2005; Oldehinkel, Hartman, De Winter, Veenstra, & Ormel, Reference Oldehinkel, Hartman, De Winter, Veenstra and Ormel2004). Children with higher levels of surgency, reflected by higher levels of impulsivity, high intensity pleasure, activity level, positive anticipation, smiling, and laughter, display more aggression in childhood (Gunnar, Sebanc, Tout, Donzella, & van Dulmen, Reference Gunnar, Sebanc, Tout, Donzella and van Dulmen2003; Tackett, Kushner, Herzhoff, Smack, & Reardon, Reference Tackett, Kushner, Herzhoff, Smack and Reardon2014), have trouble using appropriate regulatory behaviors (Fox, Henderson, Rubin, Calkins, & Schmidt, Reference Fox, Henderson, Rubin, Calkins and Schmidt2001), and have greater risk for internalizing and externalizing behavior problems later in life (Oldehinkel et al., Reference Oldehinkel, Hartman, De Winter, Veenstra and Ormel2004). Problems with self-regulation, the process that modulates emotional and behavioral reactivity (Posner & Rothbart, Reference Posner and Rothbart2000), have been linked to a variety of externalizing and internalizing behavior disorders as well as issues with social functioning, academic functioning, and disrupted measures of physiological stress reactivity (Calkins & Perry, Reference Calkins, Perry and Cicchetti2016).
Several studies report a variety of indices of maternal stress (self-report and biological indices of stress activation) relate to offspring temperamental and behavioral reactivity and regulation in infancy (Sandman, Davis, Buss, et al., Reference Sandman, Davis, Buss and Glynn2012). For example, higher maternal reports of stress and plasma cortisol during pregnancy have been shown to predict slower infant behavioral recovery (regulation) from the stress of a painful heel-stick (Davis, Glynn, Waffarn, & Sandman, Reference Davis, Glynn, Waffarn and Sandman2011). Higher levels of the maternal stress biomarker corticotropin-releasing hormone (Davis et al., Reference Davis, Glynn, Schetter, Hobel, Chicz-Demet and Sandman2005) and “pregnancy-specific anxiety” (Nolvi et al., Reference Nolvi, Karlsson, Bridgett, Korja, Huizink, Kataja and Karlsson2016) have also been shown to predict reports of infant temperamental negativity. While the methodology limits certainty about exposure timing, one small study (n = 23) conducted in a mixed socioeconomic status sample with an elevated prevalence of posttraumatic stress disorder symptomology found that 6-month-old infants of mothers with elevated perinatal (including pregnancy and postnatal period) traumatic stress (reporting experiencing effects of lifetime trauma exposure within the past year) demonstrated greater rater-coded behavioral distress and worse recovery and regulation during a stress paradigm (Bosquet Enlow et al., Reference Bosquet Enlow, Kullowatz, Staudenmayer, Spasojevic, Ritz and Wright2009). Although there is a moderate body of literature demonstrating prenatal stress effects on temperament, the studies were conducted within a handful of laboratories, and often with samples of limited sociodemographic risk and exposure to adverse life events during pregnancy. Studies that simultaneously examine stressful events and perceptions of stress are rare, precluding examination of their unique contribution. In the current study, we examine maternal exposure to stressful experiences and perceptions of stress during pregnancy to examine effects on infant temperamental negativity, surgency, and self-regulation, within a multiethnic, low-income, high-risk sample.
Infant ANS
Most studies of prenatal stress effects on infant physiologic functioning focus on impacts on infant cortisol (e.g., Davis et al., Reference Davis, Glynn, Waffarn and Sandman2011), and a few others have examined associations with measures of brain structure or function related to social and emotional processing (e.g., Buss et al., Reference Buss, Davis, Shahbaba, Pruessner, Head and Sandman2012). Although the ANS plays a prominent role in stress reactivity and regulation (Beauchaine, Reference Beauchaine2015) and is one mechanism through which exposure to early adversity affects emotional and behavioral outcomes (McLaughlin et al., Reference McLaughlin, Sheridan, Tibu, Fox, Zeanah and Nelson2015), the body of research exploring the association between prenatal stress and infant ANS function is small. This is surprising given the need to understand the etiology of its development, but also given the origins of fetal programming research in cardiovascular disease (Barker, Reference Barker1998) and fetal programing theories about maternal stress influences on the nervous system. Furthermore, the fetal ANS develops rapidly within the last trimester of pregnancy and in infancy, making it likely that exposures or experiences of stress during those periods may have a potent effect on its development and function.
The ANS consists of two branches, the parasympathetic nervous system (PNS) and sympathetic nervous system (SNS), and controls central and peripheral responses to everyday and adverse experiences (Berston, Quigley, & Lozano, Reference Berston, Quigley, Lozano, Cacioppo, Tassinary and Berntson2007). The PNS (rest and digest) and the SNS (fight and flight) operate in tandem to facilitate organismic response to the environment. Substantial withdrawal of the PNS during times of threat allows for dominance of the SNS, and moderate disengagement of the PNS during challenging situations is thought to reflect increased attention and orienting to the environment without requiring activation of the SNS. The preponderance of ANS assessment in young children, including the small body of limited prenatal programing research, is based on measures of PNS functioning such as heart rate variability (HRV) or respiratory sinus arrhythmia (RSA), or more integrated measures of PNS functioning, such as heart rate (HR) or heart period.
Most extant studies of prenatal stress effects on ANS involve fetal assessments of HR and HRV (see for review Dipietro, Reference DiPietro2012), which has been shown to correlate with HR and HRV later in infancy (RSA; DiPietro, Bornstein, Hahn, Costigan, & Achy-Brou, Reference DiPietro, Bornstein, Hahn, Costigan and Achy-Brou2007). A variety of infant studies report associations between maternal mental health (depression and anxiety) and lower newborn resting vagal tone (an index related to HRV and RSA; Field et al., Reference Field, Diego, Hernandez-Reif, Schanberg, Kuhn, Yando and Bendell2003, Reference Field, Diego, Dieter, Hernandez-Reif, Schanberg, Kuhn and Bendell2004; Jacob, Byrne, & Keenan, Reference Jacob, Byrne and Keenan2009; Jones, Fox, Davalos, Lundy, & Hart, Reference Jones, Fox, Davalos, Lundy and Hart1998; Ponirakis, Susman, & Stifter, Reference Ponirakis, Susman and Stifter1998; Propper & Holochwost, Reference Propper and Holochwost2013). Specific examination of the effects of prenatal stress exposure, rather than mood or mental health symptoms, is less common, and many findings are fairly weak and focused on PNS measures during rest, rather than “stress reactivity.” For example, although Jacob et al. (Reference Jacob, Byrne and Keenan2009) found that the number of maternal life stressors was negatively correlated with neonatal resting HRV within a sample of 87 neonates born to low-income African American mothers, stress was not uniquely predictive in adjusted models. DiPietro, Novak, Costigan, Atella, and Reusing (Reference DiPietro, Novak, Costigan, Atella and Reusing2006) found that higher maternal rating of PS during pregnancy was associated with lower child vagal tone (an indicator of PNS activity at rest) at age 2 within an upper-class sample of predominantly Caucasian women; the association, however, became marginal after infant sex was included.
Few research groups have evaluated associations between measures of prenatal stressors and/or prenatal stress perceptions and infant ANS reactivity to stressors. The small study of lifetime trauma exposure and maternal perceptions of trauma-related stress experienced during the perinatal period described earlier also found that higher levels on both measures predicted higher infant HR during the recovery phase of the Still Face stressor paradigm, suggesting less PNS recovery and regulatory capacity (Bosquet Enlow et al., Reference Bosquet Enlow, Kullowatz, Staudenmayer, Spasojevic, Ritz and Wright2009), but they did not find stress effects on calculations of HR reactivity. Alkon et al. (Reference Alkon, Boyce, Tran, Harley, Neuhaus and Eskenazi2014) tested whether exposure to psychosocial risk factors during pregnancy, such as poverty or low social support, predicted infant ANS measures between 6 months and 5 years of age. No effects of prenatal adversity on offspring ANS levels at specific ages were reported, but poverty or low social support predicted dampened HR and SNS (but not PNS) reactivity trajectories from 6 months to 5 years of age.
Rash and colleagues published two studies examining the association between maternal psychological and physiological stress and infant ANS functioning within a Canadian sample of 194 predominantly middle-class, Caucasian dyads. Rash, Campbell, Letourneau, and Giesbrecht (Reference Rash, Campbell, Letourneau and Giesbrecht2015) found that higher levels of biomarkers of maternal stress (cortisol awakening response and total cortisol output) assessed at 14 weeks of gestation were positively associated with infant RSA reactivity to a series of frustration tasks. Higher total cortisol output at 14 weeks and higher cortisol awakening response at 32 weeks were also associated with lower infant RSA at rest. These authors suggest that CNS and cardiac structure itself may be impacted by the presence of heightened maternal cortisol. Rash et al. (Reference Rash, Thomas, Campbell, Letourneau, Granger, Giesbrecht and Team2016) took a more complex approach to modeling maternal prenatal stress effects. That study found that mothers with decreasing daytime salivary alpha amylase (sAA) slopes during early pregnancy and relatively greater psychological distress during late pregnancy were more likely to have infants who exhibited combined physiology profiles of co-inhibition (sAA < 0, RSA < 0) during these frustration tasks at 6 months of age. Low psychological distress in late pregnancy was associated with reciprocal activation (sAA > 0, RSA < 0; or sAA < 0, RSA > 0).
Finally, a recent study by Suurland et al. (Reference Suurland, van der Heijden, Smaling, Huijbregts, van Goozen and Swaab2016), using a sample of 121 predominantly Caucasian mother–child dyads from the Netherlands, found that the “higher risk” group of mothers (from a sample with relatively low levels of psychosocial risk factors) had infants with increased HR and RSA withdrawal during recovery from the Still-Face Paradigm (SFP; suggesting a lack of regulation). This finding is intriguing, but a major study design problem limits confidence that the results reflect fetal programming of the ANS. The psychosocial risk factors within the cumulative risk score (e.g., psychiatric diagnosis, lack of secondary education, and maternal age < 20 years) assessed during the third trimester of pregnancy were not likely to vary 6 months postnatally, so it is not possible to infer that the associations seen were driven specifically by prenatal exposure to those risks, particularly as the study also did not adjust for postnatal stress levels. A second limitation of these data is the very low level of overall sample risk and the factors within the cumulative risk score used for defining groups, which make it difficult to understand whether the women in the high-risk group felt stressed or were experiencing stress.
In sum, there are limited data examining prenatal stress programming of offspring ANS resting, reactivity, and regulation/recovery, core risk factors for psychopathology. In particular, the extant research on prenatal stress and PNS reactivity has mostly been conducted outside of the United States with predominantly low-risk Caucasian samples. Additional research on low-income and multiethnic samples with substantial exposure to stressors and reporting chronic stress will greatly advance our understanding of this potential early pathway to risk for developing psychopathology.
The Importance of Measuring Both Objective Stressors and Perceived Stress
Although stress is a central concept in research on developmental processes and prenatal programming, there is no single measure used to assess it. A variety of measures of both objective and perceived stress are predictive of child outcomes. Different aspects of stress tend to be only weakly correlated, as they likely measure different processes, and findings suggest they may have differing effects on development and/or point to different intervention targets. Despite this, many studies examining the association between prenatal stress (rather than mood or symptoms) and maternal and child outcomes use a single measure of stress.
Measures that reflect more persistent exposures, such as chronic stress, tend to show stronger associations than do measures based on mood or daily events (DiPietro et al., Reference DiPietro, Novak, Costigan, Atella and Reusing2006). Chronic stress may partly reflect external events and may partly reflect more persistent psychological attributes of the individual that are minimally related to external events. A review by Dunkel Schetter (Reference Dunkel Schetter2011) concluded that different types of stress exposures, perceptions of stress, as well as the duration of stress (chronic vs. acute), have varying associations with infant outcomes. Similarly, other reviews (Graignic-Philippe, Dayan, Chokron, Jacquet, & Tordjman, Reference Graignic-Philippe, Dayan, Chokron, Jacquet and Tordjman2014; Nast, Bolten, Meinlschmidt, & Hellhammer, Reference Nast, Bolten, Meinlschmidt and Hellhammer2013) have concluded that examining objective measures of stressors combined with perceived measures of stress offer the best understanding for impact on birth and infant outcomes of interest.
Stressful exposures and PS are salient for understanding fetal development within US samples. Among US women, the prevalence of at least one significant life event (SLE) during pregnancy was recently estimated as 65%–70%, with one in five women reporting experiencing multiple stressors (Braveman et al., Reference Braveman, Marchi, Egerter, Kim, Metzler, Stancil and Libet2010; Burns, Farr, & Howards, Reference Burns, Farr and Howards2015). Multiple stressors were more common among pregnant women living in poverty and were more likely to be associated with adverse maternal and child health outcomes when compared to women who reported only one SLE.
Differences in both PS during pregnancy and objective measures of stress have also been found between racial/ethnic groups (Borders et al., Reference Borders, Wolfe, Qadir, Kim, Holl and Grobman2015). One large epidemiologic study in the United States showed that non-Hispanic Black pregnant women reported more PS than their White counterparts across a broad array of psychosocial domains (Grobman et al., Reference Grobman, Parker, Wadhwa, Willinger, Simhan, Silver and Reddy2016). As noted above, the limited evidence examining prenatal stress effects on offspring reactivity (particularly ANS) was derived from research conducted with predominantly low-risk, Caucasian samples, limiting generalizability to the population experiencing the greatest adversity during pregnancy. Given that women with limited financial and social resources and high exposure to past and present trauma have children at greater risk for psychopathology and a variety of health outcomes, it is important to utilize multiple measures to capture the complexity of prenatal stress exposure and perception in this population.
The Present Study
The current study advances existing science examining prenatal stress effects on infant risk for developmental psychopathology in several ways. We recruited a racially and ethnically diverse sample of low- to middle-income pregnant women, with significant exposure to adverse experiences to understand the effects of variation in prenatal stress in a chronically stressed sample. We examined effects of two levels of stress during pregnancy: objective counts of exposure to SLE across pregnancy and a repeated measure of global PS, to understand their potentially unique effects on infant development. We examined two levels of infant reactivity and regulation: parent report of temperament and assessment of RSA activity during a gold-standard infant stress paradigm, tailored to optimize stress measurement.
In light of the evidence for the positive association between prenatal stress and infant negative temperament and cortisol, and one similarly designed study finding a positive association between maternal cortisol and infant RSA reactivity (Rash et al., Reference Rash, Campbell, Letourneau and Giesbrecht2015), we hypothesized that infants born to mothers with higher stress during pregnancy would be more reactive and demonstrate lower levels of self-regulation, across both behavioral and physiologic indices. Although there is a dearth of literature contrasting event-based counts of adversity and perceptions of stress, we speculated that PS might be the stronger predictor, due to its likely association with activation of maternal biological stress responses that affect fetal development, such as cortisol.
Method
Participants and procedures
Participants were drawn from the Maternal Adiposity, Metabolism, and Stress (MAMAS) Study, a nonrandomized control trial that was designed to examine the effects of a mindfulness-based stress reduction and healthy lifestyle intervention to reduce excessive gestational weight gain (Epel et al., Reference Epel, Laraia, Coleman-Phox, Leung, Vieten, Mellin and Adler2017). Women with a singleton pregnancy, English-speaking, aged 18–45, with self-reported prepregnancy body mass index of 25–41 kg/m2, household income less than 500% of the federal poverty level (e.g., $73,550 for a family of two in 2011, a US indicator of low to middle income; US Department of Health and Human Services, 2011), and without medical conditions that might affect gestational weight gain (e.g., diabetes, abnormal glucose screen in early pregnancy, hypertension, and eating disorders) were eligible to participate. Eligibility criteria also included that women enroll between 12 and 24 weeks of pregnancy. Women were recruited from hospital-based clinics, community health centers, Supplemental Nutrition Program for Women, Infants and Children offices, organizations providing services to pregnant women, and through online advertisements (e.g., Craigslist) from August 2011 through June 2013. Details of our recruitment strategy have been published previously (Coleman-Phox et al., Reference Coleman-Phox, Laraia, Adler, Vieten, Thomas and Epel2013).
Of the 215 MAMAS participants, 13 were not eligible for enrollment in the postnatal offspring study (5 dropped out of the MAMAS study, 3 miscarriage, 1 fetal death, 1 moved out of the area, and 3 were lost to follow up prior recruiting), resulting in 202 mothers contacted postpartum for recruitment into the Stress, Eating, and Early Development (SEED) study. SEED is an offspring follow-up study, assessing the effects of prenatal factors on offspring behavioral, physiologic, and anthropometric development through age 4. Of the 202 women eligible for SEED, 162 (80%) enrolled postnatally in the offspring follow-up study. There were no differences in baseline characteristics or prenatal stress between the women who consented to postnatal follow-up compared to those who declined or who were lost to follow-up.
For the SEED study, maternal participants were 18–43 years of age at enrollment (M = 28.0, SD = 5.8). Two-thirds were married or partnered (68%) and half were multiparous (54%). Approximately 31% had completed high school or less, 50% had some college or vocational training, and 19% had earned a college degree. Annual household income was $0–$98,000 (median = $19,000), with the majority of the sample falling below the federal poverty level at the time of data collection. Eight-five percent self-reported as ethnic or racial minorities: 39% African American, 31% Latina, 2% Asian, and 13% other or multiracial. The cesarean rate was 28%, which was below the 2012 US and California rates of 33% but representative of the county regions sampled (range = 26%–29%). Average gestational age at birth was at 39.6 weeks.
For the 6-month postnatal visit, mothers were invited to complete in-person assessments either at the university clinic or in their home. Of the 162 enrolled, a total of 156 participants agreed to the 6-month in-person visit (1 refused; 2 could not complete the visit due to moving out of the study area, but 1 of those agreed to questionnaire portion via phone; and 3 were missing contact information or were unreachable for this visit). Two “6-month” visits were completed after the infant was 9 months of age, and thus were excluded from analyses, leading to a possible SEED sample of 154 infants at this time point. Of those, the 151 mother–child dyads with prenatal and postnatal questionnaire data were included in the present analyses (see Table 1 for descriptive statistics).
Table 1. Descriptive information for full sample and subsamples of children with and without RSA data
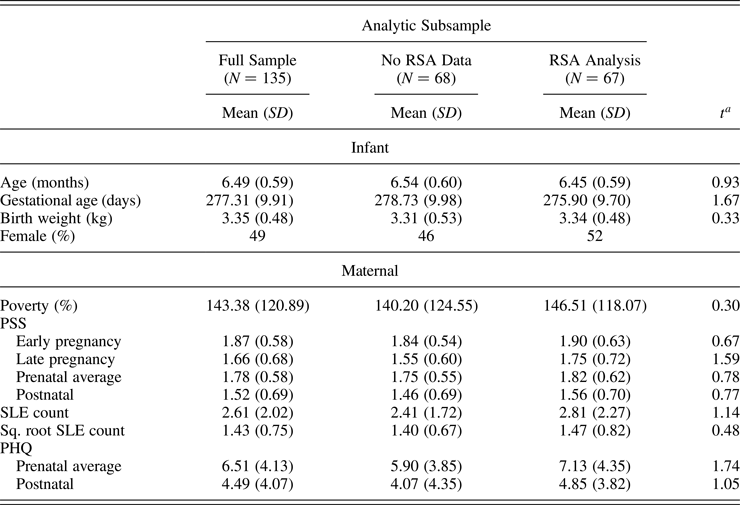
Note: RSA, respiratory sinus arrhythmia; PSS, Perceived Stress Scale; SLE, stressful life events; PHQ, Patient Health Questionnaire.
a Children in RSA analyses subsample did not differ from children without RSA data by any sample characteristics or predictor values.
Delays in funding for SEED limited our ability to collect physiologic data on women in the first half of the pregnant MAMAS cohort, and only the latter half of the infant sample was assessed for ANS response to the standardized stressor. After refinement of the ANS collection protocol and piloting its administration with this sample, ANS data was collected on a total of 67 infants at 6 months of age, using the stressor paradigm described below.
Mothers completed questionnaires in person and over the phone throughout pregnancy and the postpartum period. This study focuses on measures assessed during middle (between 12 and 20 weeks gestation) and later (20–28 weeks) pregnancy, and again at 6 months postpartum. Trained research assistants reviewed medical records to abstract data and confirm gestational age and birth weight. The infant experimental stress paradigm was conducted in person, either in the clinic or in participants' homes, in conjunction with the maternal assessment during the 6-month postpartum visit (M infant age = 6.5 months, SD = 0.6 months); visits were scheduled on days and times mothers felt their infant was well rested and fed and could be alert for the activities. All procedures were approved by the Institutional Review Board at the University of California, San Francisco.
Demographic measures
At enrollment into MAMAS prenatally, women reported age, parity, marital or partnered status, race and ethnicity, education, annual household income, and number of individuals and children in the household.
Maternal reports of stress
SLE
Maternal report of the number of SLE that occurred during pregnancy was assessed, retrospectively, at 6 months postpartum. SLE were assessed with a list of 14 events adapted from the Centers for Disease Control and Prevention (2005) PRAMS survey, a population-based postpartum survey of maternal attitudes and experiences before, during, and after pregnancy. Participants were asked to respond yes or no to statements about experiences with illness, death, relationship problems, housing difficulties, legal issues, and financial problems during pregnancy. Affirmative responses were summed. The number of SLE reported ranged from 0 to 8, with 14% reporting no events, 39% reporting 1 or 2 events, and 47% reporting 3 or more events. SLE was square-root transformed to reduce slight skewness (skew = 0.97 before transformation, –0.40 after transformation). Such measures of events are thought to have limited recall bias and be accurate over a span of years (Krinsley, Gallagher, Weathers, Kutter, & Kaloupek, Reference Krinsley, Gallagher, Weathers, Kutter and Kaloupek2003).
Perceived Stress Scale (PSS)
Self-report on the PSS (Cohen, Kamarck, & Mermelstein, Reference Cohen, Kamarck and Mermelstein1983) was assessed twice during pregnancy and again at 6 months postpartum. The PSS is a widely used, highly reliable, and valid, self-report questionnaire that assesses an individual's perceptions of his or her generalized stress and coping over the previous month (as opposed to reactions to a specific event). The PSS assesses current levels of stress and the extent to which individuals perceive their lives as “unpredictable,” “uncontrollable,” and “overloaded.” Participants responded to 10 items asking how often they had certain thoughts and feelings in the last month on a 5-point scale (never, almost never, sometimes, fairly often, and very often). Positively worded items were reverse-coded. Mean scores for each of the three time points were computed as long as greater than 75% of the items in the respective scale were answered. Internal consistency across the three time points was good (α = 0.85–0.86). Responses on this measure across the two prenatal time points were highly correlated (r = .66), and this, along with the goal of examining pre- versus postnatal stress effects led us to average those scores to create a single measure of prenatal PS.
Infant outcome measures
Infant temperament
At 6 months postpartum, mothers completed the Infant Behavior Questionnaire—Revised, a measure designed to assess temperament in infants between 3 and 12 months of age. Parents are asked to rate how often they observed a particular behavior in their infant within the last 1 to 2 weeks, on a 7-point scale ranging from 1 (never) to 7 (always). The 91 items load onto 14 scales with very good internal reliability (ranging from .70 to .90 for parent report; Gartstein & Rothbart, Reference Gartstein and Rothbart2003). In line with common practice, three “superfactor” composite variables were created. Infant regulation was computed from the mean scores of the approach; vocal reactivity; high intensity pleasure, smiling, and laughter; activity level; and perceptual sensitivity subscales (α = 0.79). Infant surgency was computed from the mean scores of the low intensity pleasure, cuddliness, duration of orienting, and soothability subscales (α = 0.88). Infant negativity was computed from the mean scores of the sadness, distress to limitations, fear, and falling reactivity subscales (α = 0.85).
Infant stress paradigm
The SFP (Tronick, Als, Adamson, Wise, & Brazelton, Reference Tronick, Als, Adamson, Wise and Brazelton1978) is one of the most widely used measures to assess infant reactivity and regulatory competency and is increasingly used in infant ANS research (Bosquet Enlow et al., Reference Bosquet Enlow, King, Schreier, Howard, Rosenfield, Ritz and Wright2014; Conradt & Ablow, Reference Conradt and Ablow2010; Holochwost, Gariepy, Propper, Mills-Koonce, & Moore, Reference Holochwost, Gariepy, Propper, Mills-Koonce and Moore2014). It provides a structured protocol designed to elicit infant self-regulation in response to parental interaction and disengagement. The SFP demonstrates good construct validity having been used to examine a number of developmental phenomena including infant attachment, temperament, sex and cultural differences, and maternal sensitivity (see for review Mesman, van Ijzendoorn, & Bakermans-Kranenburg, Reference Mesman, van Ijzendoorn and Bakermans-Kranenburg2009). It has shown good reliability when infant behavioral responses were tested over a 2-week period (Provenzi, Olson, Montirosso, & Tronick, Reference Provenzi, Olson, Montirosso and Tronick2016).
The standard SFP consists of a sequence of three, 2-min episodes (play, Still Face [SF], and play) in which the parent and the infant are seated about 1 m away from each other. During the first “play” episode, the parent is instructed to play “naturally” with the child as they normally would without toys. During the SF episode, the parent is asked to maintain a neutral expression on her face and is told not to touch or interact with the baby. The third episode, sometimes referred to as the “reunion” episode, is a resumption of play in which the parent is told to respond to the infant in the manner they choose but without removing the child from the seat. Researchers interested in capturing measures of stress physiology have increasingly chosen to administer a second SF episode and a third play episode (second reunion) to create a more persistent challenge and enhance infant stress responses (e.g., Bosquet Enlow et al., Reference Bosquet Enlow, King, Schreier, Howard, Rosenfield, Ritz and Wright2014). In line with this work, for this study, infant–mother dyads participated in a 10-min SFP protocol including five episodes: 2-min play (baseline); 2-min SF (SF 1); 2-min play (Reunion 1); 2-min SF (SF 2); and 2-min play (Reunion 2). Experimenters prompted mothers to begin and end each episode. Mothers were told that they could discontinue the task at any point if they felt the infant was overly stressed. Research assistants (RAs) were also trained to terminate the task if the infant demonstrated significant distress for longer than 1 min and the mother had not chosen to terminate.
RSA
To obtain measures of children's PNS reactivity and recovery, we assessed RSA, a reliable index of the PNS influence on cardiac functioning in adults (Berntson, Cacioppo, & Quigley, Reference Berntson, Cacioppo and Quigley1994; Sherwood, Allen, Obrist, & Langer, Reference Sherwood, Allen, Obrist and Langer1986) and in child and adolescent samples (Alkon et al., Reference Alkon, Lippert, Vujan, Rodriquez, Boyce and Eskenazi2006; Calkins & Keane, Reference Calkins and Keane2004). RSA indices were calculated using the interbeat intervals detected from electrocardiography (ECG) readings, respiratory rates detected from impedance waveforms (e.g., dZ/dt), and a bandwidth range of 0.24 to 1.04 Hz for 6-month-olds (Bar-Haim, Marshall, & Fox, Reference Bar-Haim, Marshall and Fox2000) collected continuously using BioNex hardware and BioLab acquisition software version 3.0 (Mindware Technologies, Ltd., www.mindwaretech.com) from infants throughout the SF protocol.
After infants acclimated to the assessors, trained RAs attached cardiac monitoring equipment to the infant while he or she sat on the mother's lap. The RA placed four spot electrodes on the infant's neck and trunk to collect impedance and respiratory measures, and three spot electrodes were placed on the right clavicle, lower left rib, and right abdomen for ECG measures (Bush, Caron, Blackburn, & Alkon, Reference Bush, Caron, Blackburn and Alkon2016). Infants were then placed into a secure, stationary infant seat, surrounded by a trifold, white visual barrier obstructing his or her view of the environment behind and to the sides of the seat. A 5-min waiting period was included, to allow for adequate adhesion of the electrodes and conduction of the electrical signal, as well as infant acclimation to the situation. During this waiting period, the RA explained the SFP to the mother and answered any questions. In order to ensure the infant was calm prior to beginning the SFP, the 10-min SFP protocol was preceded by a 2-min “resting” baseline assessment while the infant listened to a soothing lullaby (Bush, Caron, et al., Reference Bush, Caron, Blackburn and Alkon2016). Continuous signals were recorded during the resting lullaby and 10-min SFP. Electrodes were removed immediately after completion of the SFP.
RSA data were filtered, extracted, and then scored in 30-s intervals using Mindware software (Heart Rate Variability Analysis Software version 3.1, Mindware Technologies, Ltd, http:// www.mindwaretech.com). Thirty-second epoch data cleaning procedures involved examining for artifact, and an individual child's data were deleted if more than 25% of the epoch was unscorable. RAs who scored the data achieved at least 90% interrater reliability with an experienced investigator. Data cleaning procedures included checking all outliers (>3 SD) by interval and summary scores.
Of the 68 infants assessed after ECG equipment was available, study staff were trained in administration, and the stress protocol was finalized, one mother refused collection of the ECG data during the study visit, resulting in 67 children with any ANS data at this time period. Comparison with the rest of the sample on measures of interest is shown in Table 2. Some of the 67 children did not tolerate the application of the electrodes and subsequent lullaby and restricted seated play with the mother. Due to infant distress, the protocol was discontinued during the first play (4 subjects), during the first two 30-s epochs of SF1 (3 subjects), during the second play/reunion (16 subjects), and during SF2 (3 subjects), leading to varying sample sizes depending upon outcome. To ensure our estimates of RSA were reliable, and reflected the experience of the target episode (e.g., play and stress), rather than brief carryover from a previous episode, we focused analyses on participants with three or more scorable RSA 30-s episodes (SFP episode averages were created by averaging three or four 30-s epochs). Of the 67 children with usable ANS data, a total of 60 (90%) had scorable RSA data for at least three 30-s epochs of the play and SF1 episodes. Only 35 infants (58%) persisted through the five episodes of the SFP and completed the final second play/Reunion episode, and 34 of those cases had three epochs of usable data. Table 3 presents the descriptive information for RSA levels across the five SFP episodes, as well as the mean RSA reactivity and recovery calculations across the paradigm.
Table 2. Pearson correlations among study variables
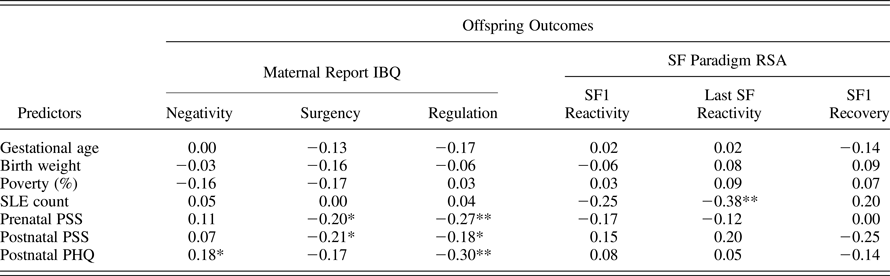
Note: IBQ, Infant Behavior Questionnaire; RSA, respiratory sinus arrhythmia; SF, Still Face; SLE, stressful life events; PSS, Perceived Stress Scale; PHQ, Patient Health Questionnaire.
*p < .05. **p < .01.
Table 3. Descriptives for RSA across resting lullaby and SF Paradigm episodes
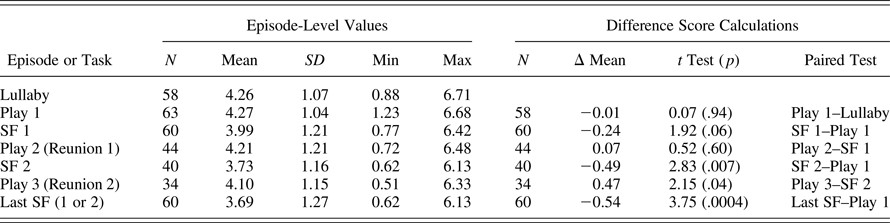
Note: RSA, respiratory sinus arrhythmia; SF, Still Face episode. See Method section for description of calculations of difference scores.
Because of the nature of the SFP, and our “enhanced stressor” version used here, which included a second SFP episode for children who were not overly distressed by the first SF episode, reactivity was calculated twice. SF1 RSA reactivity scores were calculated by subtracting the average response during the first 2-min play episode (baseline) from the average response across the first SF (stressor task). Because of the variability in individual experiences of distress in response to standardized stressor exposures, it is sometimes necessary to calibrate the stress exposure by increasing either the intensity or the duration of the stress exposure in order to elicit a stress response. Unfortunately, precipitous arousal-related task termination after SF first instance (one-third failed to continue to SF2) led to a substantial reduction in sample size during SF2. For this reason, “last SF” RSA reactivity scores were calculated for the full possible ANS sample by subtracting the average response during the last available of the two SF episodes for which the infant had three or more scorable 30-s epochs (SF1 for infants who terminated the paradigm early due to distress, SF2 for infants who persisted in the paradigm). Thus, a negative SF1 or last SF reactivity score indicates greater PNS withdrawal (stress response) during that SF relative to Play 1. Recovery to SF1 was calculated by subtracting the average response during the second 2-min play period (reunion) from the average response across the first SF (stressor task). Thus, a positive RSA recovery score indicates greater PNS activation (calming response or self-regulation) during Play 2 relative to SF1. Due to the substantial dropout during Play 3 (final reunion), and concerns about power and multiple testing, a second recovery score was not calculated.
Covariates
Gestational age was obtained via labor and delivery medical records abstraction. Birth weight was obtained via labor and delivery medical records abstraction, except in one case where records were not available and maternal report was utilized. Participants reported total household income and number of individuals living in the household at enrollment. Household income was converted to percent of US federal poverty level (US Department of Health and Human Services, 2011), which adjusts for household size. Depressive symptoms were assessed using the sum of the nine-item Patient Health Questionnaire (Kroenke, Spitzer, & Williams, Reference Kroenke, Spitzer and Williams2001), a depression screening tool commonly used in primary care settings that has been validated in pregnant women (Sidebottom, Harrison, Godecker, & Kim, Reference Sidebottom, Harrison, Godecker and Kim2012). It is a DSM-IV based measure that assesses how often participants were bothered by various depression symptoms/problems, with responses ranging from 0 to 3 (not at all, several days, more than half the days, or nearly every day).
While not the intended focus of this paper, since some of the SEED women participated in a prenatal stress-management intervention aimed at preventing excessive weight gain during pregnancy (Epel et al., Reference Epel, Laraia, Coleman-Phox, Leung, Vieten, Mellin and Adler2017), we examined whether it was necessary to covary for whether women participated the MIND program during pregnancy in relation to our infant outcomes using a dichotomous dummy code (MIND compared with the control group).
Data analysis
Analyses were performed using SAS version 9.4. Descriptive statistics were calculated for all demographic characteristics and study variables. Data were assessed for normal distributions and potential outliers. Pearson correlation coefficients were used to explore the associations between key study variables. We used ordinary least squares regression models to examine the effects of the objective (SLE) and perceived (PSS) stress exposures in relation to maternal report of infant temperament (negativity, surgency, and regulation) and measures of ANS functioning (reactivity SF1, reactivity last SF, and SF1 recovery), adjusting for covariates. Because of the high correlation between pre- and postnatal PS and the potential problems introduced by multicolinearity, each regression was run twice (the first with prenatal stress, the second with postnatal stress), and model coefficients for PS at both time points were compared. Because of the limited power due to sample size in the ANS analyses, removing nonsignificant predictors from ANS models was also explored. Finally, post hoc regression analyses examining the interactive effects of SLE and PS on the infant temperament and ANS outcomes were conducted following recommendations by Aiken and West (Reference Aiken and West1991), including centering all predictor variables prior to inclusion in the models.
Results
Preliminary analyses
To determine possible selection biases associated with availability of ANS data, we compared infants in the RSA subsample to the subsample without usable ANS data on the key maternal stress predictor variables and covariates poverty, gestational age, and birth weight. As expected, because missingness was based on funding availability (likely to be random), the subsample was representative of the larger sample, and there were no significant differences between those with and without ANS data on maternal stress measures of interest. Descriptives for the full sample, and for the sample split by availability of ANS data, are shown in Table 1.
To test for effects of participation in the MIND program, we compared outcomes for those in the intervention versus the comparison group. Because group assignment was not significantly correlated with any of the offspring outcomes (rs = –.10 to .10, ps = .24–.95), to preserve power, it was not included as a covariate.
Intercorrelations among potential study covariates and study outcomes are presented in Table 2. Although only correlated at a trend level with a few outcomes, due to theoretical and empirical rationales for their potential confounding role in tested associations, and for consistency and ease of comparison across models, gestational age, birth weight z score, and percent poverty were included as covariates within all models.
Intercorrelations among stress measures showed that maternal report of count of SLE experienced during pregnancy was weakly and nonsignificantly related to measures of PS during pregnancy (r = .21, ns) and at 6 months postpartum (r = .05, ns); longitudinal reports of PS were fairly stable from prenatal to postnatal assessment (r = .66, p < .05), as described above regarding concerns about multicolinearity within models.
Descriptive statistics for infant RSA values, by SFP episode, and paired t tests for means across episodes, are presented in Table 3. The mean level of RSA during lullaby and play were not different from each other; thus, we calculated RSA reactivity relative to levels during play, as is commonly done (Bosquet Enlow et al., Reference Bosquet Enlow, King, Schreier, Howard, Rosenfield, Ritz and Wright2014; Ritz et al., Reference Ritz, Bosquet Enlow, Schulz, Kitts, Staudenmayer and Wright2012). RSA reactivity during SF1 (SF1–Play 1) and RSA reactivity during SF2 (SF2–Play 1) were both significantly different from zero, indicating that, on average, the PNS responses were different between SF episodes and baseline play. The sample average RSA reactivity to the last SF was also different than zero, and as was intended, reflected the largest average reactivity change score across the full sample. On average, infant RSA during the first reunion episode was not different than RSA levels during SF1; this lack of recovery is consistent with some extant literature demonstrating a lack of PNS recovery during the reunion episode (e.g., Conradt & Ablow, Reference Conradt and Ablow2010; Suurland et al., Reference Suurland, van der Heijden, Smaling, Huijbregts, van Goozen and Swaab2016).
Regression models predicting infant temperament
Table 4 displays results for full-sample regression models examining pre- and postnatal stress associations with maternal report of infant temperament. Compared to bivariate associations between the stress measures and offspring outcomes in Table 2, results from covariate-adjusted regressions simultaneously modeling both stress measures were not different. After covariate adjustment for gestational age, birth weight, and percentage of poverty threshold, the count of SLE was not significantly related to any of the three temperament domains. However, higher ratings of PS, at both the pre- and postnatal period were significantly related to lower ratings of maternal report of infant surgency and regulation. The high correlation between pre- and postnatal PS in this chronically stressed sample prevented simultaneous modeling of both time points. We note that coefficients for the prenatal time point of PS were larger than that of the postnatal PS time point, especially in the prediction of infant regulation, suggesting the prenatal exposure window may be more important for that outcome. Of note, family income was the only significant predictor of infant negativity such that infants from families with greater incomes-per-household size were rated as less negative, adjusting for stress and covariates.
Table 4. Regression coefficients predicting temperamental and parasympathetic reactivity and regulation
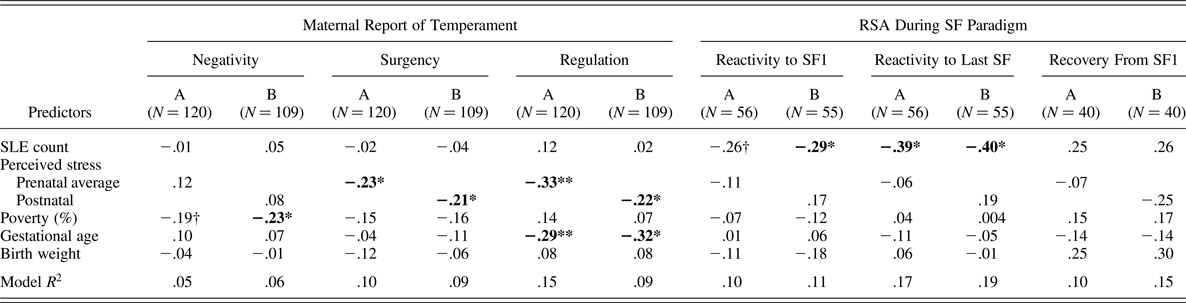
Note: RSA, respiratory sinus arrhythmia; SF, Still Face episode; Because of concern about colinearity within the models, due to the high correlation between pre- and postnatal perceived stress, Model A includes stressful life events and prenatal perceived stress and covariates, and Model B includes SLE and postnatal perceived stress and covariates.
†p < .10. *p < .05. **p < .01.
Regression models predicting PNS functioning
Table 4 displays parallel regression results for ANS-subsample models examine effects of pre- and postnatal stress associations with infant PNS functioning, after covariate adjustment for gestational age, birth weight, and percentage of poverty threshold. Compared to bivariate associations between the stress measures and infant outcomes in Table 2, results from covariate-adjusted regressions simultaneously modeling both stress measures were only slightly different in that SLE was associated with both RSA reactivity calculations, rather than one.
RSA reactivity to SF1
In contrast to the models predicting temperament, the number of objective stressful events reported as occurring during pregnancy was significantly negatively related to RSA reactivity. Higher counts of SLE predicted greater withdrawal of RSA during the first SF exposure at the trend level when prenatal PS was in the model (β = –0.26, p = .06) and significantly when postnatal PS was in the model (β = –0.29, p < .05; note the first coefficient rose to significance, when the nonsignificant prenatal PS was removed from the model: β = –0.30, p < .05; results not shown). Neither prenatal nor postnatal PS was significantly related to RSA reactivity during the first SF.
RSA reactivity to last SF
Findings from this model paralleled those of the model predicting SF1 reactivity, although the coefficients for SLE effects were larger. The number of objective events during pregnancy was significantly negatively related to RSA reactivity such that higher counts of SLE predicted greater withdrawal of RSA during the last SF exposure when either prenatal or postnatal PS was in the model (β = –0.39, p < .05; β = –0.40, p < .05; respectively). PS was not significantly related to RSA reactivity during the last SF exposure. Neither prenatal nor postnatal PS was significantly related to RSA reactivity during the last SF.
RSA recovery from SF1
Although several of the coefficients within the models were magnitudes of .25 or greater, none reached significance in the prediction of RSA recovery. This is likely because of the substantial reduction in sample size due to infant distress from the SF1 episode and the need to discontinue the task with those infants.
Post hoc examination of the interaction between objective and perceived stress
Although not originally planned, examination of the findings and consideration of literature on coping during pregnancy (Guardino & Schetter, Reference Guardino and Schetter2014) led us to wonder about the possible synergistic association of high objective exposure count coupled with high PS with offspring reactivity. We therefore conducted post hoc tests for interaction effects in the prediction of the infant outcomes. Tests of the interaction between SLE count and prenatal PS were not significant in the prediction of the temperament outcomes or in the prediction of RSA recovery. Follow-up analyses revealed a significant interaction effect between prenatal PS and SLE in relation to RSA reactivity to last SF (β = –0.33, p < .05), and inclusion of this interaction term explained an additional 9% of the variance in RSA reactivity (R 2 = 26%, relative to 17%). Using the approach outlined by Aiken and West (Reference Aiken and West1991), we examined the relationship between SLE and RSA at selected values of PS, average PS and ±1 SD (see Figure 1a). The tests of the simple slope for the sample average PS (b = –0.66, p < .001) and higher PS (b = –1.11, p < .001) indicated significant inverse relations with RSA reactivity. The slope between SLE and RSA reactivity was not significant at lower levels of PS (b = –0.21, p = 0.31). An alternative and complimentary approach allows us to precisely compute the boundaries of moderating effect in which a significant slope between our SLE and RSA is found (Preacher, Curran, & Bauer, Reference Preacher, Curran and Bauer2006). Examining the range of PS within this “regions of significance” framework (see Figure 1b) confirmed that SLE significantly predicts RSA reactivity for the majority of the sample: when PS scores (centered) are greater than –.42 (this is slightly less than 1 SD below the sample mean).

Figure 1. (Color online) (a) The interaction between stressful life events and perceived stress in the prediction of respiratory sinus arrhythmia reactivity, plotted at three levels of perceived stress. (b) The regions of significance for this interaction.
Discussion
The findings of our study suggest that variation in maternal psychological stress during pregnancy in a population of racially and ethnically diverse low-income women is prospectively associated with infant reactivity and regulation at 6 months of age, and that effects persist after adjusting for postpartum maternal stress and other key covariates during the postnatal period. The pattern varied by whether the measure of maternal stress was “objective” (exposures) or “subjective” (appraisals), and also by whether the measure of infant reactivity and regulation was based on maternal perception or infants' physiological responses to a standardized stressor. Overall, mothers who perceived themselves as being more stressed during pregnancy and postpartum reported that their infants were higher in temperamental surgency and had lower self-regulatory abilities, adjusting for exposure to SLE during pregnancy. These ratings of PS were unrelated to infant PNS stress reactivity and recovery. In contrast, higher counts of SLE during pregnancy were associated with greater infant PNS reactivity. Interaction findings suggest that the average effect of SLE on offspring physiology was significant, but that it was particularly salient among offspring of women with moderate to high levels of PS (i.e., PS appeared to moderate the effect of life event stress on offspring physiology). The findings are novel, in that there is relatively little data examining the unique contributions of both objective and perceived stress effects on offspring reactivity, and the majority of the few studies reporting tests of prenatal stress programming effects on offspring PNS reactivity have involved advantaged, Caucasian, non-US samples.
Given the uniqueness of the study population and the novelty of findings related to infant ANS reactivity, we focus our discussion first on these findings. Our ANS findings parallel those of Rash et al. (Reference Rash, Campbell, Letourneau and Giesbrecht2015), who found that a higher maternal cortisol awakening response (a biological indication of greater stress, as well as other behavioral and biological processes) was associated with greater RSA reactivity for 6-month-old infants during a frustration paradigm. They also found it predicted lower baseline RSA, but that was not replicated in our study. Although more difficult to compare due to their use of multisystem profiles, Rash, et al.’s (Reference Rash, Thomas, Campbell, Letourneau, Granger, Giesbrecht and Team2016) finding from the same sample is also consistent with ours in that their mothers with relatively greater psychological distress during late pregnancy (in combination with decreasing daytime sAA slopes) were more likely to have infants who exhibited “coinhibition” of SNS and PNS during the stressors. Our findings are in contrast to Suurland et al. (Reference Suurland, van der Heijden, Smaling, Huijbregts, van Goozen and Swaab2016), who found that a cumulative psychosocial risk score (including maternal psychiatric status, substance abuse, maternal education, marital status, social support, and maternal age) was not associated with infant RSA reactivity in the same infant stress paradigm used here. Instead, they found “higher risk” infants demonstrated greater RSA withdrawal during the recovery phase, suggesting they were less able to regulate after the stressor. Discrepancies in Suurland et al. may be due to the large differences in the constructs tapped by their “stress” measure, the inability to tease apart effects of pre- and postnatal exposure, or their sample with more limited adversity. It is notable that these three papers resulted from two low-risk, Caucasian samples residing within countries with exceptional social services for pregnant women and mothers. In our sample of pregnant women experiencing high levels of adversity (recall 84% reported at least one major SLE during gestation; 47% reported three or more events), variation in adversity exposure predicted variation in offspring physiological reactivity, after adjusting for concurrent report of maternal stress.
This reactivity finding complements the more robust evidence base for prenatal programming of maternal stress effects on infant resting levels of PNS functioning and integrated measures of ANS functioning such as HR, which has been conducted on more diverse samples with broad ranges of adversity. It also suggests that associations demonstrated between maternal prenatal experience and fetal PNS (e.g., DiPietro, Costigan, & Gurewitsch, Reference DiPietro, Costigan and Gurewitsch2003; Sandman et al., Reference Sandman, Glynn, Wadhwa, Chicz-DeMet, Porto and Garite2003) mark physiological impacts that appear to be sustained postnatally, at least through 6 months of age.
There is a broader evidence base for prenatal programming effects than was the focus here, if measures of depression, anxiety, and other mental health symptomatology are included in the conceptualization of stress. A number of studies have found that infants of depressed or anxious mothers have lower resting PNS activity (Dierckx et al., Reference Dierckx, Tulen, van den Berg, Tharner, Jaddoe, Moll and Tiemeier2009; Field et al., Reference Field, Diego, Hernandez-Reif, Schanberg, Kuhn, Yando and Bendell2003; Jacob et al., Reference Jacob, Byrne and Keenan2009), although others find no association between maternal mood and infant vagal tone (Field et al., Reference Field, Diego, Dieter, Hernandez-Reif, Schanberg, Kuhn and Bendell2001; Kaplan, Evans, & Monk, Reference Kaplan, Evans and Monk2008). DiPeitro et al. (Reference DiPietro, Novak, Costigan, Atella and Reusing2006) found that adding depression and anxiety to a composite score with stress clouded the unique effect of PS on child vagal tone. We did not find that pre- or postnatal depression was associated with our outcomes, and those variables were dropped from final models to preserve power. Rash et al. (Reference Rash, Campbell, Letourneau and Giesbrecht2015) also reported that maternal depression in early or late pregnancy did not predict infant RSA. Although related, the physiological consequences of stress can be different than those of depression (Gold & Chrousos, Reference Gold and Chrousos2002), and findings here suggest that they may have different patterns of transmission to the fetus, at least in terms of ANS development.
In terms of stress paradigm methodology, our data are consistent with that of the two other studies we are aware of that have used two SF episodes to elicit RSA responses (Bosquet Enlow et al., Reference Bosquet Enlow, King, Schreier, Howard, Rosenfield, Ritz and Wright2014; Ritz et al., Reference Ritz, Bosquet Enlow, Schulz, Kitts, Staudenmayer and Wright2012). Our results were similar in that infants demonstrated PNS withdrawal to the SF episodes (with stronger reductions during the second SF) and some PNS recovery during reunion without full return to the original level during play. Other studies have found that infants from high-risk populations did not recover from the SF during reunion (Conradt & Ablow, Reference Conradt and Ablow2010), or experienced even lower RSA in the reunion (Suurland et al., Reference Suurland, van der Heijden, Smaling, Huijbregts, van Goozen and Swaab2016), suggesting that physiological effects of stress can be sustained, at least for a short while.
Our findings regarding maternal report of temperament are theoretically consistent with extant literature (Bosquet Enlow et al., Reference Bosquet Enlow, Kullowatz, Staudenmayer, Spasojevic, Ritz and Wright2009; Davis et al., Reference Davis, Glynn, Waffarn and Sandman2011; Sandman et al., Reference Sandman, Davis and Glynn2012), in that greater maternal pregnancy stress and postpartum stress have been associated with more difficult infant temperaments (such as high surgency and low regulation, found here), except that maternal stress did not predict negativity, which appears to be the most commonly documented association. Higher PS scores have been correlated with higher levels of cortisol (Pruessner, Hellhammer, & Kirschbaum, Reference Pruessner, Hellhammer and Kirschbaum1999); poor eating, drinking, and sleeping practices (Cohen & Williamson, Reference Cohen and Williamson1988; Gibson, Reference Gibson2006); and general health behaviors during pregnancy (Guardino & Schetter, Reference Guardino and Schetter2014), which can affect fetal development. In the prediction of infant regulation in our study, the effects of prenatal stress were larger than those of postnatal stress and the prenatal stress model accounted for 6% more of the variance, so it is tempting to infer that prenatal exposure to maternal PS is particularly relevant. Although important to examine perceptions, the stability of maternal report of PS across pregnancy and the postnatal period within this highly stressed sample may not be optimal for discerning prenatal from postnatal effects, as it prevented optimal modeling for determination of which exposure period was most important.
Extant theoretical and empirical literature suggests the timing of stress exposure is important for prenatal programming. Rash et al. (Reference Rash, Campbell, Letourneau and Giesbrecht2015) found that maternal total cortisol assessed at 14 weeks of gestation, but not 32 weeks, was positively associated with infant RSA reactivity, and suggest that the effects of maternal cortisol on infant vagal tone appear to be sensitive to timing. Our assessment of exposure to SLE during pregnancy did not allow for determination of exposure timing. However, PS was assessed at two different time points during pregnancy (roughly 8 weeks apart). Although results were not presented here, exploratory analyses showed that the coefficients for “average prenatal stress” were stronger than those for either time point alone.
Limitations and strengths
In addition to the limitations described above, other factors merit consideration when interpreting findings presented here. First, although our sample size was larger than that of many ANS studies with infants (Bosquet Enlow et al., Reference Bosquet Enlow, King, Schreier, Howard, Rosenfield, Ritz and Wright2014; Feldman, Singer, & Zagoory, Reference Feldman, Singer and Zagoory2010; Moore, Reference Moore2010; Ritz et al., Reference Ritz, Bosquet Enlow, Schulz, Kitts, Staudenmayer and Wright2012), funding time lines led to a relatively small sample, and a larger sample size is desirable. Second, the self-report measures of maternal stress and offspring temperament introduce potential bias and minimize confidence in those findings, yet others have found similar patterns using more objective measures of temperament. The setting for the assessment data described here also presents a possible limitation in that roughly half of assessments were completed in participant homes and the others were completed in our laboratory. This potential limitation is balanced by the successful completion of data collection with participants who were unable or unwilling to travel to our lab. Further, analyses revealed no difference in RSA values by home or clinic, as has been found in other home/clinic infant ANS studies (Haley, Handmaker, & Lowe, Reference Haley, Handmaker and Lowe2006). Third, as our focus was on understanding these phenomena within a multiethnic sample of low-income women, our study population did not have a full range of stress levels; specifically, it included few women with low levels of exposure to major adverse events. Despite this narrowed range, there was considerable variation in both of our stress predictors, and associations with offspring development were found.
These limitations are offset by a range of important study strengths. This study is one of few that examine infant RSA reactivity, and we used a gold-standard stress reactivity paradigm to assess reactivity and regulation. The study was conducted in a racially and ethnically diverse sample with a high level of exposure to life stressors, a population that is understudied and at increased risk for adverse infant development, including psychopathology. Moreover, the inclusion of both counts of adverse exposures and repeated measures of perceptions of stress provide an opportunity to investigate these unique sources of stress in vulnerable populations with complex challenges.
Implications and future directions
The specific role of PNS functioning within the etiology of early life psychopathology is still being understood, but weak PNS withdrawal to challenging contexts during infancy and early childhood has been shown to predict internalizing and externalizing symptoms; high levels of resting PNS activation and flexible withdrawal of the PNS in challenging contexts during early infancy and childhood have been shown to predict better regulation of attention and affect and more optimal social functioning (Beauchaine, Reference Beauchaine2001, Reference Beauchaine2015; Beauchaine, Gatzke-Kopp, & Mead, Reference Beauchaine, Gatzke-Kopp and Mead2007; Boyce et al., Reference Boyce, Quas, Alkon, Smider, Essex and Kupfer2001; Calkins & Keane, Reference Calkins and Keane2004; Graziano & Derefinko, Reference Graziano and Derefinko2013), although this can vary by sample type (Graziano & Derefinko, Reference Graziano and Derefinko2013). The greater RSA withdrawal demonstrated by infants born to mothers with higher levels of exposure to adverse events may actually be adaptive, preparing the offspring for flexible responding to a stressful environment. Moreover, RSA reactivity is an important marker of biological sensitivity to context in which a highly reactive child is more sensitive to both positive and negative environments (see Bush & Boyce, Reference Bush, Boyce and Cicchetti2016), so understanding potential prenatal sources of influence for PNS development is an important goal for the field. Chronic exposure to stress (in utero and postnatally) with concomitant high PNS reactivity may have long-term consequences for infant stress regulation across the life course.
We found a very high level of exposure to major life stressors and high levels of reported PS among our low-income sample of pregnant women, yet the two measures were only weakly (and nonsignificantly) correlated. It is striking that only maternal self-report of PS predicted her report of infant temperament, whereas only the more objective measure of maternal exposure to adverse events predicted infant stress physiology, particularly as both stress measures were included in all models. It is possible that in a sample of women with limited access to financial and other resources, some mothers experiencing high levels of adverse major life events may have habituated to such events and may not perceive exposures as distressing or may choose to underreport their level of stress. Minimizing acknowledgement of stress may be adaptive for high-risk populations or part of a cultural context (Kuo, Reference Kuo2014). Some mothers may also have had sufficient support and coping skills to maintain a sense of calm in the face of adversity.
Our data, at first glance, suggest that exposure to SLE impacts offspring PNS reactivity, and maternal perceptions of stress are not relevant. However, the interaction found reveals the potential that the effect of exposure to adverse events is only significant for mothers with moderate to high levels of PS. Although distinction between various components or dimensions of psychological stress as discrete entities may be clarifying, it is also necessary to recognize that objective stressors and psychological stress often co-occur and can be interrelated. The impact of an acute circumstance, such as a SLE (e.g., death of a family member), varies considerably across individuals in the nature, intensity, and duration of its psychological and physiologic consequences. This variation in impact of adverse exposures is likely to depend upon many factors, including whether they occur in the context of a period of chronic psychological distress (e.g., if the death of the family member occurred in the midst of an ongoing contentious divorce) or in the context of low levels of stress (perhaps due to secure housing, high levels of social support, and adaptive coping skills). Accordingly, and based on the precedent from literature reviewed earlier, there are strong arguments for why examining the combination of perception and exposure might reveal distinct patterns of association with infant developmental outcomes. Nevertheless, we are cautious in interpreting one significant interaction out of six tested, but such a pattern, if replicated, points to the possibility of providing resources to reduce the experience of stress or improve adaptive coping for pregnant women exposed to adverse events (Guardino & Schetter, Reference Guardino and Schetter2014), as a means of minimizing impact on the fetus. That said, as noted above, a more reactive PNS may be adaptive in a variety of stressful and optimal contexts, and such efforts should not be made without a deeper understanding of these phenomena.
Our findings add to the evidence demonstrating that stressful events and maternal levels of PS during pregnancy are associated with infant temperament and PNS functioning. This has a variety of potential clinical implications. Stress exposures during pregnancy should be evaluated and monitored, and findings here suggest they merit intervention to improve public health. The American College of Obstetrics and Gynecology recommends screening for psychosocial stressors (American College of Obstetrics and Gynecologists Committee on Health Care for Undeserved Women, 2006) and depression (Siu et al., Reference Siu, Bibbins-Domingo, Grossman, Baumann, Davidson and Pignone2016) to identify severe cases. In 2016, the United States Preventive Service Task Force recommended depression screening for all pregnant and postpartum women along with provision of adequate systems of care to provide treatment for those who screen positive. Many states and federal programs across the country such as the Comprehensive Perinatal Services Program recognize the importance of psychosocial stress and provide extensive screening as a part of routine prenatal care. Pregnant women experiencing economic hardships and SLE likely need multifaceted support including accessible and integrated assistance for their social and healthcare needs in order to have optimally healthy pregnancies.
In light of the moderately high stability of PS across the pre- and postnatal period (at least in our sample), and the high probability that women experiencing significant environmental adversity during pregnancy will continue to be at high risk for exposures after the birth of their child, consideration of intervention need not be restricted to the pregnancy period. Interventions to support low-income, highly stressed women postpartum are also likely to be good investments. For example, in families with a history of neglecting their infants, child–parent psychotherapy (CPP) and the Psychoeducational Parenting Intervention have been shown to decrease maternal perceived parenting stress, and, for families receiving CPP, those reductions in maternal stress were associated with more adaptive regulation in maternal basal cortisol (Toth, Sturge-Apple, Rogosch, & Cicchetti, Reference Toth, Sturge-Apple, Rogosch and Cicchetti2015). Such improvements in maternal psychological and physiological function may serve as mechanisms for the demonstrated CPP and Psychoeducational Parenting Intervention effects on children, such as normalized infant hypothalamus–pituitary–adrenal axis regulation across infancy and early childhood (Cicchetti, Rogosch, Toth, & Sturge-Apple, Reference Cicchetti, Rogosch, Toth and Sturge-Apple2011), and point to potential preventative interventions that may improve infant ANS physiologic functioning as well.
In addition, it is important to consider the critical importance of the quality of the mother–child relationship (attachment) in postnatal life for a diverse set of mental and physical health outcomes (Bakermans-Kranenburg & van IJzendoorn, Reference Bakermans-Kranenburg and van IJzendoorn2009; Cassidy, Jones, & Shaver, Reference Cassidy, Jones and Shaver2013; Jones-Mason, Allen, Bush, & Hamilton, Reference Jones-Mason, Allen, Bush and Hamilton2016). The quality of this relationship and the experience of parenting is dependent on not only what the mother brings to the interaction (which can be influenced by her levels of stress, among many other things) but also what the child brings to the interaction; during infancy, this predominantly consists of her or his temperament. Although a comprehensive literature review on the issue concluded that attachment relationships cannot explain individual differences in temperament and visa versa (Vaughn, Bost, & van IJzendoorn, Reference Vaughn, Bost, van IJzendoorn, Cassidy and Shaver2008), empirical evidence suggests parenting might impact infant reactivity and regulation. For example, Haley and Stanbury (Reference Haley and Stansbury2003), using procedures similar to those used in this study, found that infants with more responsive parents demonstrated HR recovery during the SF reunion episode while infants with less responsive parents showed increased HR during that transition. Bosquet Enlow et al. (Reference Bosquet Enlow, King, Schreier, Howard, Rosenfield, Ritz and Wright2014) found that the infants of mothers who were insensitive during play episodes show lower levels of RSA and higher levels of infant affective distress throughout the SFP. In a lower risk sample, high maternal sensitivity predicted a decrease in infant RSA from baseline to reunion (Moore et al., Reference Moore, Hill-Soderlund, Propper, Calkins, Mills-Koonce and Cox2009), whereas in a high-risk sample maternal sensitivity during reunion was found to be associated with an increase in RSA during reunion (Conradt & Ablow, Reference Conradt and Ablow2010). Accordingly, the postnatal environment, and particularly the attachment relationship, may play a significant role in shaping infant reactivity. RSA has also been shown to interact with parenting behaviors to impact the attachment relationship; Holochwost et al. (Reference Holochwost, Gariepy, Propper, Mills-Koonce and Moore2014) posited that high infant RSA confers environmental sensitivity and found an interaction between levels of RSA (during play and reunion) and maternal negative intrusiveness coded at 6 months of age predicted disorganized attachment at 12 months. Such findings suggest that effects of maternal prenatal stress on infant ANS function may make infants more or less vulnerable to differences in parenting after birth, and provide additional support for the need to consider the context when inferring the adaptive nature of reactivity and regulation. Regardless of maternal factors, infant temperament can affect parental mood and levels of stress. Collectively, to the extent that infant development is partly shaped, by prenatal influences such as maternal stress, the effect of maternal stress during pregnancy on infant biological or behavioral reactivity and regulation could impact the quality of one of the most important postnatal determinants of child health and well-being.
In conclusion, future research in this area should consider inclusion of both objective and subjective measures of maternal stress and both reports and biological measures of child functioning as each can provide different insight and different opportunities for intervention strategies (Cicchetti & Gunnar, Reference Cicchetti and Gunnar2008). It also appears important to distinguish the impact of mood from stress and to advance evidence within populations more representative of our nation's racial/ethnic and socioeconomic composition. Finally, it is critical that this field unpacks the complex concepts of stress, which exists across a continuum and is a normal part of human experience. For example, Glynn and Sandman (Reference Glynn and Sandman2011) articulate the potential importance of prenatal hormone exposures in programing mothers' own brain structure and function (and resultant behavior and mood), in preparation for motherhood. It will be important to understand whether stress and stress hormones during pregnancy are important for a mother's own readiness for and adaptation to pregnancy and parenting within her own environment. In addition, some studies suggest moderate distress during pregnancy can be associated with better offspring mental and psychomotor development (DiPietro et al., Reference DiPietro, Novak, Costigan, Atella and Reusing2006), particularly when levels of adversity during and after pregnancy are congruent (Sandman, Davis, & Glynn, Reference Sandman, Davis and Glynn2012). Greater understanding of contexts and thresholds for maladaptive effects is needed, particularly as our societal structural is not likely to provide “stress-free pregnancies” for most individuals. This type of clarification will contribute to a deeper understanding around the interaction between the effects of prenatal and postnatal experience, and advance understanding of multilevel mechanisms of the effects of adversity on offspring development. If the notion that prenatal and early experience have lifelong health consequences for risk of psychopathology and physical health is correct, then advancing our understanding in these areas will support the development of public-health scale preventative interventions.







