Automated room disinfection technologies are increasingly being used as an adjunct to standard cleaning and disinfection in healthcare facilities. Ultraviolet (UV) radiation devices have been most widely adopted owing to the efficiency and well-documented efficacy of UV irradiation.Reference Conner-Kerr, Sullivan, Gaillard and Jones 1 – Reference Owens, Deal and Shoemaker 7 Several UV room disinfection devices are now being marketed. Most of these devices use low pressure mercury gas bulbs, but recently pulsed xenon flash bulbs have also been incorporated into disinfection systems. UV radiation has peak germicidal effectiveness in the wavelength range from 240 to 280 nm.Reference Conner-Kerr, Sullivan, Gaillard and Jones 1 – Reference Owens, Deal and Shoemaker 7 Mercury gas bulbs primarily emit UV-C at 254 nm, whereas xenon gas bulbs produce a broad spectrum of radiation that encompasses the UV (100–280 nm) and visible (380–700 nm) spectra.Reference Jinadatha, Quezada, Huber, Williams, Zeber and Copeland 8 – Reference Umezawa, Asai, Inokuchi and Miyachi 12 The UV-C radiation emitted by low pressure mercury bulbs is delivered in a continuous stream that gradually accumulates to lethal doses depending on duration of exposure and distance from the primary field of radiation.Reference Conner-Kerr, Sullivan, Gaillard and Jones 1 – Reference Owens, Deal and Shoemaker 7 The broad-range UV delivered by xenon bulbs is emitted in short, high-intensity pulses, possibly requiring a shorter duration of exposure to achieve lethal doses.Reference Jinadatha, Quezada, Huber, Williams, Zeber and Copeland 8 – Reference Umezawa, Asai, Inokuchi and Miyachi 12
Given the increasing use of UV devices and variations in recommended cycle times, there is a need for evaluations of the real-world performance and comparative effectiveness of different devices. We previously demonstrated that a mobile, automated room disinfection device that utilizes mercury bulbs for emitting UV-C radiation is effective for reducing the frequency of positive methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant Enterococcus (VRE), and Clostridium difficile culture results on high-touch surfaces in hospital rooms (Tru-D Rapid Room Disinfection device; Lumalier).Reference Nerandzic, Cadnum, Pultz and Donskey 13 For disinfection of vegetative bacteria and C. difficile spores, the manufacturer recommends cycles in standard hospital rooms of approximately 15 and 45 minutes, respectively. Here we examined the effectiveness of a mobile, automated pulsed xenon ultraviolet (PX-UV) device (Xenex; Xenex Disinfection Services) at a substantially shorter disinfection cycle (10 minutes, as suggested by manufacturer). The efficacy of the device was assessed for killing of MRSA, VRE, and C. difficile spores on carriers placed in hospital rooms and for reducing naturally occurring contamination on high-touch surfaces in hospital rooms.
METHODS
C. difficile, MRSA, and VRE Strains
Two clinical isolates each of C. difficile, MRSA, and VRE were studied. The MRSA strains were a pulsed-field gel electrophoresis type USA300 and USA800. The VRE strains were a VanA-type isolate (C37) and a VanB-type isolate (C68). The C. difficile strains were VA 17, a restriction endonuclease analysis type BI strain, and VA 11, a restriction endonuclease analysis type J strain.
Preparation of C. difficile Spores
Spores were prepared as previously described.Reference Sorg and Sonenshein 14 Spores were stored at 4°C in sterile distilled water until use. Prior to testing, spore preps were confirmed by phase contrast microscopy and malachite green staining to be at least 99% dormant, bright-phase spores.
Microbiology
VRE, MRSA, and C. difficile were cultured on selective media as previously described.Reference Nerandzic, Cadnum, Pultz and Donskey 13 , Reference Nerandzic and Donskey 15 For specimens collected with sterile, premoistened swabs, the swabs were applied directly to the surface of the appropriate selective agar. To detect lower levels of C. difficile spores, 10 mL of pre-reduced cycloserine-cefoxitin-brucella broth containing 0.1% taurocholic acid and lysozyme 5 mg/mL (CDBB) was poured into a sterile culture tube containing specimens collected with sterile gauze pads.Reference Nerandzic and Donskey 15 Positive broth cultures were subcultured onto selective agar for identification of C. difficile. To quantify total heterotrophic bacteria, swabs were plated on trypticase soy agar containing 5% sheep blood and incubated at 37°C for 48 hours. VRE and MRSA colonies with unique morphology were subjected to identification and susceptibility testing in accordance with Clinical Laboratories Standards Institute guidelines. 16 C. difficile was confirmed on the basis of odor and appearance of colonies and by a positive reaction using C. difficile latex agglutination (Microgen Bioproducts).
The Pulsed Ultraviolet Disinfection Device
Figure 1 is a photograph of the PX-UV device (Xenex; Xenex Disinfection Services). The device contains a xenon gas flash bulb that operates at 2 Hz and emits a broad spectrum of radiation covering the UV-C spectrum of 200 to 280 nm as well as the visible light spectrum. The device is designed for manipulation by a single operator and is approximately 1.6 ft wide by 2.3 ft long by 3.3 ft high and weighs 150 pounds. It is operated remotely outside the room and includes motion sensors, which turn off the device if the door is opened. The device is wheeled into a strategic position located near high-touch surfaces in the room and set to irradiate for 5 to 7 minutes as suggested by the manufacturer. Then, the device is wheeled to a second location in the room and run for an additional 5 to 7 minutes. The disinfection process takes approximately 15 to 20 minutes, which includes setup, radiation cycles, and repositioning.

FIGURE 1 Photograph of the Xenex pulsed xenon ultraviolet device.
The Impact of Pathogen Concentration and Organic Load on the Efficacy of PX-UV Disinfection on Carriers in Hospital Rooms
Initial experiments were conducted to determine whether pathogen concentration (ie, colony-forming units [CFU] per cm2) or organic load influenced the disinfection efficacy of the PX-UV device. Ten µL aliquots of C. difficile spores, MRSA, and VRE suspended in phosphate buffered saline were inoculated onto glass microscope slides and spread to cover a 1-cm2 area. For each pathogen, the inoculum applied to the slide was adjusted such that 2 to 5 or more log10CFU/cm2 were recovered from the positive control specimens after desiccation. For a subset of samples, the organisms were suspended in 5% fetal calf serum.
The slides were placed on a table positioned centrally over the bed in a hospital room, 4 feet within the direct field of radiation delivered by the PX-UV device. Baseline slides were left untreated outside of the room (ie, positive controls). The PX-UV device was run for a total of 10 minutes, 5 minutes on the left side of the bed and 5 minutes on the right, as suggested by the manufacturer and standard protocol in the facility utilizing the device.
To quantify viable organisms, the slides were submersed in 25 mL of sterile phosphate buffered saline and vortexed vigorously, and dilutions of the suspensions were plated onto selective media. Following 48 hours of incubation, log10CFU reductions were calculated by comparing the log10CFU recovered from slides after PX-UV disinfection to untreated controls. All experiments were performed 3 times.
The Impact of Distance on the Efficacy of PX-UV Disinfection on Carriers in Hospital Rooms
The killing efficacy of the PX-UV device was evaluated at increasing distances from the primary field of radiation. Slides were prepared and processed as described previously. However, the inoculum was altered such that each glass slide yielded 5 log10 CFU at baseline. Additionally, slides were placed 6 inches, 4 feet, and 10 feet within the direct field of radiation, and also 4 feet shaded from direct radiation (under bedside table).
Comparison of Pulsed Xenon Versus Continuous Mercury UV for Killing of Pathogens
We compared the efficacy of PX-UV versus UV-C delivered by mercury bulbs for reduction of pathogens inoculated onto slides in similarly sized hospital rooms. This comparison was performed in separate facilities because the devices were housed in separate hospitals. The experiments were performed in similar rooms with equivalent dimensions, and the experimental samples were placed at the same distances from the UV devices. Slides were prepared as described previously; the inoculum was altered such that each glass slide yielded 5 log10 CFU at baseline. The slides were placed 4 feet from each device within the direct field of radiation. The UV-C was delivered by the Tru-D device (Lumalier); each device was run for a total of 10 minutes. Slides were processed as described previously. The experiments were performed 3 times.
Disinfection of Environmental Surfaces in Hospital Rooms
The efficacy of the PX-UV device was assessed in rooms (~10×20 feet) of discharged patients in a tertiary care facility. In phase 1, the PX-UV device was run in rooms that had not yet been cleaned. In phase 2, the device was run after standard terminal cleaning by environmental services personnel that included use of bleach for high-touch surfaces in all discharge rooms; a subset of the rooms had previously been occupied by patients with C. difficile infection. Swabs and gauze pads premoistened with saline were used to collect cultures for MRSA, VRE, C. difficile, and total heterotrophic bacteria from high-touch surfaces (ie, call light, bedside table, telephone, chair, intravenous poles, portable keyboards, and bed rail) before and after use of the PX-UV device for 10 minutes (5 minutes on each side of the bed). An approximately 10×10-cm area was cultured before PX-UV disinfection and adjacent areas of the same size were cultured after disinfection. Specimens were cultured and identified as described previously.
Data Analysis
Data were analyzed using STATA, version 9.0 (StataCorp). Continuous data were analyzed using paired t tests and categorical data were assessed using the Fisher exact test.
RESULTS
Figure 2 shows the mean log10CFU/cm2 reductions of 2 strains of C. difficile, MRSA, and VRE on glass slides after the use of the PX-UV device. There were no significant differences between the log10CFU reductions of the 2 strains of each pathogen tested. Therefore, in subsequent experiments, data for the 2 strains was pooled. Pathogen concentration did not have a significant impact on the killing efficacy of the PX-UV device. Irradiation delivered 4 feet from the PX-UV device for 10 minutes reduced C. difficile spores by 0.55±0.34 log10CFU/cm2, MRSA by 1.85±0.49 log10CFU/cm2, and VRE by 0.6±0.25 log10CFU/cm2. Organic load (5% fetal calf serum) did not significantly impact the efficacy of the PX-UV device (data not shown).

FIGURE 2 The effect of pathogen concentration on the efficacy of the pulsed xenon ultraviolet (PX-UV) device.
The log10CFU reduction/cm2 of 2 strains each of Clostridium difficile spores, methicillin-resistant Staphylococcus aureus (MRSA), and vancomycin-resistant Enterococcus (VRE) inoculated onto carriers is shown. Carriers contained either >5, ≤5 and >3, or ≤3 log10CFU of each pathogen. The carriers were irradiated for 10 minutes at a distance of 4 feet from the PX-UV device. The means of the data from experiments conducted in triplicate are presented. Error bars indicate standard error.
As shown in Figure 3, the efficacy of PX-UV decreased as distance from the device increased. For each pathogen, significantly less reduction was achieved at 4 feet versus 6 inches and at 10 feet versus 4 feet (P<.05 for each comparison). At 4 feet from the device, shading the organisms from the direct field of radiation did not have a significant impact on efficacy (P>.05 for each comparison). At 10 feet from the device, the log10CFU reduction was less than 1 log10CFU/cm2 for each pathogen.

FIGURE 3 The effect of distance on the efficacy of the pulsed xenon ultraviolet (PX-UV) device.
The log10CFU reduction/cm2 of Clostridium difficile spores, methicillin-resistant Staphylococcus aureus (MRSA), and vancomycin-resistant Enterococcus (VRE) at increasing distances and shaded from the direct field of radiation delivered by the PX-UV device is shown. Carriers contained 5 log10CFU of each pathogen. The carriers were irradiated for 10 minutes at a distance of 6 in, 4 feet, 4 feet shaded, and 10 ft from the PX-UV device. The means of the data from experiments conducted in triplicate are presented. Error bars indicate standard error.
Figure 4 shows the mean log10CFU/cm2 reductions of C. difficile, MRSA, and VRE on slides after the use of the UV-C and PX-UV devices for 10 minutes at a distance of 4 feet from the devices. The UV-C device achieved significantly greater log10CFU reductions than the PX-UV device (P<.001 for each pathogen).

FIGURE 4 The efficacy of pulsed xenon ultraviolet (PX-UV) versus continuous mercury UV-C for killing of pathogens.
A comparison of the log10CFU reduction/cm2 of Clostridium difficile spores, methicillin-resistant Staphylococcus aureus (MRSA), and vancomycin-resistant Enterococcus (VRE) by PX-UV and continuous mercury UV-C is shown. Carriers contained 5 log10CFU of each pathogen. The carriers were irradiated for 10 minutes at a distance of 4 feet from the devices. The means of the data from experiments conducted in triplicate are presented. Error bars indicate standard error.
Table 1 provides a summary of the results of 2 phases of PX-UV disinfection on high-touch surfaces in hospital rooms. For 16 rooms that were cultured before and after use of PX-UV without cleaning (phase 1), PX-UV resulted in statistically significant reductions in the percentages of sites positive for each of the 3 pathogens, the number of CFU recovered for each pathogen, and the heterotrophic plate counts. For 24 rooms that were cultured before and after standard cleaning plus PX-UV (phase 2), there were also statistically significant reductions in percentages of sites positive for each of the pathogens, the number of CFU recovered, and the heterotrophic plate counts.
TABLE 1 Clostridium difficile, Methicillin-Resistant Staphylococcus aureus (MRSA), Vancomycin-Resistant Enterococcus (VRE), and Total Heterotrophic Plate Counts (HPC) on Hospital Surfaces before and after Pulsed Xenon Ultraviolet (PX-UV) Disinfection
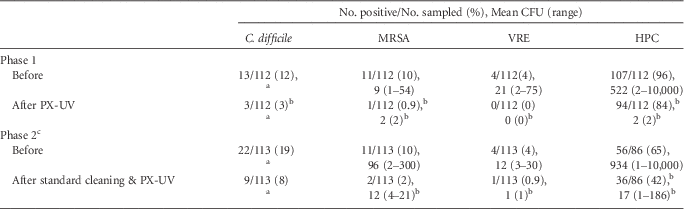
NOTE. CFU, colony-forming units.
a Broth enrichment positive only, no data available for mean CFU.
b Indicates a significant reduction, P<.01.
c In phase 2, a total of 42% of rooms housed patients with C. difficile infection (in phase 1, no rooms housed such patients).
DISCUSSION
We found that the PX-UV device reduced recovery of MRSA, C. difficile, and VRE on carriers and on frequently touched surfaces in hospital rooms with a 10-minute exposure time. Increasing the distance from the device dramatically reduced the killing efficacy of PX-UV irradiation, whereas pathogen concentration, organic load, and shading from the direct field of radiation did not. PX-UV was less effective than continuous UV-C in reducing pathogen recovery on glass slides with a 10-minute exposure time in similar hospital rooms.
Our findings are consistent with previous studies demonstrating the efficacy of PX-UV disinfection for reduction of VRE, MRSA, and heterotrophic bacteria from surfaces in healthcare facilities.Reference Jinadatha, Quezada, Huber, Williams, Zeber and Copeland 8 , Reference Stibich, Stachowiak and Tanner 11 In addition, our study provides 2 assessments not included in previous publications on PX-UV (ie, log reductions achieved by PX-UV on carriers and a comparison with continuous UV). Although the log reductions achieved by PX-UV on carriers at 10 minutes were relatively modest, this exposure time was sufficient to reduce contamination on real-world surfaces. We have previously demonstrated that contaminated surfaces in hospital rooms yield relatively low concentrations (<1–3 log10CFU per site sampled using swabs) of healthcare-associated pathogens.Reference Nerandzic, Cadnum, Pultz and Donskey 13 , Reference Nerandzic, Cadnum, Eckart and Donskey 17 This observation is corroborated in the current study and may contribute to the efficacy of the PX-UV device in real-world settings.
The PX-UV device has some important potential advantages over other UV disinfection devices. First, unlike continuous UV-C devices, xenon flash lamps do not contain mercury. Therefore, there are no safety hazards associated with disposal or exposure to mercury. Second, the manufacturer recommends a relatively brief disinfection cycle (10–20 minutes per room versus up to 45 minutes for spore-killing cycles of some UV-C devices) which may facilitate greater use of the devices. However, our results suggest that continuous UV-C devices might be similarly effective or more effective than PX-UV with a 10-minute exposure time. Moreover, previous studies have demonstrated that optimal killing of C. difficile spores by UV-C is likely to be achieved with longer cycle timesReference Nerandzic, Cadnum, Pultz and Donskey 13 , Reference Nerandzic, Fisher and Donskey 18 ; for 2 continuous UV-C devices, reductions in C. difficile spores at 10, 20, and 40 minutes of exposure were ~1, 2, and 3 log10CFU, respectively.Reference Nerandzic, Fisher and Donskey 18 Finally, organic load did not impact the efficacy of the PX-UV device. PX-UV has previously been shown to be more effective at penetrating organic load present in waste water than UV-C emitted by low pressure mercury bulbs.Reference Otaki, Okuda, Tajima, Iwasaki, Kinoshita and Ohgaki 10 Thus, it is possible that the organic burden present on real-world hospital surfaces might have less impact on the killing efficacy of PX-UV than UV-C. However, we have previously demonstrated that real-world organic material collected from hospital surfaces only modestly reduced the effectiveness of continuous UV-C for killing of C. difficile spores.Reference Zhang, Nerandzic, Kundrapu and Donskey 19
The PX-UV device also has some potential limitations. The efficacy of PX-UV was dramatically reduced as the distance from the device was increased. Therefore, it is recommended that commonly touched surfaces (eg, bedside table, call button, telephone) be arranged close to the device for optimal exposure to irradiation. Although the PX-UV device reduced contamination on surfaces, residual contamination was not uncommon. In contrast, technologies such as hydrogen peroxide vapor may be more effective in eliminating pathogens.Reference Barbut, Menuet, Verachten and Girou 20 , Reference Boyce, Havill and Otter 21 Further studies are needed to determine whether the level of reduction in contamination provided by the PX-UV device is sufficient to reduce rates of infection. Two recent quasi-experimental studies have reported reductions in rates of C. difficile infection with the use of PX-UV.Reference Levin, Riley, Parrish, English and Ahn 9 , Reference Haas, Menz, Dusza and Montecalvo 22 Randomized trials are needed to determine whether use of PX-UV or other UV devices is effective in reducing infection rates.
Our study has some limitations. First, the use of swabs and direct plating to quantify the concentrations of bacteria is imprecise at higher concentrations. In addition, recovery and release of bacteria from swabs is less than 100% and therefore we may not have detected lower levels of bacteria on surfaces. However, methods were standardized for processing all samples so any limitations in the methodology would be equally shared by baseline and experimental groups. Second, because the PX-UV and continuous UV-C devices were housed in separate hospitals, it was not feasible to perform the comparative evaluation in the same room. We cannot completely rule out the possibility that some of the differences in results for the 2 devices were due to unappreciated differences in room characteristics. However, the experiments were performed in similar rooms with equivalent dimensions, and we found similar results for the PX-UV device when testing was done in 3 different rooms. Finally, for the evaluation of pathogen reduction in hospital rooms, we did not monitor the thoroughness of standard environmental disinfection practices.
Acknowledgments
Financial support. Department of Veterans Affairs (Merit Review grant to C.J.D.) and Agency for Healthcare Research and Quality (grant 11073-H44 to C.J.D).
Potential conflicts of interest. All authors report no conflicts of interest relevant to this article.







