For children with mitral valve disease, mitral valve replacement carries a high mortality, ranging from 10 to 36% Reference Elmahrouk, Mashali and Ismail1,Reference Brancaccio, Trezzi and Chinali2 and poor long-term prognosis, given it often requires redo valve replacement as the child undergoes somatic growth. For this reason, mitral valve repair is often the initial treatment strategy for children with surgical mitral valve disease; however, valve replacement is performed when a durable repair is not anatomically feasible.
Currently, the two options for valve replacement are mechanical and bioprosthetic. Mechanical valves are resistant to structural valve deterioration. Reference Head, Çelik and Kappetein3 The major downside to mechanical valve replacement is the requirement for long-term anticoagulation to prevent valve thrombosis. This is increasingly problematic in female patients who desire to become pregnant, given that the only approved oral anticoagulant is Warfarin, a known teratogen. Reference Economy and Valente4 Additionally, mechanical valve re-replacement rates are higher in paediatric populations, with reported rates in excess of 25%. Reference Ibarra, Spigel and John5 The other option for valve replacement is bioprosthetic valves. These do not require life-long anticoagulation but are commonly associated with higher rates of structural valve deterioration. Reference Head, Çelik and Kappetein3
Currently, there are two leading designs for bioprosthetic valves, valves with bovine pericardial tissue and valves with porcine pericardial tissue. Reference Beute, Goehler and Parker6 Recent studies have found the porcine-based mitral valves to have decreased rates of deterioration and longer time between reoperation. Reference Beute, Goehler and Parker6
Due to the high morbidity and mortality associated with paediatric mitral valve replacement, surgeons have tried various techniques and collaborated with industry in hopes to develop innovative strategies and improved valve options. Our institution is the first in the country to successfully implant the MITRIS RESILIA mitral valve (Edwards Lifescience, Irvine, CA) in a patient with a history of congenital mitral valve disease.
Case
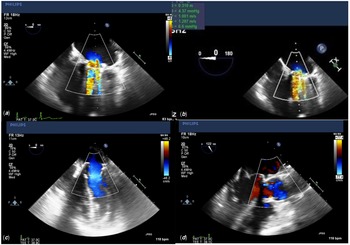
A 20-year-old female presented with exercise intolerance. Her medical history was significant for mitral valve replacement at 6 years of age, with a 23-mm mechanical valve due to severe mitral valve insufficiency secondary to rheumatic heart disease. A thorough pre-operative evaluation revealed mitral valve stenosis. Transthoracic echo demonstrated mitral valve stenosis secondary to pannus formation, effecting both the effective orifice area of her mechanical mitral valve and partially obstructing the mechanical leaflet movements (Fig 1a).
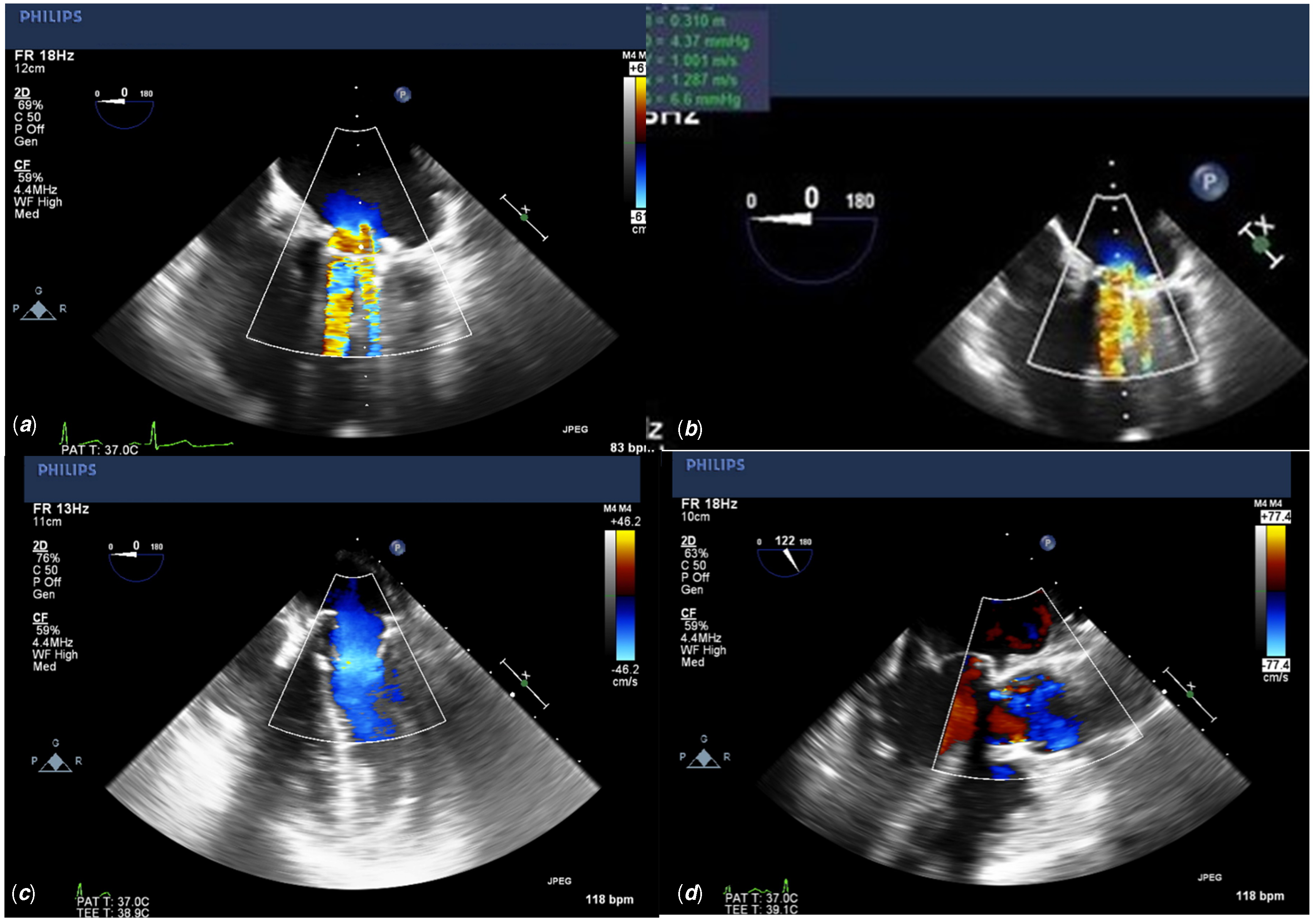
Figure 1. ( a ) Pre-operative transesophageal echo apical 4 chamber view demonstrating a severely dilated left atrium with flow aliasing across the mechanical valve, consistent with mitral stenosis. Echogenic pannus tissue can be seen on the ventricular side of the valve. ( b ) Pre-operative transesophageal echo apical 4 chamber view demonstrating a severely dilated left atrium with flow aliasing across the mechanical valve, consistent with mitral stenosis. Echogenic pannus tissue can be seen on the ventricular side of the valve. ( c ) Post-operative transesophageal echo apical 4 chamber view with colour flow doppler demonstrating a patent MITRIS RESILIA mitral valve with no flow aliasing and no insufficiency. ( d ) Post-operative transesophageal echo parasternal long axis with colour flow Doppler demonstrating an unobstructed left ventricular outflow tract.
After redo sternotomy and bicaval cannulation, her mechanical valve was removed via a transeptal approach. The patient’s valve annulus was sized for a 27-mm MITRIS RESILIA tissue valve. Pledgeted sutures were placed along the underside of the annulus except for the region of mitral aortic continuity where everting sutures pattern was used. The valve was easily seated, and the sutures were secured with COR KNOTS®. The case was done under normothermia with a total cross-clamp time of 52 minutes. The patient was extubated in the operating room, and the post-operative course was unremarkable.
Post-operative transesophageal echocardiogram demonstrated a well-seated valve with good function, no perivalvular leak, no residual mitral stenosis, trivial mitral insufficiency, and the left ventricular outflow tract was unobstructed (Fig 1c, 1d).
Discussion
The Carpentier-Edwards company has a history of safe and effective mitral valves. Reference Bourguignon, Lhommet and El Khoury7 With their latest valve, the MITRIS RESILIA, the Carpentier-Edwards Company has built upon the well-known and effective PERIMOUNT platform, first introduced over four decades ago. This platform boasts three bovine pericardial leaflets, flexible stenting, and a mathematically optimised geometric design. Unique to this valve is the utilisation of RESILIA tissue for leaflet construction. As reported in the COMMENCE trial, patients who received the RESILIA tissue valve and were followed over 5 years demonstrated sustained improvements in transvalvular gradients and effective orifice area, in addition to a 0% incidence of valve thrombosis. Reference Johnston, Griffith and Puskas8 Furthermore, the MITRIS RESILIA valve utilises unique processing and dry storage technology found in other successful Carpentier-Edwards valves such as the INSPIRIS aortic valve, which resists oxidation and calcification of the pericardial leaflets. Reference Ushijima, Kimura and Shiose9 Unlike other valves on market such as the Epic Plus mitral valve (Abbot Laboratories, Chicago IL), this eliminates the need for valve rinsing prior to implantation.
The MITIRS RESILIA valve also showcases all new adaptive technologies. The valve is externally marked along the posteromedial and anterolateral commissures, as well as the anterior valve segment, allowing for fast and easy orientation. Additionally, to facilitate seating the valve within a limited anatomical space, the stents can be temporarily adjusted inward to a 55-degree angle (Fig 2). Given the anatomic limitations of the mitral annulus, the contours of the annular ring have been designed to better fit the geometric shape of the mitral annulus throughout the cardiac cycle. The valve’s effective orifice area of 1.5 ± 0.5 cm2 Reference Bourguignon, Lhommet and El Khoury7 is similar to the 1.56 ± 0.3 cm2 effective orifice area of the Mosaic mitral valve Reference Celiento, Blasi, De Martino, Pratali, Milano and Bortolotti10 and the 1.4 ±0.7 cm2 effective orifice area of the Epic mitral valve (St. Jude Medical, Saint Paul MN). Perhaps most importantly, the stent height has been lowered to 7 mm in order to minimise the risk of left ventricular outflow tract obstruction. This technological advancement is noticeable lower than other mitral valves on market, such as the Mosaic mitral valve (Medtronic, Minneapolis, MN) which has a minimum stent height of 11 mm. This adaptive technology is important in minimising left ventricular outflow tract while maximising valve flow dynamics. These advances are especially important in young patients with CHD or in patients with suboptimal annular or left ventricular outflow tract dimensions.
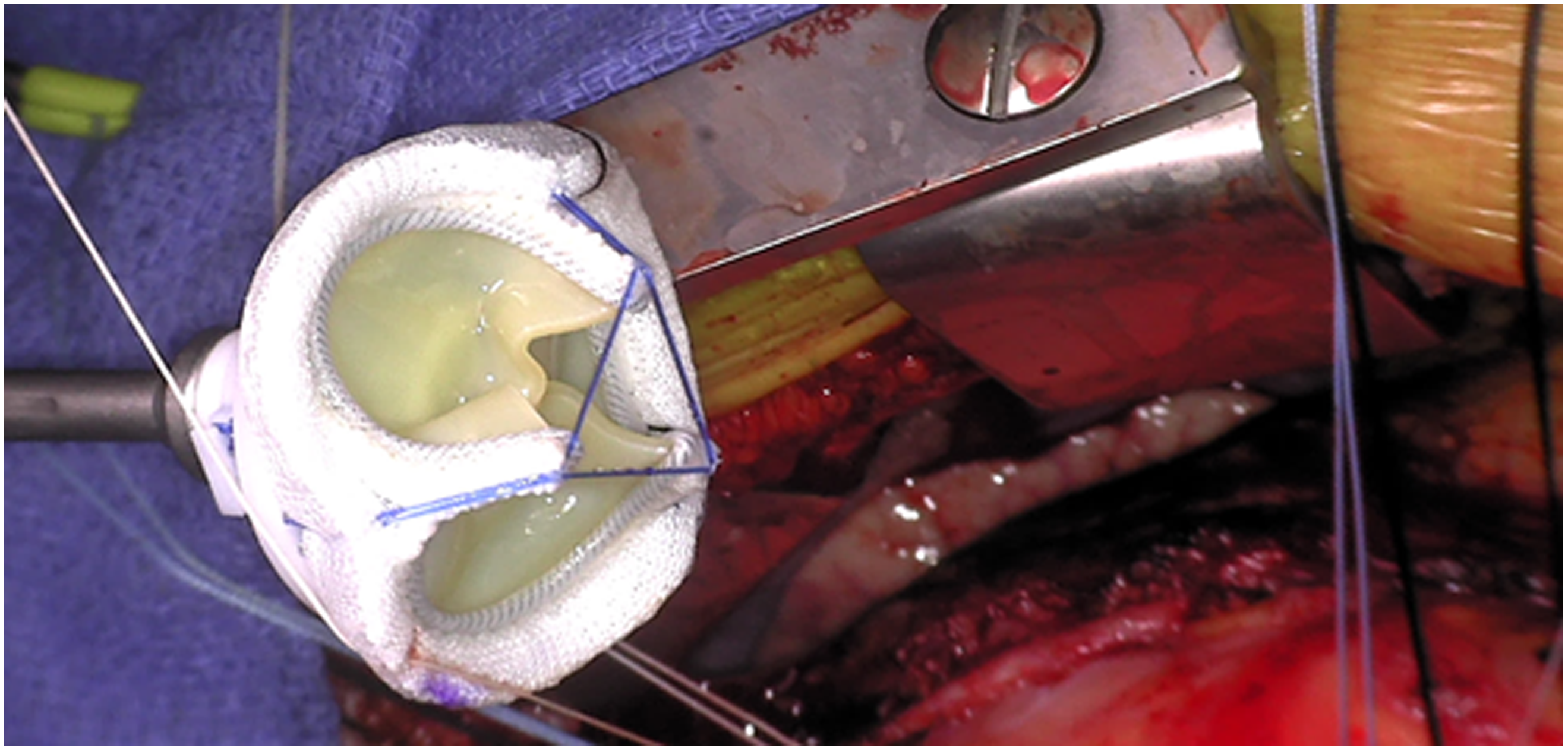
Figure 2. Operative image of the MITRIS RESILIA valve showcasing the low-profile stents in the folded strut position.
We believe the MITRIS RESILIA mitral valve is a safe and effective option for mitral valve replacement in young patients. The unique aspects of its design allow for easier implantation, and the low-profile stents minimise risk of obstruction to the left ventricular outflow tract, in addition to improved leaflet durability and haemodynamic performance. We believe that the outlined advantages of this valve, in addition to its proven durability, make it an advantageous prosthesis to consider when evaluating a patient for mitral valve replacement.