Anti-muscarinic drugs are intended for short-term use only (World Health Organization, 1990; Reference BarnesBarnes, 1990; Reference BazireBazire, 1998). They are widely used in psychiatric practice for the treatment of neuroleptic induced extrapyramidal side-effects (Reference Barnes and McPhillipsBarnes & McPhillips, 1996). However, these drugs have their own side-effects (British National Formulary, 1999) and in the long term may exacerbate serious movement disorders (Reference Perris, Dimitrijevic and JacobssonPerris et al, 1979). In addition such drugs are sometimes misused by patients (Reference Crawshaw and MullenCrawshaw & Mullen, 1984; Reference Marken, Stoner and BunkerMarken et al, 1996). Therefore, monitoring of anti-muscarinics in psychiatric practice would seem to be of considerable importance.
Two audits of anti-muscarinic use were carried out at the State Hospital, Carstairs - the sole provider of psychiatric care, in conditions of special security, in Scotland and Northern Ireland (Reference Snowdon, Chiswick and CopeSnowdon, 1995). The audit in 1996 indicated that some patients were prescribed these drugs for extended periods without clear indication. Approved standards for the correct usage of these drugs was circulated in 1996. The audit was repeated in 1998 and compared with these standards.
Although patients are reviewed regularly at the State Hospital, there is a system for a more comprehensive multi-disciplinary review of the whole treatment/care package of each patient, at three monthly intervals. For this reason the standards that relate to anti-muscarinic prescribing focus on their continued prescription beyond three months.
Approved standards
-
1 After three months regular anti-muscarinic treatment, a reduction in dose or complete discontinuation of the anti-muscarinic should be considered at the patients case review. Following the initial review, the continued use of the anti-muscarinic should be considered at subsequent case reviews. Patients taking a uniform dose and type of anti-psychotic, and an anti-muscarinic for three months or more, must be reviewed.
-
2 Only one anti-muscarinic should be prescribed in each patient.
-
3 The dose of anti-muscarinic should not exceed British National Formulary guidelines.
-
4 An anti-muscarinic should not be prescribed with clozapine unless an additional anti-psychotic is prescribed, or it is being used to treat hypersalivation.
-
5 The anti-muscarinic of choice at the State Hospital is procyclidine.
Objectives
-
(a) To assess adherence to the standards set for the use of anti-muscarinic drugs in 1996, and identify if a problem of inappropriate usage exists.
-
(b) To assess if the extended use of anti-muscarinics is occurring and if so, what reasons for this are given in the clinical notes or by the consultant.
-
(c) To see if the introduction of an approved standard improved prescribing practice.
The study
All patients on anti-muscarinic drugs on the 5 May 1998 were reviewed. These patients were identified with the assistance of the hospital pharmacist. Using the prescription kardexes, anti-muscarinic drug use was compared with the approved Standards 1-5. Where the approved standards were not met, the case notes were reviewed. If reasons for prescribing outwith the standards were not documented, the responsible consultant was approached to try and determine the reasons, if any. The results of this audit were compared with those from the earlier 1996 audit. In the 1996 audit, all patients on anti-muscarinic drugs were identified, on a given day, by the hospital pharmacist. Once again the prescription kardexes of these patients were used to compare with the Standards 1-5. However, in this first audit the case notes were not examined, nor were the consultants approached, regarding findings outwith the standards.
Findings
Adherence to the standards for the use of anti-muscarinic drugs
Standard 1 (see Table 1)
Standard 2
No patients were prescribed more than one anti-muscarinic in either the 1996 or 1998 audits.
Table 1. Numbers of patients on anti-muscarinics falling outwith the Standard 1 at the State Hospital
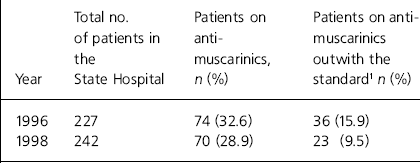
| Year | Total no. of patients in the State Hospital | Patients on anti-muscarinics, n (%) | Patients on anti-muscarinics outwith the standard1 n (%) |
|---|---|---|---|
| 1996 | 227 | 74 (32.6) | 36 (15.9) |
| 1998 | 242 | 70 (28.9) | 23 (9.5) |
Standard 3
No patients exceeded the recommended British National Formulary dosage guides in either the 1996 or 1998 audits.
Standard 4
All patients on clozapine alone and on an anti-muscarinic had had problems of continuing hypersalivation in both the 1996 and 1998 audits (see Table 2).
Table 2. Use of clozapine in combination with anti-muscarinics andadditional neuroleptics at the State Hospital

| Year | Patients on clozapine n (%) | Patience on clozapine alone and an anti-muscarinic, n (%)n (%) | Patients on clozapine, an anti-muscarinic and an additional neuroleptic,n (%) | ||||
|---|---|---|---|---|---|---|---|
| 1996 | 25 (11.0) | 2 (0.9) | Not available | ||||
| 1998 | 34 (14.0 | 4 (1.6) | 2 (0.8) | ||||
Standard 5
The preferred anti-muscarinic drug was procyclidine in both the 1996 and 1998 audits (see Table 3).
Table 3. Numbers of patients on various types of anti-muscarinics used at the State Hospital
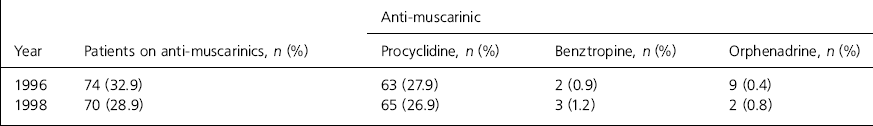
| Anti-muscarinic | ||||
|---|---|---|---|---|
| Year | Patients on anti-muscarinics, n (%) | Procyclidine, n (%) | Benztropine, n (%) | Orphenadrine, n (%) |
| 1996 | 74 (32.9) | 63 (27.9) | 2 (0.9) | 9 (0.4) |
| 1998 | 70 (28.9) | 65 (26.9) | 3 (1.2) | 2 (0.8) |
Assessing if the extended use of anti-muscarinic drugs occurs and the reasons for it
A review of the last case conference on each of the 70 patients on anti-mucarinics in 1998 was made. Out of the 70 patients, three did not have a case conference as they had not been in the hospital long enough. For the remaining 67 patients the medication the patient was on, including anti-muscarinics, was documented clearly in the case conference notes. Medication changes made at the time of the case conference and proposed future changes were also documented. However, out of the 67 cases, specific reference to neuroleptic induced side-effects and continued prescription of anti-muscarinics was made in only three cases. Reasons for continued anti-muscarinic prescriptions outwith Standard 1 were sought from the relevant consultant (see Table 4).
Table 4. Reasons for prescribing anti-muscarinics outwith Standard 1 at the State Hospital
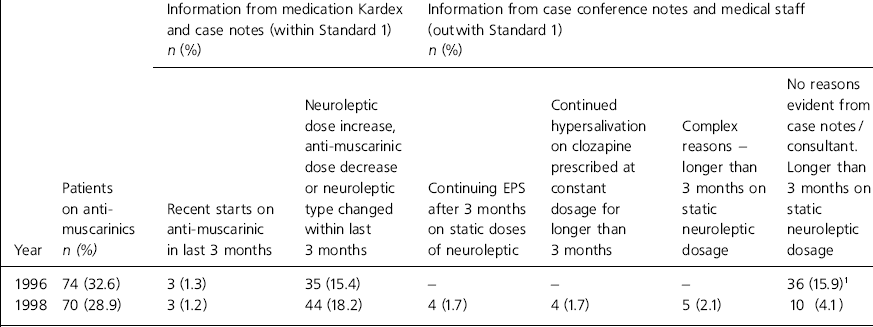
| Information from medication Kardex and case notes (within Standard 1) n (%) | Information from case conference notes and medical staff (outwith Standard 1) n (%) | ||||||
|---|---|---|---|---|---|---|---|
| Year | Patients on anti-muscarinics n (%) | Recent starts on anti-muscarinic in last 3 months | Neuroleptic dose increase, anti-muscarinic dose decrease or neuroleptic type changed within last 3 months | Continuing EPS after 3 months on static doses of neuroleptic | Continued hypersalivation on clozapine prescribed at constant dosage for longer than 3 months | Complex reasons - longer than 3 months on static neuroleptic dosage | No reasons evident from case notes/consultant. Longer than 3 months on static neuroleptic dosage |
| 1996 | 74 (32.6) | 3 (1.3) | 35 (15.4) | - | - | - | 36 (15.9)1 |
| 1998 | 70 (28.9) | 3 (1.2) | 44 (18.2) | 4 (1.7) | 4 (1.7) | 5 (2.1) | 10 (4.1) |
Complex reasons given for prescribing outwith the standards at the State Hospital included:
-
(d) Previous experiences of severe extrapyramidal side-effects in patients when their anti-muscarinics were reduced.
-
(e) Short-term future plans to change a patients neuroleptic or dosage.
-
(f) Difficulty with particular patients being controlling over their medication/treatment. In these cases the benefits of neuroleptic medication were felt to out-weigh the risks of non-adherence if the anti-muscarinic was reduced as dictated by the standard.
-
(g) Movement of patients within the State Hospital meaning they have acquired a new clinical team and are awaiting a case conference with that team.
Comment
The proportion of patients at the State Hospital on regular anti-muscarinics has fallen slightly since 1996. Perhaps more significantly, there has been a reduction in the percentage of prescriptions of anti-muscarinics, which fall outwith Standard 1, between the 1996 and 1998 audits. This may be a reflection of an increasing awareness of the prescribing guidelines or it may be a reflection of the increasing use of certain atypical neuroleptics which have fewer extrapyramidal side-effects. It may be the case that some anti-muscarinic prescriptions that fell within the standards are not clinically required, but this is outside the remit of this audit.
A direct comparison of patients outwith the anti-muscarinic prescribing Standard 1 in the 1996 and 1998 audits cannot be made other than to compare the initial number. This is because the 1996 audit did not look at case notes or seek medical staff opinion. In the 1998 audit it would seem that 10 out of the 23 patients outwith the Standard 1, have no clear clinical reason to continue their anti-muscarinic at its present dose without review. The remaining 13 have reasons given for the extended continuation of the anti-muscarinic at its present dose. Reasons such as continuing extrapyramidal side-effects or hypersalivation (clozapine only) would seem pharmacologically valid. Other reasons given, relate to other aspects of the treatment ‘package’ as a whole and their validity would be a matter for debate.
In terms of medical documentation at the State Hospital, it is the case that the three monthly reviews document all medications prescribed. They also record changes to medication at the time of the review, and for the short-term after the review. As stated before specific reference to anti-muscarinic prescription is rarely made. This is a problem which could easily be addressed, and doing so would aid prescribing practice.
Referring back to the objectives, it would seem there was an improvement in the practice of prescribing anti-muscarinics since the 1996 audit, and the introduction of the prescribing standards at this time. In both audits Standards 2-5 were adhered to rigorously. However, extended use of anti-muscarinics still occurred in 1998, although to a lesser degree than in 1996. This problem, which relates to Standard 1, involves a small but nevertheless, important, number of patients. In the 1998 audit reasons for the extended prescription of anti-muscarinics are given in over 50% of these cases. However, 10 patients are left requiring immediate review. The introduction of standards in 1996 may be responsible for this improvement although it is well recognised that despite publication of prescribing surveys/audits there is a general inertia of change to prescribing habits (Reference Clarke and HoldenClarke & Holden, 1987).
Recommendations
There is a need for greater attention to anti-muscarinic prescribing at case conferences and the alteration of case conference treatment plans to incorporate this at the State Hospital. There is also a need for further audit or monitoring of anti-muscarinic prescribing practice at the State Hospital.
Acknowledgements
Sincere thanks to Morag Wright, State Hospital Pharmacist and her staff, for identifying the patients for the audit. I am also very grateful to Dr David Reid for his encouragement and constructive criticism of subsequent drafts which led to this paper being written.







eLetters
No eLetters have been published for this article.