Maintenance of cognitive function has a crucial role in the well-being when an individual ages. As our understanding of the pathophysiology of dementia increases, this has stimulated interest in identifying modifiable lifestyle factors that could reduce, or at least delay, the cognitive impairment associated with ageing( Reference Imtiaz, Tolppanen and Kivipelto 1 ). The Mediterranean diet is the most extensively studied dietary pattern related to cognition; it has been found to reduce the rate of cognitive decline with ageing and to lower the risk of developing Alzheimer's disease( Reference Lourida, Soni and Thompson-Coon 2 ) in addition to improving global cognitive function( Reference Martinez-Lapiscina, Clavero and Toledo 3 ). Due to differences in food culture and available resources, it is important to study whether other dietary patterns, typical of certain populations, are associated with cognition. One such potential dietary pattern is the Nordic diet, a typical healthy Northern diet following the Nordic Nutrition Recommendations( 4 ). The traditional Nordic diet is based on commonplace local Scandinavian food items, characterised by high consumption of vegetables, fruit and berries, fish and whole-grain products as well as low-to-moderate consumption of meat and alcohol, and rapeseed oil being the recommended source of fat( Reference Kanerva, Kaartinen and Schwab 5 ). The Nordic diet primarily differs from the Mediterranean-type diets in that it has different sources of vegetables, grain products and dietary fat, consisting of products readily available in the Nordic countries. In brief, the Nordic diet is characterised by a wide selection of berries, providing a variety of polyphenols, antioxidants and other bioactive compounds. The major sources of grain products are rye, oat and barley being eaten in bread and porridge, i.e. products that have high fibre contents. An important source of unsaturated fatty acids is rapeseed oil that contains the essential fatty acids linolic acid and α-linolenic acid 2- and 20-fold amounts, respectively, compared with olive oil, a major food component in the Mediterranean diet. Previously, the Nordic diet has shown to be associated with reduced cardiovascular risk factors( Reference Kanerva, Kaartinen and Schwab 5 , Reference Uusitupa, Hermansen and Savolainen 6 ); however, no data are available on its impact on cognition. Since a better cardiovascular risk profile has been associated with a lower risk of vascular dementia( Reference Gorelick, Scuteri and Black 7 ), it seemed reasonable to postulate that the Nordic diet could reduce the rate of cognitive decline with ageing. In addition, the majority of the components of the Nordic diet have previously been associated with preserved cognition. For example, extensive consumptions of vegetables and fish as well as a high intake of PUFA have been postulated to protect against cognitive decline( Reference Eskelinen, Ngandu and Helkala 8 , Reference Loef and Walach 9 ), whereas the amounts of SFA consumed have been inversely associated with cognition( Reference Eskelinen, Ngandu and Helkala 8 ). Further, n-3 fatty acids have beneficial effects on mild cognitive impairment( Reference Mazereeuw, Lanctot and Chau 10 ), and low-to-moderate alcohol use has been associated with a reduced risk of dementia( Reference Peters, Peters and Warner 11 ).
In the present study, we examined both cross-sectional and longitudinal associations of the baseline Nordic diet with cognitive function at baseline and after a 4-year follow-up in a randomly selected population-based sample of older men and women.
Methods
Study population
The data presented here represent the secondary analyses from the Dose-Responses to Exercise Training Study (the DR's EXTRA), which is a population-based, randomised, controlled 4-year trial on the health effects of regular physical exercise and diet (ISRCTN45977199, http://isrctn.org). This report describes observational data collected during the 4 years of monitoring the subjects. Since the aim of the present study was to examine the association of the Nordic diet with cognition, the study intervention groups were pooled in the analyses and thus study design can be considered to represent a 4-year follow-up. However, the original study group assignment was adjusted for as a covariate in the analyses.
Subjects were identified from the Finnish Population Register (Fig. 1), as described previously( Reference Komulainen, Kivipelto and Lakka 12 ). Altogether, 1479 men and women participated in baseline examinations conducted in 2005–6. Due to exclusion criteria (health conditions that impair safe exercise training, malignancies and conditions preventing co-operation, e.g. existing dementia, as judged by a physician) or other reasons (i.e. moving elsewhere or refusal), sixty-nine individuals were excluded, and 1410 were randomised into an intervention group (aerobic exercise, resistance exercise, diet, aerobic exercise+diet or resistance exercise+diet) or to the reference group. A total of 1199 individuals of original participants completed the 4-year follow-up in 2009–2011. There were 211 (15·0 %) dropouts during the intervention. In the present study, after excluding fifty-nine individuals with missing or insufficient data on baseline diet (n 8), cardiorespiratory fitness tests (n 46) or both (n 2), or 4-year cognition assessment (n 3), complete data were available for 1140 individuals (567 men and 573 women). Complete data on the 4-year change in diet and cognition were available for 1132 individuals. Those individuals excluded from the analyses (211 dropouts and fifty-nine with missing data) were older (mean 68·3 (sd 5·7) v. 66·1 (sd 5·2) years, P< 0·001), had lower scores in Consortium to Establish a Registry for Alzheimer's Disease total score (CERAD-TS) (mean 78·7 (sd 10·5) v. 83·5 (sd 8·6) points, P< 0·001) and in the Mini-mental State Examination (MMSE) (mean 27·0 (sd 3·0) v. 28·0 (sd 2·0) points, P< 0·001), reported more depressive symptoms (mean 10·3 (sd 6·6) v. 8·3 (sd 6·3) points, P< 0·001), were less educated (mean 10·4 (sd 3·9) v. 11·3 (sd 3·8) years, P< 0·001), had higher BMI (mean 28·6 (sd 5·4) v. 27·5 (sd 4·3) kg/m2, P< 0·001), lower cardiorespiratory fitness (mean 20·9 (sd 5·9) v. 24·2 (sd 6·2) ml/kg per min, P< 0·001) and lower energy intake (mean 6·4 (sd 1·8) v. 7·1 (sd 1·9) MJ, P< 0·001) at baseline than those who completed all of the analyses. The present study was conducted according to the guidelines laid down in the Declaration of Helsinki. The study protocol was approved by the Research Ethics Committee of the Northern Savo Hospital District. Written informed consent was obtained from all the study participants.

Fig. 1 Dose-Responses to Exercise Training Study (DR's EXTRA) flow chart in the present study.
Assessment of diet
Dietary intake was assessed at baseline and at the 4-year follow-up evaluation by a 4-d food record that was predefined to include three weekdays and one weekend day, as described previously( Reference Kouki, Schwab and Lakka 13 ). The participants were given detailed written and verbal instructions on how to complete the food record. All food records were reviewed and checked for completion by either a clinical nutritionist or a trained nurse. Portion sizes were estimated by the subjects using a picture booklet( Reference Pietinen, Hartman and Haapa 14 ), household gauges or actual weighing. Data from food records were analysed using the MicroNutrica® nutrient calculation software for group analysis, version 2.5 (recipes updated in 2007)( Reference Rastas, Seppanen and Knuts 15 ).
The Nordic diet score was obtained by a modification of the method of Kanerva et al. ( Reference Kanerva, Kaartinen and Schwab 5 ). This Nordic diet score consists of the following eight variables: consumption of fish (g/d; including fatty and lean fish and processed fish products); vegetables (g/d; including roots, non-root vegetables, mushrooms, legumes and nuts, but not potatoes); fruit and berries (g/d); whole-grain bread (g/d); meat (g/d; including beef, pork, poultry, game, sausage and giblets); alcohol (g/d), and intake of α-linolenic acid (g/d; to represent the consumption of rapeseed oil); unsaturated fatty acids:total fat ratio. Changes were made to the original Nordic diet score( Reference Kanerva, Kaartinen and Schwab 5 ) due to either the availability of limited data or in an attempt to refine the quality of the score. The original Nordic diet score included low-fat milk products, whereas in the present study, all milk products were excluded from the score. Due to the data given by the nutrient calculation software, we were not able to separate milk products according to their fat content. The original variables regarding fat intake (total fat and PUFA:SFA ratio in the original score) were also changed. Nowadays, quality of fat is considered more important than the amount of fat( Reference Schwab, Lauritzen and Tholstrup 16 ); thus, we did not include total fat intake into the score. We decided to describe the quality of dietary fat by estimating the unsaturated fatty acids:total fat intake ratio, and this was included into the score because in the Finnish nutrition guideline, the recommended proportion of unsaturated fatty acids is at least 2/3. In addition, rapeseed oil is a common and recommended source of fat in the Nordic diet. However, we did not have data available about the consumption of rapeseed oil. Thus, the intake of α-linolenic acid as a surrogate marker of the consumption of rapeseed oil was included in the modified score. In addition, nuts were included in the vegetable group, in contrast to their classification in the original score. In the nutrient calculation software, legumes and nuts were categorised as one food group, and they could not be separated. The Nordic diet score was calculated according to the sex-specific quartiles of each dietary component (with the exception of alcohol). For each component (fish, vegetables, fruit and berries, whole-grain bread, α-linolenic acid and unsaturated fatty acids:total fat ratio), the lowest quartile was coded as 0, the two middle quartiles as 1 and 2 and the highest quartile as 3. For meat, the highest quartile was coded as 0, the two middle quartiles as 1 and 2 and the lowest quartile as 3. Consumption of alcohol was two-point scale based on specific cut-points in accordance with the Dietary Guidelines for Americans( Reference McGuire 17 ). Non-alcohol drinkers ( < 1 g of alcohol/d) as well as heavy alcohol consumers (>24 g/d in men and>12 g in women) received 0 points. Mild-to-moderate drinkers (1–24 g/d in men and 1–12 g in women) received 1 point. Hence, the total Nordic diet score ranged from 0 to 22 points, with higher points indicating better adherence to a desirable Nordic diet. The sex-specific medians were used to dichotomise the score. Men with poor adherence had 0–11 points and men with good adherence scored 12–21 points. Similarly, women with poor adherence had 1–10 points and women with good adherence had 11–21 points.
Assessment of cognitive function
Cognitive function was assessed using the standardised Finnish version of CERAD neuropsychological battery( Reference Morris, Heyman and Mohs 18 ) and the MMSE( Reference Folstein, Folstein and McHugh 19 ) at baseline and after 2 and 4 years (only the baseline and 4-year results are reported in the present study). Trained nurses performed the assessments under the supervision of a neuropsychologist. Tests were performed in the same order on every study visit. The CERAD-TS was calculated, as previously described, including Verbal Fluency, Modified Boston Naming Test, Word List Learning, Constructional Praxis, Word List Recall and Word List Recognition Discriminability( Reference Chandler, Lacritz and Hynan 20 ). The score ranged from 0 to 100 points, with a higher score indicating better performance, and thus, an individual scoring ≤ 70 points was classified as being impaired cognition( Reference Paajanen, Hanninen and Tunnard 21 ).
Other assessments
Symptoms of depression were assessed with the Center for Epidemiological Studies Depression Scale( Reference Radloff 22 ). Cardiorespiratory fitness was assessed as maximal oxygen uptake (VO2max, ml/kg per min) measured by the VMax respiratory gas analyser (SensorMedics) during a maximal symptom-limited, exercise stress test on an electrically braked cycle ergometer (Ergoline). Prevalent use of medications, smoking status and education were assessed from a self-administered questionnaire, and BMI was calculated from height and weight.
Statistical analyses
Statistical analyses were performed using the IBM SPSS statistics for Windows, version 19.0 (IBM Corporation). Associations with a P< 0·05 were considered as statistically significant. Differences between the groups and between baseline and 4-year examinations were analysed using t test, Mann–Whitney U test, Wilcoxon signed-rank test or χ2 test as appropriate. These values are presented as mean and standard deviations for normally distributed variables and as median and interquartile range (IQR) for non-normally distributed variables. The assumption of normality was verified using the Kolmogorov–Smirnov test and visual inspection of histograms, with the latter receiving greater emphasis. ANCOVA was used to assess the association of the baseline Nordic diet with the CERAD-TS, its subtests and MMSE at 4 years among all individuals and among those with normal cognition at baseline. The Nordic diet score was used as a continuous variable. Interactions between the Nordic diet and sex on CERAD-TS and MMSE were analysed via an interaction term in all models. A hierarchical approach was used to reveal the effect of confounding factors on the association between the Nordic diet and cognition. Covariates were chosen based on the current knowledge of factors associated with cognitive function during ageing. Model 1 included adjustments for basic demographic factors (age, sex and education), and baseline cognitive scores and study group. Model 2 was additionally adjusted for different lifestyle factors including smoking, cardiorespiratory fitness (maximal oxygen uptake, ml/kg per min), medications (antihypertensive, lipid-lowering and antidiabetic) and symptoms of depression. Model 3 was additionally adjusted for energy intake.
Results
Baseline characteristics are presented in Table 1, and food consumption and nutrient intake data are summarised in Table 2. At baseline, 8·6 % (n 98) showed evidence of impaired cognition. These individuals were older (mean 69·4 (sd 5·1) v. 65·8 (sd 5·1) years, P< 0·001), less educated (mean 9·0 (sd 3·0) v. 11·5 (sd 3·8) years, P< 0·001), reported more depressive symptoms (median 9·0 (IQR 9·0) v. 7·0 (IQR 8·0) points, P= 0·028) and had a lower energy intake (mean 6·8 (sd 1·9) v. 7·2 (sd 1·9) MJ, P= 0·050), as well as using more antihypertensive medication (52·0 v. 39·0 %, P= 0·012) and antidiabetic medication (13·3 v. 6·0 %, P= 0·005) than those with normal cognition. The Nordic diet score (median 10·0 (IQR 5·0) v. 11·0 (IQR 6·0) points, P= 0·076) and BMI (mean 27·8 (sd 3·8) v. 27·5 (sd 4·3) kg/m2, P= 0·522) did not differ between individuals with impaired or normal cognition.
Table 1 Baseline characteristics of all participants and according to adherence to the Nordic diet (Mean values and standard deviations; medians and interquartile ranges (IQR))

CERAD, Consortium to Establish a Registry for Alzheimer's Disease; MMSE, Mini-mental State Examination; CES-D, the Center for Epidemiological Studies Depression Scale; VO2max, maximal oxygen uptake.
* Men with poor adherence had 0–11 points in the Nordic diet score and men with good adherence had 12–21 points. Women with poor adherence had 1–10 points and women with good adherence had 11–21 points.
† P values are from t test and refer to the difference between groups of poor and good adherence to the Nordic diet.
‡ Non-normally distributed variables.
§ P values are from Mann–Whitney U test or χ2 test and refer to the difference between groups of poor and good adherence to the Nordic diet.
Table 2 Baseline food consumption and nutrient intake of all participants and according to adherence to the Nordic diet (Mean values and standard deviations; medians and interquartile ranges (IQR))

E%, percentage of energy; UFA, unsaturated fatty acids (including MUFA and PUFA).
* Men with poor adherence had 0–11 points in the Nordic diet score and men with good adherence had 12–21 points. Women with poor adherence had 1–10 points and women with good adherence had 11–21 points.
† P values are from t test, and refer to the difference between groups of poor and good adherence to the Nordic diet.
‡ Non-normally distributed variables.
§ P values are from Mann–Whitney U test, and refer to the difference between groups of poor and good adherence to the Nordic diet.
∥ Including roots, non-root vegetables, mushrooms, legumes and nuts, but not potatoes.
During the 4 years, the cohort's CERAD-TS improved from 83·4 (sd 8·5) to 84·4 (sd 10·0) points (P< 0·001). The total Nordic diet score did not change (median 11·0 (IQR 6·0) points at baseline and 11·0 (IQR 5·0) points after 4 years, P= 0·367). However, increased consumption was observed in the amounts of fruit and berries (from median 207 (IQR 187) to 231 (IQR 193) g/d, P< 0·001), fish (from median 37·5 (IQR 60·8) to 42·2 (IQR 56·3) g/d, P= 0·019) and α-linolenic acid (from mean 1·7 (sd 0·9) to 1·9 (sd 1·0) g/d, P< 0·001), whereas declines were noted in the consumption of alcohol (from median 0·9 (IQR 8·0) to 0·0 (IQR 5·0) g/d, P< 0·001) and in the unsaturated fatty acids:total fat ratio (from mean 0·58 (sd 0·07) to 0·53 (sd 0·07), P< 0·001).
The Nordic diet and cognition
At baseline, the adherence to the Nordic diet had not been associated with CERAD-TS in the total study cohort (β 0·10 (95 % CI − 0·02, 0·22), P= 0·114) or in individuals with normal cognition (β 0·05 (95 % CI − 0·05, 0·15), P= 0·300) after adjustment for age, sex, education and study group. In addition, at baseline, the Nordic diet score had not been associated with either the MMSE or with the individual cognitive domains in the CERAD-TS (data not shown). However, the Nordic diet score at baseline had been positively associated with the CERAD-TS at 4 years in the total cohort and in individuals with normal baseline cognition in Model 1 (Table 3). In Model 2, these associations became weakened, but remained statistically significant (P< 0·05) in individuals with normal cognition but not in the entire cohort. After further adjustment for energy intake, these associations were no longer statistically significant. Similar associations were observed between the baseline Nordic diet and the MMSE at 4 years (Table 3). Age (β − 0·33 (95 % CI − 0·41, − 0·25), P< 0·001), sex (women v. men 1·69 (95 % CI 0·64, 2·75), P= 0·002), education (0·12 (95 % CI 0·02, 0·23), P= 0·002) and baseline CERAD-TS (0·81, (95 % CI 0·76, 0·85), P< 0·001) were the only covariates associated with CERAD-TS at 4 years in Model 3 in all individuals. Similar associations of covariates were observed with MMSE. In addition, no interaction was observed between the Nordic diet score and sex with respect to CERAD-TS or MMSE either in the entire cohort or in those with normal baseline cognition (P>0·05 in all Models).
Table 3 Association of the baseline Nordic diet score with the Consortium to Establish a Registry for Alzheimer's Disease (CERAD) total score and the Mini-mental State Examination (MMSE) after the 4-year follow-up (β Coefficients and 95 % confidence intervals)
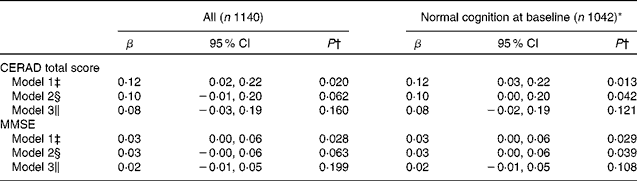
* Individuals with impaired cognition (CERAD total score ≤ 70 points) at baseline were excluded.
† Values are from ANCOVA.
‡ Model 1: age, sex, education, study group and baseline CERAD total score or MMSE.
§ Model 2: Model 1+symptoms of depression, smoking, VO2max (ml/kg per min), antihypertensive medication, lipid-lowering medication and antidiabetic medication.
∥ Model 3: Model 2+energy intake.
The Nordic diet score at baseline had been positively associated with the Verbal Fluency at 4 years in all individuals in Model 1 (β 0·10 (95 % CI 0·03, 0·18), P= 0·009) and in Model 2 (β 0·08 (95 % CI 0·00, 0·16), P= 0·039), but not in Model 3 (β 0·04 (95 % CI − 0·04, 0·13), P= 0·308). Similarly, a positive association had been found in individuals with normal cognition in Model 1 (β 0·10 (95 % CI 0·03, 0·18), P= 0·010) and in Model 2 (β 0·09 (95 % CI 0·01, 0·17), P= 0·033), but not in Model 3 (β 0·05 (95 % CI − 0·04, 0·14), P= 0·270). Furthermore, the Nordic diet score at baseline had been positively associated with the Word List Learning at 4 years in the entire group in Model 1 (β 0·07 (95 % CI 0·02, 0·12), P= 0·004) and in Model 2 (β 0·06 (95 % CI 0·01, 0·10), P= 0·022), but not in Model 3 (β 0·04 (95 % CI − 0·02, 0·09), P= 0·172). A similar positive association had been found in individuals with normal cognition in Model 1 (β 0·06 (95 % CI 0·02, 0·11), P= 0·009) and in Model 2 (β 0·05 (95 % CI 0·00, 0·10), P= 0·044), but not in Model 3 (β 0·03 (95 % CI − 0·02, 0·09), P= 0·205). Finally, there were no associations detected between the Nordic diet score at baseline and the other subtests of the CERAD-TS (i.e. Modified Boston Naming Test, Constructional Praxis, Word List Recall and Word List Recognition Discriminability) at 4 years (data not shown).
Discussion
The present study revealed that better adherence to the baseline Nordic diet had been associated with higher scores in global cognition and also in two subtests, i.e. those assessing memory and language, which are the earliest domains to reveal problems in cognition( 23 ), over the 4-year study period after adjustment for demographic and lifestyle factors in individuals with normal cognition. However, after adjustment for dietary energy intake, none of the associations found between the Nordic diet and cognition remained statistically significant. This may reflect the overall importance of different kinds diets, i.e. ensuring that an individual consumes a sufficient amount of energy to maintain energy balance and prevent malnutrition, and in that way to reduce the cognitive decline that can occur during ageing.
However, there are sources of bias in the adjustment for energy intake, which need to be considered before one can draw any final conclusions. Underreporting of energy intake is a common source of error in nutritional assessments( Reference Poslusna, Ruprich and de Vries 24 ), and this was also evident in the present study. In addition, undereating (so-called ‘Anorexia of ageing’) tends to increase with age, and this is reflected in nutrition assessments as a lower energy intake( Reference de Boer, Ter Horst and Lorist 25 ). In the present study, the energy intake was lower among individuals with impaired cognition in comparison to those with normal cognition; this is likely to be due to both underreporting and undereating.
The statistically significant association between the Nordic diet score and global cognition, before adjustment for the energy intake, was observed only in individuals with normal cognition. We postulated that a stronger association would be found between the Nordic diet score and cognition in the analysis involving all individuals. Partly, these differences may reflect the dietary misreporting in participants with impaired cognition. In addition, the fact that associations between the Nordic diet and the change in cognition were weakened, or in the case of global cognition disappeared, after adjustment for lifestyle factors may reflect the accumulation of beneficial factors in a healthy lifestyle. However, no clinically significant differences in the extent of the functional cognitive decline could be detected in these analyses.
Limited data are available about the association between consumption of a Nordic diet and the level of cognition. Most of the studies examining the effect of dietary patterns on cognition have investigated the Mediterranean diet, characterised by its high consumption of plant foods (vegetables, fruit, legumes, cereals, nuts and seeds), moderate consumption of fish and dairy products, relatively low consumption of red meat, low-to-moderate consumption of alcohol, particularly in the form of red wine, and with olive oil being the principal source of fat( Reference Willett, Sacks and Trichopoulou 26 ). In prospective observational studies, the Mediterranean type diet has been shown to decrease the risk of experiencing cognitive decline( Reference Feart, Samieri and Rondeau 27 Reference Tangney, Kwasny and Li 28 Reference Wengreen, Munger and Cutler 29 ) and Alzheimer's disease( Reference Scarmeas, Stern and Tang 30 ). However, not all studies have found positive associations( Reference Psaltopoulou, Kyrozis and Stathopoulos 31 , Reference Cherbuin and Anstey 32 ). Only a few observational studies have been conducted in the actual Mediterranean countries( Reference Feart, Samieri and Rondeau 27 , Reference Psaltopoulou, Kyrozis and Stathopoulos 31 ), most studies originate from the USA. In a randomised trial conducted in Spain, consumption of a Mediterranean diet with added extra-virgin olive oil or nuts had been associated with better cognitive function in comparison to the control diet( Reference Martinez-Lapiscina, Clavero and Toledo 3 ). A limitation of this trial was that cognitive function was assessed only at the end of the intervention; thus, the effect of intervention on the actual change in cognition could not be estimated. With respect to other dietary patterns, prospective observational studies have revealed both positive( Reference Wengreen, Munger and Cutler 29 , Reference Eskelinen, Ngandu and Tuomilehto 33 ) and neutral( Reference Tangney, Kwasny and Li 28 ) findings related to cognitive decline and dementia. All these dietary patterns, including the Nordic diet, emphasise the importance of high consumption of vegetables and fruit. Most, but not all, also recommend high consumption of fish and whole-grain products and low-to-moderate consumption of meat and high-fat dairy products. There may be some differences in the definitions of dietary fat quality, but the tendency has been to prefer unsaturated fatty acids over SFA. Hence, although the recommended dietary patterns share some similarities, there are also significant differences. In other words, a diverse and healthy diet can be constructed in many ways. The above-mentioned dietary patterns, including the Nordic diet, are all descriptions of a healthy diet with different nuances attributable to local food culture, preferences and resources. Since the adherence to dietary patterns is usually estimated with population-based cut-point values (e.g. medians), even the same dietary pattern will not be directly comparable in different countries and populations. In addition, a diet consisting of familiar and widely available food items will be easier to adopt and therefore the practical effectiveness of health promotion actions can be improved when they emphasise the benefits of this kind of diet. Therefore, it is important to study the effects of different dietary patterns in different populations.
No clear mechanism to explain the beneficial effects of healthy diets on cognitive function has been formulated as yet( Reference Frisardi, Panza and Seripa 34 ). However, it is likely that a healthy diet and its components can influence cognition via their beneficial effects on vascular risk factors, inflammation and oxidative stress. High consumption of fish( Reference Virtanen, Siscovick and Longstreth 35 ) and high levels of circulating n-3 fatty acids( Reference Virtanen, Siscovick and Lemaitre 36 ) have been associated with a lower prevalence of subclinical infarcts and white matter abnormalities. In addition, certain nutrients (n-3 fatty acids, B-vitamins and antioxidants) present in healthy diets have been proposed to interfere with the processing of β-amyloid in the brain( Reference Otaegui-Arrazola, Amiano and Elbusto 37 ).
The improvement in cognitive function during the 4-year study period was small but unexpected in this age group. In these kinds of longitudinal studies, the changes in cognitive function are typically minor( Reference Salthouse 38 ) and cognition may even improve( Reference Van Dijk, Van Gerven and Van Boxtel 39 ). One potential explanation in the present study is that the actual participation into the intervention study led to improvements in the lifestyle factors as well as providing social and mental stimulation. The cognitive tests were performed three times during the study; thus, a learning effect might account for the better performance in the tests similarly as found in other studies( Reference Salthouse 38 , Reference Van Dijk, Van Gerven and Van Boxtel 39 ). The retest effect may have conferred some bias in the present results by underestimating the age-related cognitive decline. It should also be borne in mind that the progression from normal cognition to dementia may require several decades( 23 ); thus, there may be limitations on our capabilities to detect clinically relevant changes in cognition over a 4-year period. Since changes in cognition may be minor, it may also be difficult to link then with the effects of the diet. Thus, even a small association showing that an improvement in cognition could be related to diet may be clinically relevant.
The strength of the study is the large representative population-based sample of older men and women. A 4-d food record is an open-ended dietary assessment method filled in at the time when food is being eaten; thus, it does not rely on memory and in that way is superior to the more commonly used retrospective FFQ or recalls( Reference Shim, Oh and Kim 40 ). The accuracy of these methods is highly dependent on the respondents' motivation and their ability and willingness to report their actual food consumption. A cognitive impairment may lead to a decline in the ability to perceive and process the relevant information( Reference Petersen 41 ), which, in turn, probably impairs the ability of an individual to record his/her food intake, independent of the methodology used. Thus, as mentioned earlier, underreporting is a common source of error in food records, especially in older individuals( Reference Poslusna, Ruprich and de Vries 24 ). Seasonal variations in diet may not have been captured at an individual level; however, as the surveys in the present study population were spread out over 1·5 years, it is most unlikely that this is a source of bias. Cognitive function was evaluated using the CERAD neuropsychological battery, which is recognised for its good interviewer and test-retest reliability( Reference Morris, Heyman and Mohs 18 ). The CERAD-TS is an accurate measure of global cognitive status in normal ageing and in the early stages of dementia( Reference Paajanen, Hanninen and Tunnard 21 ). It should also be kept in mind that responses to the diet may vary between individuals due to genotype( Reference Egert, Rimbach and Huebbe 42 ), a factor for which we were unable to control.
The Nordic diet score has some limitations; it was not possible to assess the impact of the contents of food groups incorporated in the diet score, because they were estimated by nutrient calculation software (MicroNutrica®)( Reference Rastas, Seppanen and Knuts 15 ). Problematic food groups were fish and whole-grain bread, which were classified as being healthy, while non-recommended food items, e.g. processed fish products and biscuits, were also included. Despite the fact that meat was classified as a non-recommended food group, it is an important source of good quality protein, especially in elderly people and it can be viewed as part of a healthy diet, if consumed in moderate amounts. Although the analyses were adjusted for the randomised study group, we cannot exclude the possibility that changes in lifestyle factors during the intervention may have affected the observed associations. In addition, the adjustments for potential confounders were performed only at baseline; thus, we cannot exclude the possibility of residual confounding. The dropout rate during the study was low (15 %), despite the long and demanding intervention period. The non-participants and dropouts were older and had more cardiometabolic risk factors and worse CERAD-TS scores than the study participants. This may have diluted our ability to reveal the potential effect of the Nordic diet on cognition in the entire population.
In conclusion, based on this present large sample of middle-aged and elderly men and women, consumption of a Nordic diet appears to display a positive association with cognition in individuals with normal levels of cognition.
Acknowledgements
The present study was supported by grants from the Ministry of Education and Culture in Finland (grant numbers 722, 627; 2004–2010); Academy of Finland (grant numbers 104943 and 123885); European Commission FP6 Integrated Project (grant number LSHM-CT-2004-00527; EXGENESIS); the City of Kuopio; Finnish Diabetes Association; Finnish Foundation for Cardiovascular Research; Kuopio University Hospital; the Social Insurance Institution of Finland; Päivikki and Sakari Sohlberg Foundation; Juho Vainio Foundation (R. M.); Aarne and Aili Turunen Foundation (R. M.), The Finnish Graduate School on Applied Bioscience: Bioengineering, Food and Nutrition, Environment (R. M.) and Finnish Cultural Foundation, North Savo Regional fund (R. M.). None of these funding sources had any role in the design and analysis or in the writing of this article.
None of the authors has any conflict of interest to declare.
The authors' contributions are as follows: R. R., P. K., R. M., U. S. and M. K. planned and designed the study; R. M., P. K., H. M. H., K. S., M. H. and R. R. implemented the acquisition of the data; R. M., P. K., U. S., K. S., M. H., T. H. and R. R. analysed and interpreted the data; R. M. wrote the original manuscript, and subsequently all of the other authors have critically revised the manuscript. All authors have read and approved the final manuscript.







