Endothelium-derived NO has several effects that are expected to slow the development of atherosclerosis. These include reduction of LDL oxidation(Reference Hogg, Kalyanaraman and Joseph1), endothelial adhesion molecule expression(Reference De Caterina, Libby and Peng2), smooth muscle cell proliferation(Reference Garg and Hassid3) and platelet adhesion(Reference de Graaf, Banga and Moncado4). Nevertheless, studies of the effects of NO on experimental atherosclerosis in rabbits have given equivocal results. l-Arginine, the precursor of NO, has sometimes but not always significantly reduced lesions(Reference Cooke, Singer and Tsao5–Reference Hayashi, Juliet and Matsui-Hirai12). Similarly, l-arginine analogues that inhibit NO production have significantly increased lesions in some trials(Reference Cayatte, Palacino and Horten13, Reference Naruse, Shimizu and Muramatsu14) but not in others(Reference Nakamura, Abe and Tanaka15).
The pathogenesis of experimental atherosclerosis in rabbits is age-dependent. In the immature aorta, lesions develop downstream of the origins of intercostal arteries(Reference Barnes and Weinberg16) and are restricted to fatty streaks(Reference Spagnoli, Orlandi and Mauriello17). In mature animals, however, lesions have been observed at the sides and upstream of these ostia(Reference Barnes and Weinberg16), and they progress to advanced types(Reference Spagnoli, Orlandi and Mauriello17). These differences have been attributed to changes with age in the NO pathway(Reference Staughton, Weinberg and Clarke18, Reference Orlandi, Marcellini and Spagnoli19). In the present study, we investigated whether inconsistencies in the effects of dietary l-arginine on lesion prevalence could be explained by the use of rabbits of different ages. A preliminary report has been published(Reference Weinberg and Cremers20).
Methods
Animals and diet
All animal procedures complied with the Animals (Scientific Procedures) Act 1986 and were approved by the Local Ethical Review Panel of the University of Reading (Reading, Berkshire, UK). A total of sixteen immature (64 (sem 0·14) d) and eighteen mature (183 (sem 0·12) d) male New Zealand white rabbits (HSDIF strain; Harlan, Bicester, Oxford, UK) were individually housed at 18 ± 2°C under a 12 h light cycle. They were fed 75 g/d of a normal diet (9603 TRB Rabbit; Harlan Teklad, Bicester, Oxford, UK) supplemented for 8 weeks with 1 % (w/w) cholesterol (Sigma, St Louis, MO, USA). The cholesterol was added in diethyl ether (inhibitor-free spectrophotometer grade; Sigma), which was then evaporated. For half of the rabbits in each age group, the diet was additionally supplemented with 6 % (w/w) l-arginine (Sigma). The arginine was added in water, which was then allowed to evaporate. Rabbits were weighed in weeks 0 (i.e. before cholesterol or arginine was added to the diet), 3 and 7. Blood was collected from the marginal ear vein into EDTA in the same weeks after an overnight fast. Plasma obtained from the blood samples was assayed for total cholesterol, TAG, nitrite plus nitrate, cyclic guanosine monophosphate (cGMP) and l-arginine by the following methods.
Plasma lipid concentrations
Total cholesterol and TAG were measured by an automated method (ILAB 600; Instrumentation Laboratories, Warrington, Cheshire, UK) using chemicals and standards from the same supplier.
Plasma concentrations of nitrite plus nitrate
Concentrations were measured using a commercial kit based on the Griess reaction (Colorimetric Nitric Oxide Assay kit; Calbiochem, La Jolla, CA, USA) according to the manufacturer's instructions with additional centrifugation steps to avoid turbidity. All samples were analysed in duplicate.
Plasma concentrations of cyclic guanosine monophosphate
cGMP concentration was determined using a commercial cGMP (low pH) immunoassay (R&D Systems Europe Limited, Abingdon, Oxon, UK). Plasma samples were first deproteinised by using centrifuge filters (50 kDa nominal cut-off value, Millipore). All samples were analysed in duplicate.
Plasma concentrations of l-arginine
l-Arginine was measured by HPLC (Autosampler Model AS-2057plus; Jasco GmbH Deutschland, Umstadt, Germany), using o-phthaldialdehyde as the precolumn derivatisation reagent(Reference Jones and Gilligan21). Plasma samples at each time point in each experimental group were pooled. The pooled samples were analysed at least in triplicate.
Surgical procedures
Each animal was given heparin (2000 USP units, intravenously; Sigma) and, 2 min later, an overdose of pentobarbital (100 mg/kg, intravenously, Euthatal; Rhône Mérieux, Harlow, Essex, UK). The aorta was cannulated below the origin of the superior mesenteric artery, flushed by retrograde perfusion with 50 ml of Ringer's solution (composition: NaCl, 9·0 g/l; KCl, 0·2 g/l; Ca2Cl, 0·2 g/l; NaHCO3, 0·1 g/l) from a reservoir 90 cm above the animal and fixed in the same way with 10 % buffered formalin for 10 min. The aorta from the point of cannulation to the descending arch was than excised, cleansed of loose adventitial tissue and divided into descending thoracic and upper abdominal segments. The third pair of intercostal branches and the left renal branch were removed for a separate spectroscopic study of lesion composition(Reference Palombo, Cremers and Weinberg22), discussed later. The remaining parts were stored in Karnovsky's fixative (4 % glutaraldehyde and 5 % formaldehyde) before being used for lesion mapping.
Staining and imaging
Each aortic segment was equilibrated with PBS (0·15 mol/l, pH 7·4), stained for 1 h at 4°C in a 1 % (w/v) solution of Oil Red O in 60 % (v/v) triethyl phosphate, destained for 30 min in 60 % triethyl phosphate and re-equilibrated with PBS. It was then counterstained with 0·003 % (w/v) Evans blue for 1 h and destained for 30 min in PBS. The thoracic aorta was opened along the ventral wall and the abdominal aorta along its dorsal wall. Each segment was placed, luminal surface down, on a flatbed scanner (Perfection 1660 Photo; Epson, Nagano, Japan) and scanned at a nominal resolution of 3200 dpi using Twain software (Vol. 1.00; Epson)(Reference Cremers and Weinberg23). The lesions appeared red against a blue background. This technique gives results that are comparable with established lesion imaging methods(Reference Cremers and Weinberg23).
Lesion mapping
The distribution of lesions was quantified with a frequency-mapping technique. Lesion frequency was analysed at high resolution around the branches and at lower resolution in the whole aortic segments (including the same branches); the branches were analysed separately because it is in these locations that changes in lesion distribution with age have been observed. For the regions around the branches, areas of the scanned images, equivalent to 2·4 × 3·6 mm before magnification and centred on the branch ostium, were selected using V++ software (Digital Optics, Auckland, New Zealand). For the whole segments, the entire scanned image was used. Each image or sub-image was imported into spreadsheet software (Excel, Microsoft, Redmond, WA, USA) as a background, and the spreadsheet was adjusted to give a superimposed transparent grid(Reference Cremers and Weinberg23). For the branches, a grid of 20 × 30 squares, each equivalent to 120 μm in length before magnification, was used. For the descending thoracic or upper abdominal segments, a coarser grid (each element equivalent to 600 μm in length) was used.
The presence of lesions was recorded by manually entering the value ‘1’ into the grid squares that had lesions covering more than 50 % of their area. One person (S. G. C.) constructed all maps in order to eliminate inter-operator variability. Our previous study has shown that manual scoring of disease is repeatable(Reference Barnes and Weinberg24). Coding the presence of disease as ‘1’ permitted mean maps and mean frequencies to be calculated by the spreadsheet software using simple mathematical operations(Reference Cremers and Weinberg23).
To obtain the average distributions near the branches, maps were combined using the centre of the ostium as a datum. To obtain the average distributions for the whole descending thoracic or upper abdominal segments, maps were combined using alignments that gave the best superimposition of the branch points. Since the segments had been excised with slightly different lengths, this procedure resulted in the summary map for each group lacking data from increasing numbers of the rabbits towards its proximal and distal ends. When calculating the mean frequencies, such regions were not included if they lacked data from more than two animals. The aortic segments also had slightly different widths. This variation was too small to warrant correction; it gave rise only to minor inaccuracies at the lateral margins of the summary maps.
Statistics
Sample sizes were chosen before the trial commenced. Data are presented as means with their standard errors. Comparisons between the groups were analysed using Student's unpaired t test, or ANOVA where data were available from more than one time point.
Results
Rabbit weights and plasma composition are given in Table 1. Mean lesion frequencies are given in Table 2.
Table 1 Weights and plasma composition of immature and mature rabbits fed cholesterol or cholesterol+l-arginine
(Mean values with their standard errors)

cGMP, cyclic guanosine monophosphate.
* Total cholesterol integrated over the whole duration of the trial by a linear interpolation of the weeks 0, 3 and 7; data were 19·2 (sem 2·28), 22·0 (sem 2·78), 7·23 (sem 2·21) and 8·66 (sem 2·23) mmol × d/l for the immature cholesterol, immature cholesterol+l-arginine, mature cholesterol and mature cholesterol+l-arginine groups, respectively.
Table 2 Mean lesion frequencies at different anatomical sites in immature and mature rabbits fed cholesterol or cholesterol+l-arginine
(Percentages of aortas or branches in which lesions occurred, averaged over all the squares in a map, with their standard errors)
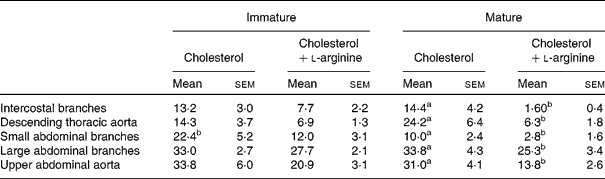
a,b Mean values with unlike superscript letters within a row were significantly different (P<0·05).
Body weight and food consumption
The weights of the mature rabbits were approximately stable, while the weights of the immature rabbits increased during the trial. There was no significant effect of dietary l-arginine supplementation on body weight in immature (P = 0·062) or mature (P = 0·617) rabbits during weeks 3–7. All groups consumed essentially all the food given to them.
Plasma cholesterol concentrations
On the normal diet (week 0), plasma cholesterol concentrations were higher in immature rabbits than in mature rabbits (P < 0·001), as found previously(Reference Barnes and Weinberg16, Reference Barnes and Weinberg24). Concentrations rose in all groups during the trial. There was no significant effect of dietary l-arginine supplementation in immature (P = 0·309) or mature (P = 0·355) animals during weeks 3–7, but there were significant effects of age for both control (P < 0·001) and l-arginine-supplemented (P < 0·001) animals, with the immature animals having higher concentrations.
Plasma TAG concentrations
Pre-trial concentrations in immature and mature rabbits were similar (P = 0·659). Concentrations rose in all groups after week 3, but not more than twofold. There was no significant effect of dietary l-arginine in immature (P = 0·578) or mature (P = 0·614) animals for weeks 3–7, and no significant effect of age in the control (P = 0·105) or l-arginine-supplemented (P = 0·776) animals.
Plasma l-arginine concentrations
There was no pre-trial difference between immature and mature animals (P = 0·91). Concentrations fluctuated, but l-arginine supplements significantly elevated plasma l-arginine concentrations in both immature (P = 0·024) and mature (P < 0·001) animals, averaged over weeks 3–7. Over the same period, there was no significant effect of age in either the control (P = 0·527) or the l-arginine-fed (P = 0·359) animals.
Plasma nitrite plus nitrate concentrations
Pre-trial nitrite plus nitrate concentrations were essentially identical in immature and mature rabbits (P = 0·787). Concentrations rose in all groups during the trial. l-Arginine supplements had no significant effect in immature (P = 0·112) or mature (P = 0·082) animals during weeks 3–7, but there was a non-significant trend for levels in the l-arginine-supplemented groups to be higher (by 14 % on average). During the same period, there was no significant difference between immature and mature control animals (P = 0·784), but there was a significant difference between immature and mature animals given l-arginine supplements (P = 0·006), with the immature animals having higher values.
Plasma cyclic guanosine monophosphate concentrations
cGMP concentrations were determined for week 7 only. There was no significant effect of age with (P = 0·345) or without (P = 0·607) dietary l-arginine, and dietary l-arginine itself had no statistically significant effect in immature (P = 0·469) or mature (P = 0·760) animals. However, as with nitrite plus nitrate concentrations, there was a non-significant trend for l-arginine-supplemented groups to have higher concentrations than those in their respective controls (by 41 % on average), and for l-arginine to have a stronger influence in immature animals.
Lesion patterns and frequencies
Intercostal branch ostia
Fig. 1 shows typical lesion patterns for the four experimental groups. Fig. 2 shows the corresponding maps of lesion frequency. Lesions occurred in an arrowhead-shaped area surrounding the downstream half of immature ostia, but they occurred mainly at the lateral margins of mature ostia. l-Arginine did not appear to change the patterns, although lesion frequencies in the mature l-arginine-fed group were so low that a pattern was hard to discern.

Fig. 1 Typical lesion patterns near the intercostal branch ostia of immature and mature rabbits fed cholesterol with or without l-arginine supplements. All images show the aortic luminal surface en face. Oil Red O staining of fatty streaks appears darker than non-lesioned areas counterstained with Evans blue dye.

Fig. 2 Maps of the lesion frequency of Oil Red O staining near the intercostal branch ostia of immature and mature rabbits fed cholesterol with or without l-arginine supplements. Maps show the same area in the same orientation as in Fig. 1. Shading indicates the percentage of branches in which lipid staining was observed at each location. ![]() , 40–100 %;
, 40–100 %; ![]() , 30–39 %;
, 30–39 %; ![]() , 20–29 %;
, 20–29 %; ![]() , 10–19 %;
, 10–19 %; ![]() , 0–9 %; ×, ostial centre.
, 0–9 %; ×, ostial centre.
Mean lesion frequencies within this region were not significantly different for immature (13·2 (sem 3·0) %) and mature (14·4 (sem 4·2) %) rabbits fed cholesterol without added l-arginine (P = 0·832). In immature animals, l-arginine supplementation did not have a significant effect on mean frequency (13·2 (sem 3·0) % control v. 7·7 (sem 2·2) % arginine; P = 0·159), but in the mature rabbits, it caused a highly significant reduction of approximately an order of magnitude (from 14·4 (sem 4·2) to 1·60 (sem 0·4) %; P = 0·007).
Descending thoracic aorta
Typical lesion patterns in the descending thoracic aortas are shown in Fig. 3. Fig. 4 shows the corresponding maps of lesion frequency for each group. (Gaps at the edges of the maps are artefacts arising from the variable width of the aortas.) In both age groups, lesion frequencies were higher in the proximal than in the distal part of the segment; this trend was stronger for the immature animals. In the proximal part, lesions affected most of the circumference of the vessel. More distally, lesions occurred in a dorsal stripe and also, further down the thoracic segment, in a stripe that started on the anatomical left side, switched to the ventral region and finally approached the right side. This spiral pattern is clearly visible in the mature control aorta shown in Fig. 3. It was less obvious in immature aortas, where lesions were concentrated around the downstream margin of branches. There is some suggestion of a spiral streak in the immature control map (Fig. 4(c)), but it was not as pronounced as for the mature control group, in which such a streak was visible. Dietary l-arginine supplements did not change the pattern in either age group.

Fig. 3 Typical lesion patterns in the descending thoracic aortas of immature and mature rabbits fed cholesterol with or without l-arginine supplements. The luminal surface of the segment, opened ventrally, is shown en face. (The aortas are divided at the third pair of intercostal ostia, which was removed for a different study.) Staining is the same as shown in Fig. 1.

Fig. 4 Maps of the lesion frequency of Oil Red O staining in the descending thoracic aortas of immature and mature rabbits fed cholesterol with or without l-arginine supplements. Orientation is the same as in Fig. 3, and shading as in Fig. 2. Because aortic geometry varied between animals, branches are only approximately superimposed. Similarly, not all aortas were of the same length; maps were truncated to show only the regions where data were available from three or more animals. ![]() , 80–100 %;
, 80–100 %; ![]() , 60–79 %;
, 60–79 %; ![]() , 40–59 %;
, 40–59 %; ![]() , 20–39 %;
, 20–39 %; ![]() , 0–19 %.
, 0–19 %.
Mean lesion frequencies were not significantly dependent on age in the animals fed cholesterol without additional l-arginine (14·3 (sem 3·7) % immature v. 24·2 (sem 6·4) % mature; P = 0·199). The mean frequency was not significantly reduced by arginine in the immature rabbits (14·3 (sem 3·7) % control v. 6·9 (sem 1·3) % l-arginine; P = 0·078), but there was a large, significant reduction in the mature animals (24·2 (sem 6·4) % controls v. 6·3 (sem 1·8) % arginine; P = 0·016).
Small abdominal branch ostia
Patterns were similar to those for intercostal ostia but showed substantial skewing to the anatomical left side at both ages (data not shown). Again, l-arginine did not appear to affect these distributions, although for the mature group, the frequencies were too low to be certain.
Mean lesion frequencies on the control diet were significantly higher in immature than in mature rabbits (22·4 (sem 5·2) % v. 10·0 (sem 2·4) %; P = 0·04). This is the only region for which such a difference occurred. In immature rabbits, dietary l-arginine supplementation had no significant effect on mean frequency (22·4 (sem 5·2) % controls v. 12·0 (sem 3·1) % arginine; P = 0·11), but in mature rabbits, it caused a significant reduction (10·0 (sem 2·4) % v. 2·8 (sem 1·6) %; P = 0·025).
Large abdominal branch ostia
Maps for the larger abdominal branches (coeliac, superior mesenteric and renal) are given in Fig. 5. Lesion patterns differed from those at the intercostal and small abdominal branches. In immature control animals, lesions occurred in an arrowhead-shaped region at the sides and downstream of branches, but frequencies were lower along the midline than at other locations within this area. (The full arrowhead is visible in the maps and examples of the entire abdominal segment, described below.) In mature control animals, lesions were still present in these locations but additionally occurred upstream of the branches, particularly on the anatomical right side. The left lateral margin was least affected. The distributions were not changed by l-arginine supplements.

Fig. 5 Maps of the lesion frequency of Oil Red O staining near the large abdominal branch ostia of immature and mature rabbits fed cholesterol with or without l-arginine supplements. Orientation, size and frequency coding are as described for Fig. 2 except that the segment was opened dorsally. ![]() , 80–100 %;
, 80–100 %; ![]() , 60–79 %;
, 60–79 %; ![]() , 40–59 %;
, 40–59 %; ![]() , 20–39 %,
, 20–39 %, ![]() , 0–19 %; ×, ostial centre.
, 0–19 %; ×, ostial centre.
Mean frequencies were particularly high around these larger branches. They were essentially identical in the immature and mature control animals (33·0 (sem 2·7) % immature v. 33·8 (sem 4·3) % mature; P = 0·285). l-Arginine supplements did not have a significant effect on mean frequency in the immature animals (33·0 (sem 2·7) % control v. 27·7 (sem 2·1) % arginine; P = 0·144) but significantly reduced disease in the mature animals (33·8 (sem 4·3) % control v. 25·3 (sem 3·4) % l-arginine; P = 0·026).
Upper abdominal aorta
Typical images are shown in Fig. 6, and corresponding maps of the lesion frequency are shown in Fig. 7. Lesions occurred particularly frequently in the vicinity of the coeliac, superior mesenteric and renal branches, so the proximal part of the upper abdominal aorta was more affected than the distal part. At both ages, a ventral stripe of high frequency was observed between the coeliac and superior mesenteric branches. A stripe of high prevalence was also seen on the right of the proximal part of the map for mature animals; this is a continuation of the spiral pattern seen in the thoracic segment (the discontinuity in the maps arising because the thoracic segment was opened ventrally, while the abdominal segment was opened dorsally). l-Arginine supplements did not change the pattern at either age.

Fig. 6 Typical lesion patterns in the proximal abdominal aortas of immature and mature rabbits fed cholesterol with or without l-arginine supplements. The segment was opened dorsally and divided at the left renal artery branch, which was removed for a different study. Staining is the same as in Fig. 1.
There was no effect of age on the mean frequency of lesions in the control groups (33·8 (sem 6·0) % immature v. 31·0 (sem 4·1) % mature; P = 0·700). These means are higher than those for the thoracic segment. l-Arginine supplements had no significant effect in immature animals (33·8 (sem 6·0) % control v. 20·9 (sem 3·1) % arginine; P = 0·078) but did in mature animals (31·0 (sem 4·1) % control v. 13·8 (sem 2·6) % arginine; P = 0·003).
Discussion
Animals fed cholesterol without additional l-arginine are considered first. In immature rabbits, lesions occurred in an arrowhead-shaped region surrounding the downstream half of intercostal branch ostia. Since almost all studies of experimental atherosclerosis in rabbits have used immature animals ( < 6 months old), this pattern has been widely reported. In mature rabbits, however, the downstream region was spared and lesions instead occurred at the lateral margins of the ostia. Such a switch has been inferred from earlier reports(Reference Barnes and Weinberg16, Reference Staughton, Weinberg and Clarke18) but has not previously been demonstrated in a single trial. The rare spontaneous lipid deposits affecting weanling and aged rabbits show a similar switch(Reference Barnes and Weinberg24). The patterns correlate spatially with age-related variations in wall permeability(Reference Sebkhi and Weinberg25, Reference Ewins, Majewicz and Staughton26) and may be determined by them.
The effect of age on diet-induced lesions at abdominal branches has also not previously been examined in a single trial. Lesions occurred around the downstream half of the large ostia in immature rabbits, with some sparing along the centreline. In mature animals, lesions again developed at these sites but additionally occurred upstream and to the right of the branches. Again, a parallel switch in the distribution of spontaneous lesions has been observed(Reference Barnes and Weinberg24). At the small segmental branches of the abdominal aorta, patterns were more similar to those seen at intercostals, although strongly skewed to the left at both ages.
There were no large-scale changes with age in the non-branch regions of the rabbit aorta. The effects of age thus appear to be branch-related, and consequently may reflect alteration of local factors such as flow or wall strain rather than global changes in pathogenetic mechanisms. However, one feature of the distribution in non-branch regions – the spiral pattern of lesions – was age-dependent; it was more evident in mature animals. It may have been caused by helical flows arising from the non-planar curvature of the aortic arch or the influence of major branches(Reference Caro, Doorly and Tarnawski27). A spiral pattern of lesions has been reported in human coronary arteries(Reference Fox, James and Morgan28) but not in the human aorta(Reference Svindland and Walloe29, Reference Cornhill, Herderick and Stary30).
Near the intercostal and large abdominal branches, and in the upper abdominal segment as a whole, mean lesion frequencies did not change with age. In the descending thoracic segment, there was a tendency for higher mean frequencies in the mature group, whereas at the small segmental branches of the abdominal aorta, mean frequencies were lower in these animals (the only such difference that reached statistical significance). Overall, mean frequencies were remarkably constant. The mature animals had plasma cholesterol concentrations approximately 40 % of those in the immature animals despite an identical dietary intake. The invariant mean lesion frequency is therefore consistent with older animals, similar to older people, being more lesion-prone, as previously observed(Reference Spagnoli, Orlandi and Mauriello17). This trend means that the effects of age cannot simultaneously be assessed at the same plasma cholesterol concentration and at the same lesion area. In the present study, comparisons were made in animals having the same lesion area.
The normal diet contained approximately 1 % l-arginine by weight so the groups receiving dietary l-arginine supplements had a sixfold increase in intake, similar to earlier studies. The effect of such supplements on plasma arginine concentrations has been highly variable within and between these studies, with increases ranging from 0- to 4·5-fold(Reference Jeremy, Mc Carron and Sullivan10). Pharmacokinetic studies have demonstrated that plasma levels drop rapidly after bolus or repeat bolus dietary intake (t 1/2 = 1–2 h in people(Reference Boger and Bode-Boger31)), so measured plasma levels may critically depend on the interval between ingestion and blood sampling. In the present study, blood was collected after a 16 h fast in order to avoid the short-term effects of diet on plasma lipid and nitrite plus nitrate levels. There was no pre-trial difference in plasma l-arginine concentration between immature and mature rabbits. This lack of difference with age was maintained throughout the trial for animals on both the cholesterol and cholesterol plus l-arginine diet. Plasma l-arginine concentrations did increase in the groups receiving l-arginine supplements, as expected; the detected increase may underestimate the time-averaged increase as a result of the 16 h fast.
l-Arginine had no detectable effect on the pattern of lesions in either age group. (We have previously shown that inhibitors of NO synthesis also have no significant effect in mature animals(Reference Staughton, Weinberg and Clarke18).) In contrast, it had profound effects on the mean frequency of lesions, and these effects were age-dependent. At all three types of branch and in both aortic segments, l-arginine significantly reduced mean frequencies in the mature but not in the immature rabbits. There were trends towards a reduction in all regions in immature animals that might have attained statistical significance with a substantially larger sample size, but the reductions in the mature animals were consistently much bigger. Notably, mean frequencies at intercostal branch ostia in mature animals were reduced by an order of magnitude.
Instead of confirming these frequency-mapping results by conventional histology, we applied micro-attenuated total reflection–Fourier transform infrared spectroscopic imaging to sections of the wall, in a separate study(Reference Palombo, Cremers and Weinberg22). This technique, dubbed ‘chemical photography’, gives the spatial distribution of infrared absorbance due to specific chemical bonds, without the need for staining. It revealed high levels of lipid esters downstream of the intercostal branch points in immature rabbits fed cholesterol or cholesterol plus l-arginine, high levels lateral to the intercostal branches in mature rabbits fed cholesterol and undetectable levels in mature rabbits fed cholesterol plus l-arginine(Reference Palombo, Cremers and Weinberg22). (In the first three groups, the highest levels were seen in cell-sized clumps, as would be expected if the esters were concentrated within foam cells(Reference Palombo, Cremers and Weinberg22).) These data are consistent with the results shown in Figs. 1 and 2, but additionally demonstrate that the trends apply across the thickness of the intima-media and are not restricted to the more superficial layers visualised by en face techniques.
The change in the efficacy of l-arginine could be explained by a decline in the NO pathway with age, which has been documented in rabbits(Reference Orlandi, Marcellini and Spagnoli19) as well as in humans(Reference Gerhard, Roddy and Creager32). The decline might make the pathway more susceptible to the adverse effects of hypercholesterolaemia and lipid deposition, and more susceptible to rescue by l-arginine. A corollary of this hypothesis is that NO synthase inhibitors should have more effect on lesion development in immature animals, where the NO pathway functions better. An alternative hypothesis is that the higher plasma cholesterol concentrations in immature animals blocked the restorative effect of l-arginine. (Plasma TAG concentrations were unaffected by age or l-arginine supplementation and hence cannot be involved in any such effect; they are not considered further.) The spread in cholesterol concentrations within each experimental group allows us to test this second hypothesis. Fig. 8 plots disease prevalence against plasma cholesterol concentration on a rabbit-by-rabbit basis. (Since each point now shows data for a single rabbit, we increased accuracy by using the lesion data for intercostal branches, many of which were examined in each animal, and by using terminal cholesterol concentrations, which were measured in larger blood samples than those obtainable from conscious animals during the trial.) This graph confirms that lesion prevalence was the same in the immature and mature groups not given l-arginine, and that cholesterol concentrations were on average higher in the former group. Considering next the l-arginine-treated animals, if the atheroprotective effect had decreased with increasing plasma cholesterol, an increase in lesion prevalence with increasing cholesterol concentration would be expected, regardless of age. If, on the other hand, the atheroprotective effect was dependent on age rather than cholesterol concentration, a discontinuity would be expected in the trend for lesion prevalence at the cholesterol concentration separating the mature from the immature animals. The data unequivocally show the latter trend, and we therefore conclude that the atheroprotective effect of l-arginine varies with age per se.

Fig. 8 Mean lesion frequencies around intercostal branch ostia are plotted against terminal plasma concentrations of total cholesterol for immature (■, □) and mature (▲, Δ) rabbits fed cholesterol (▲ ■, —) or cholesterol plus l-arginine (Δ □, – – –). The sharp discontinuity in the trend between immature and mature animals given l-arginine is consistent with an effect of age rather than cholesterol concentration on the atheroprotective effect of this supplement.
The bioactivity of basal (i.e. flow-mediated) NO release in large arteries in vivo is the key parameter, but there are currently no practicable, widely accepted methods for assessing it; surrogate indices have to be used. We chose not to measure the response of arterial rings to acetylcholine, used in many earlier studies, since it involves a signalling pathway that is distinct from the flow-stimulated one(Reference Fleming, Bauersachs and Busse33) and differs in its sensitivity to hypercholesterolaemia(Reference Hutcheson, Smith and Griffith34). Instead, we measured plasma concentrations of nitrite plus nitrate and of cGMP. Nitrite and nitrate are the oxidation products of NO. Differences in NO production are therefore reflected in their plasma concentration, regardless of how rapidly NO is inactivated. NO binds to the haem moiety of soluble guanylate cyclase and thereby increases the production of cGMP. Concentrations of cGMP are consequently a measure of NO activity.
Concentrations of nitrite plus nitrate increased in all groups during the trial. This could reflect enhanced synthesis of NO by endothelial NO synthase to overcome its increased inactivation during hypercholesterolaemia(Reference Minor, Myers and Guerra35) or by the inducible NO synthase, which is known to be up-regulated during the development of disease. Although nitrite plus nitrate and cGMP concentrations were higher on average in animals given l-arginine supplements, these trends did not reach significance and were more pronounced in the immature than in the mature animals. These results do not necessarily contradict the hypothesis that l-arginine rescues the NO pathway more effectively in mature animals because l-arginine could have directly increased NO synthesis by endothelial NO synthase and indirectly decreased NO synthesis by inducible NO synthase (by reducing disease), giving rise to complex effects. Alternatively, l-arginine could have affected mean lesion frequency through other pathways. For example, it has antioxidant effects and stimulates growth hormone, insulin, glucagon and prolactin release(Reference Boger and Bode-Boger31). Further investigation of the mechanisms involved will require the development of new techniques for assessing NO bioactivity in vivo.
In conclusion, the present study demonstrated a stronger effect of dietary l-arginine on experimental atherosclerosis in mature than in immature cholesterol-fed rabbits. This finding may account at least in part for discrepancies in the literature concerning the protective effect of l-arginine. The precise mechanisms remain to be established. Since human aortas show similar age-related changes in lesion location and type to those seen in rabbits, and a similar decline in the NO pathway with age, the data may also have relevance to the atheroprotective effects of l-arginine in human aortas.
Acknowledgements
The technical assistance of J. del Rio and R. Blank is gratefully acknowledged. There are no conflicts of interest. The study was supported by British Heart Foundation project grant PG 02/046 awarded to P. D. W. The work was conducted by S. G. C. with assistance from P. D. W.; S. J. W. conducted the l-arginine analyses. S. G. C. and P. D. W wrote the manuscript.













