INTRODUCTION
Infections by Campylobacter spp. are the single biggest cause of bacterial gastroenteritis in the developed world. Reported cases exceeded 46 000 in the United Kingdom in 2004 [1] and there is evidence that disease is under-reported [Reference Charlett2, Reference Wheeler3]. Cases of Campylobacter infections in The Netherlands alone have been estimated at costing €21–36 million annually [Reference Mangen, de Wit and Havelaar4]. Identification of Campylobacter spp. as pathogens was noted in the 1970s [Reference Butzler5], and the subsequent search for cause of infection in humans identified poultry as a risk factor over 20 years ago [1]. Production of broilers for the United Kingdom in 2002 was about 1·2 million tonnes of carcasses [Reference Sheppard6]. A survey of meat on retail sale in the United Kingdom in 2002 [Reference Meldrum, Tucker and Edwards7] found that between 46% and 52% of raw poultry was contaminated with Campylobacter. On this basis, potential sources of entry of the bacteria into the human food chain are great. Although negative flocks can be cross contaminated with Campylobacter during poultry processing, the number of organisms on these carcasses is far less than on carcasses originating from Campylobacter-positive flocks [Reference Allen8]. Thus the farm on which they are reared is the most important place for control to reduce carcass contamination. Identifying risk factors for infection of chickens on the farm is the first point for addressing this problem.
There have been many studies of risk factors for Campylobacter in broilers and a key feature of many of these is the collation of data describing the prevalence of infection in flocks and measures of a series of putative covariates deemed to play a role in this process. Many factors have been identified as contributing to risk of infection and these factors are associated with processes that operate over several scales. Many of these factors interact, with processes at one scale impacting on factors more proximal to the infection of the individual broiler chicken, indicating that pathways to infection are multifactorial and complex (Fig. 1). Campylobacter forms long-term associations with its host [Reference Young, Davis and DiRita9] and the organism is found in a wide variety of wild mammals and birds and the faeces they produce. The outside environment therefore acts as the ultimate source of infection for housed broiler flocks and unsurprisingly flock infection has been related to these external sources, such as the presence of livestock outside [Reference Bouwknegt10–Reference van de Giessen14] and wild rodents [Reference McDowell15]. However, the incidence of infection in humans [Reference Kovats16, Reference Patrick17] and broilers [Reference Wallace18] also shows seasonality. In humans cases peak in spring, earlier following mild winters [Reference Kovats16]. This suggests that larger scale processes involving weather and climate impact on the sources and survival of the pathogen in the outside environment. Weather may also have indirect effects on the processes involved in introduction of the pathogen to the broiler house, since the activity of fly vectors is dependent on temperature. Management of the external environment and the broiler house itself also impact on the incidence of infection. External manure storage has been recorded as having a protective [Reference Guerin19] as well as promoting effect [Reference Cardinale11], although the effects in the former case may have been related to local storage practice rather than a beneficial effect of storage sensu lato. Risk of infection has also been positively associated with the size of the enterprise, specifically the number of sheds and the size of the flocks [Reference Guerin19]. Internal management within the shed may also mitigate the impacts of environmental sources through such activities as disinfection [Reference McDowell15, Reference Evans and Sayers20] and removal of internal sources of infection such as dead birds [Reference Evans and Sayers20] and ventilation [Reference Barrios21]. Broiler houses are likely to be continuously surrounded by many potential sources of contamination and, as such, there are many risk factors that could contribute to individual infection events. Disentangling cause and effect is made complex because of the interaction of the processes over different scales. One approach that might provide more insight into the relative significance of different risk factors would be to focus on longitudinal studies of incidence of infection on a restricted number of farms, where many of the covariates associated with environmental risk can be measured, held constant or accounted for in the design of the monitoring. A longitudinal study allows for investigation of fixed farm and house effects, and analyses of seasonality all of which have been previously associated with Campylobacter infection in poultry [Reference Patrick17].
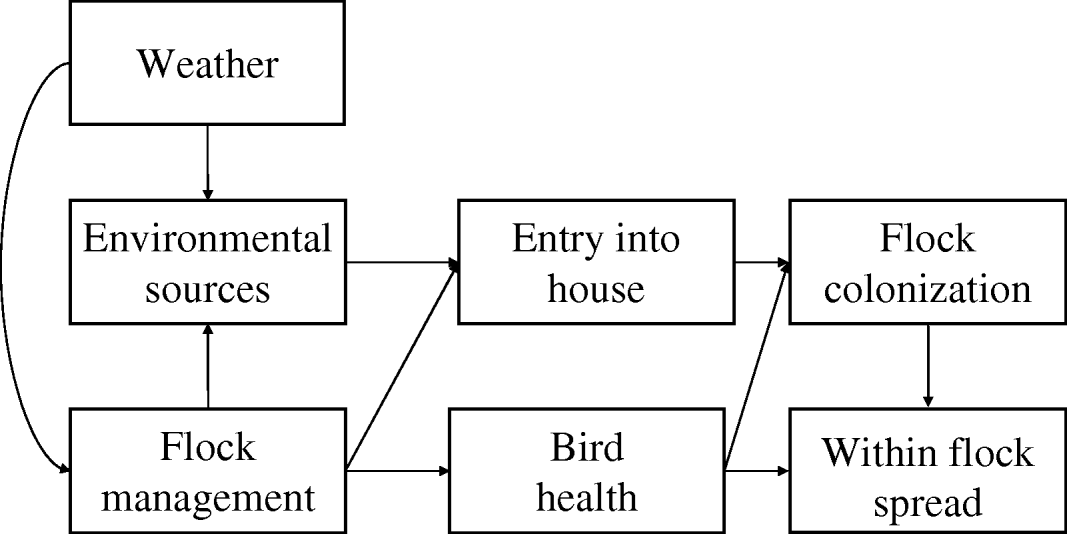
Fig. 1. Causal path map showing likely pathways to infection of broiler chickens by Campylobacter.
We recently completed a large study on the incidence of Campylobacter in 789 housed broilers flocks in Great Britain. Aspects of that study will be published elsewhere and will include an assessment of the impact of bird health and season. Flocks from 214 farms were examined at least once. However, 30 farms were tested up to 13 times. In the present study we analyse data on the incidence of Campylobacter in chickens from this longitudinal subset of farms from one integrated poultry company using Generalized Linear Mixed Effect Models (GLMMs) and survival analysis. We used GLMMs to investigate the extent to which the occurrence of Campylobacter infection in broiler flocks was related to environmental and management risk factors. We then used survival analysis to analyse the time to infection using two underlying conceptual models. In the first of these we tested the extent to which infection events on farms were opportunistic and independent, whilst in the second we tested the extent to which events were predicated by previous events.
METHODS
Study population and data collection
The sample design for this study follows that described in Bull et al. [Reference Bull22] and differs from it insofar as only those farms subjected to a sequence of repeated sampling are considered here. Briefly, samples of chickens were collected from indoor flocks reared on 30 farms supplying one integrated poultry company over the period 2004–2006. The majority of flocks sampled were Ross or Cobb standard broilers. Where possible, farms were visited in successive crop cycles. In practice this was not possible and the dataset was unbalanced with the maximum number of flocks per farm sampled ranging from six to 13 times.
Thirty birds were sampled at random at slaughter from each flock at first full or partial harvest and intact pairs of caeca were collected from each immediately after evisceration. Each bird was tested separately for the presence of Campylobacter. This sampling protocol allowed us to detect infection where the prevalence was >10% in the flock with 95% confidence [Reference Thrusfield23]. In our study, caecal contents from each pair collected were sampled by immersing a swab (Medical Wire and Equipment, Wiltshire, UK) into a caecum, which was opened by an incision at the blind end. The swab was streaked onto modified charcoal cefoperazone deoxycholate agar (mCCDA) (Oxoid CM739 with SR155 supplement), which was incubated at 37°C for 48 h in a microaerobic atmosphere. The atmosphere was achieved by evacuating the air from gas jars (Don Whitley Scientific Ltd, West Yorkshire, UK; Launch Diagnostics Ltd, Kent, UK) and replacing it with a gas mixture that resulted in an atmosphere comprising: 5–6% O2, 3–7% CO2 and 7% H2, in a balance of nitrogen. After incubation, mCCDA plates were examined for the presence of typical Campylobacter colonies (greyish, flat and moist colonies, with a tendency to spread). One to three typical colonies per sample were subcultured onto duplicate plates of Columbia blood agar with 5% (v/v) defibrinated horse blood (COLBA; Oxoid Ltd, Hants, UK) and confirmed as Campylobacter using standard tests.
Selection of covariates
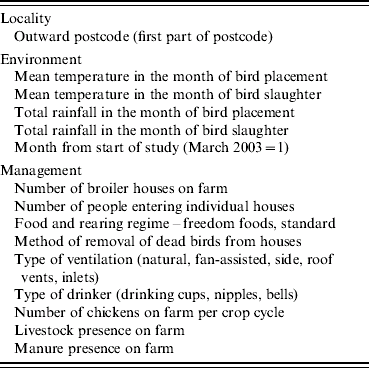
We selected covariates for inclusion in the modelling on the basis of their reported role in colonization and subsequent within-flock spread and their likely theoretical involvement in both processes. The key variables were potential sources of Campylobacter in the outside environment (land use patterns, dung heaps, etc.); weather which might impact on survival of Campylobacter in the environment or influence ingress to broiler houses (specifically temperature and rainfall); and management factors that might allow ingress of the bacteria (people entering the house) and those that might accentuate spread amongst birds in the house (the form of water provision). These farm- and flock-specific covariates were collected at the time of flock harvest and they included indicators of bird health collected post mortem which has been considered a risk factor for colonization and spread (see Table 1 and ref. [Reference Bull22]).
Table 1. List of covariates collected from farms
Data analysis
We used GLMMs and survival analysis to investigate the longitudinal nature of infection in chicken flocks. In the mixed-effect modelling we undertook two types of analysis. First we used the number of positive cases out of 30 birds tested in each flock as an indicator of flock prevalence of infection. We did not use a Poisson error structure in these models because the number of chickens sampled was finite and all chickens in the sample on some farms were colonized with Campylobacter (offending the underlying assumptions of the Poisson model). Second, we used the presence or absence of infection in a flock as a categorical variable, in effect a simple descriptor of whether or not the flock was infected. Therefore, the flock prevalence analyses were undertaken with binomial error structures, the former with numbers of cases and a denominator of total number of birds tested, the latter simply a binomial trial of yes/no the flock being infected. We adopted a progressive model-building strategy following Pinheiro & Bates [Reference Pinheiro and Bates24]. We first used a step-down modelling strategy with simple binomial regression to identify covariates associated with the presence of Campylobacter in chickens and flocks (with binomial error structure and relevant weights), removing those that were non-significant in simple models. Models and parameter estimates derived with this analysis might be biased because of serial dependency in the response variable and the potential impact of random effects arising from unmeasured farm-specific factors. We went on to investigate farm effects as a factor using GLMMs with a binomial error structure and the inclusion of different random effects for the intercepts at the level of the farm. We then tested whether the inclusion of random effects for individual covariates on farms improved model fit under the assumption that the response to the covariates varied with farm. We assessed the extent to which there was serial correlation in the data by assessing autocorrelation in the models and then re-fitted the models with a correlation structure allowing for serial dependence where appropriate. We assessed the distributional assumptions of the models by analysis of the residuals, and abandoned models if distributional assumptions were not met. Models were fitted in the statistical package R [25] using glm and the glmmML library of Broström [Reference Broström26].
In order to investigate the hazard of farms becoming infected with Campylobacter over time, we used Cox proportional hazard models to assess the extent to which the timing of infection events on farms could be explained in terms of the measured covariates. In these analyses we assumed that the detection of Campylobacter in a flock at slaughter constituted an ‘event’. The Cox model assumes that there is an underlying unspecified baseline hazard of events occurring, which stays constant through time (in this case of farms becoming infected) that is influenced by covariates that enhance or mitigate the risk of the event occurring. We used three models that made different assumptions about the infection process on the farms. We used time to the first event as a baseline model. We investigated the effects of event number on individual farms as a covariate. We then compared the baseline model against two models which treated incidents of infection at the flock level as ordered events. In the first of these we assumed that each infection event on a farm was independent of the others; the independent increment or Andersen–Gill model. In the second we used a conditional model in which it was assumed that infection events were predicated by preceding events (the conditional or Prentice–Williams–Petersen model). The two models effectively assess the dependency of infection events on individual farms. The independent increment and conditional models treat the data as time-ordered outcomes and differ in their use of stratification [Reference Therneau, Grambsch, Dietz, Gail, Krickeberg, Samet and Tsiatis27]. In the latter model the data are stratified by the event number, i.e. the sequential numbering of infected status since the start of the study. We used step-wise reduction to identify the parsimonious model from a full model with all covariates. We tested assumptions of proportionality in the parsimonious models in two ways. First, we plotted time-dependent coefficients for each covariate against time and assessed the change in coefficients with time visually. Second, we undertook a formal test correlating the scaled Schoenfeld residuals for each model with time for each covariate and assessed significance with a two-sided χ2 test (significance denoting evidence for deviation from a constant hazard of infection in relation to that covariate through time). All models were fitted with the Survival library of Therneau in R [25].
RESULTS
Of the flocks from 30 farms tested, four farms never produced a Campylobacter-positive flock. A total of 289 flocks were sampled, of which 94 (32·5%) were positive for Campylobacter. The number of Campylobacter-positive flocks recorded over the study period at each farm ranged from two to 10 (median 3). The distribution of the within-flock prevalence data had a tendency to bimodality with numbers of positive birds in individual flocks being low (mean of 3·94 out of 30) or being very high with all animals testing positive.
Incidence of Campylobacter at farm level
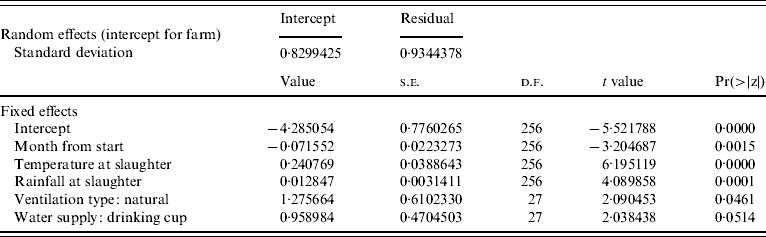
Results of a simple GLM analysing the incidence of Campylobacter at the level of the farm, with a binomial error structure (Table 2) suggested that incidence of infection declined with time and that weather conditions during the month of slaughter were important predictors. The residual deviance for the model was similar to the number of degrees of freedom suggesting that the binomial error model was a reasonable error structure for the data. The residuals from the parsimonious model (Table 3) containing the mean monthly temperature at slaughter and the total rainfall during the month of slaughter and the use of natural ventilation, were not serially correlated when farm was included as a factor, suggesting that the incidence of Campylobacter in a flock was not dependent on previous incidence of the pathogen. The results of the parsimonious GLMM, where farm was included as a random effect, are shown in Table 4. The mean monthly temperature and total rainfall during the month of slaughter, the use of natural ventilation, the use of nipples with drinking cups and the month from the start of the study were significant predictors. None of these variables were significant when modelled as random effects at the farm level; the best model included random effects for intercept only. This means that there were no differences in the farm-level response to the driving variables, but only differences in the underlying risk of infection on each farm. In other words the increase in risk of infection per unit increment in mean monthly temperature and rainfall was the same for all farms. Analysis of the residuals from the models showed that there was no serial dependency in that none of the residuals were significantly correlated up to a lag of 12 months. Inclusion of an off-diagonal error structure to account for serial dependency, whilst not necessary on this evidence, did not markedly alter the magnitude of the regression coefficients. This suggests that flock infections were independent of each other in time. The observed incidence of Campylobacter over time along with the predicted probability of incidence from the mixed-effect model is shown in Figure 2. Whilst it is difficult to make a simple comparison between categorical (0, 1) and binomial data (0 to 1), there is a close match between predicted and observed for many of the farms with peak and low incidences and the predicted probability roughly overlapping over time. The correlation between observed and predicted Campylobacter status of flocks was 0·646 (t=14·35, P<2·2×10−16) and this provides a crude estimate of the utility of the model. Clearly, weather (temperature and rainfall) is a key predictor in this model. Of the 289 flocks sampled the residuals were >1·97 standard deviations from zero for only nine flocks. Five of these occurred at the last sampling date in 2006, when Campylobacter was observed but not predicted. Analysis of the random-effect intercepts for the farms from the GLMM showed that these were negatively correlated with the easting geographical position of the farm (r=−0·406, t=−2·314, P<0·0282). This suggests that the incidence of infections was generally higher in flocks on western farms, even though the farms were all drawn from a small geographical region.

Fig. 2. Observed incidence (presence or absence in a flock) of Campylobacter over the period 2003–2006 in flocks on 30 farms compared with predicted probability of occurrence derived from a Generalized Linear Mixed Model with binomial error structure relating incidence to temperature and rainfall at slaughter, month, use of natural ventilation and drinking cups. ○- - -○, Observed data; ×–––×, predictions from best model.
Table 2. Regression diagnostics for a GLM relating presence or absence of Campylobacter infection in individual flocks to different covariates for 289 flocks sampled from 30 farms, 2003–2006

Null deviance: 364·59 on 288 degrees of freedom; residual deviance: 255·77 on 303 degrees of freedom; Akaike's Information Criterion: 287·77.
Significant covariates are shown in bold.
Table 3. Regression diagnostics for a parsimonious GLM relating presence or absence of Campylobacter infection in individual in flocks to different covariates for 289 flocks sampled from 30 farms, 2003–2006
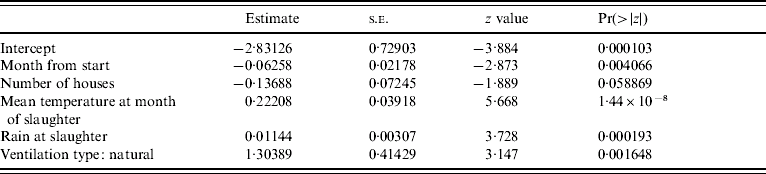
Null deviance: 364·59 on 288 degrees of freedom; residual deviance: 283·64 on 283 degrees of freedom; Akaike's Information Criterion: 295·64.
Table 4. Parsimonious binomial GLMMs relating incidence of Campylobacter spp. (presence or absence in flock) in 289 flocks sampled from 30 farms, 2003–2006
Prevalence of Campylobacter within chicken flocks
Analyses of the prevalence of Campylobacter in the 30 chickens sampled from each flock indicated that whilst most covariates were apparent significant predictors of the prevalence in flocks, the residual deviance (the unexplained variation) in the model was approximately an order of magnitude greater than the degrees of freedom. The data were markedly overdispersed suggesting that either the binomial error structure postulated was inappropriate and/or there were key variables missing from the model. Inclusion of a factor for farm did decrease the residual deviance by about 20% but still left the simple GLM overdispersed and effectively invalid. Further extension of the modelling to include farm as a random effect did not decrease the residual deviance. Extension to include a negative binomial error structure (not shown) markedly decreased the residual deviance in a parsimonious GLM, rendering it underdispersed, suggesting that the incidence of positive status in birds in flocks was aggregated. This error distribution was not strictly appropriate for the data given the upper constraint on the number of birds sampled. Given the large amount of overdispersion in the flock data and the intractability of modelling mixed-effect models where the response shows aggregation, no further analyses of prevalence of infection in flock were considered.
Survival analysis
A survival curve showing the flock infection over time across farms is shown in Figure 3. The ordinate represents the inverse of the hazard of infection (i.e. avoidance of infection) in flocks and is plotted on a log scale. In effect this shows that the probability of there not being an infection event on a farm declined exponentially with time. The most obvious feature is that the curve is steeper in the period up to 250 days, suggesting that hazard varied with time – with infections greater than expected (or those after 250 lower than expected) from an exponential model. However, there was a significant negative correlation between mean temperature at month of slaughter and the time of slaughter over the course of the study (r=−0·5459, P<1·8×10−11) indicating that the temperature at slaughter was generally higher in the first year of the study. The extent to which hazard of infection varied with time and temperature is analysed more fully below.
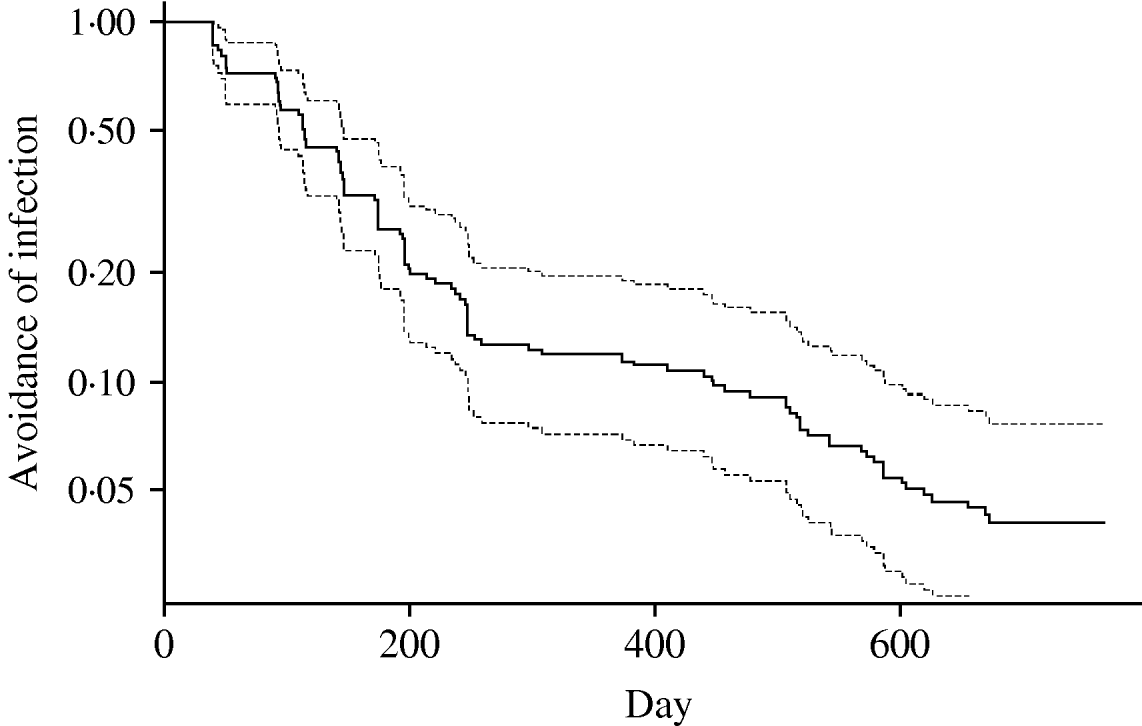
Fig. 3. Survival function for Campylobacter infections on the study farms, with associated 95% confidence limits. Curve represents probability of flock on a farm not becoming infected over time.
For the time to first-event model, with each farm included once only, mean temperature and rainfall at month of slaughter were significant positive predictors (not shown) whilst the presence of stock was also a significant predictor having a protective effect (odds ratio 0·494, 95% confidence interval 0·346–0·703). Inclusion of event number as a factor in a simple Cox model showed that the magnitude of coefficients for the event number (not shown) did not increase monotonically with the event number as might be expected if there was a serial dependency. Incidence of the third and fourth infections had negative coefficients relative to the first suggesting that there was a reduced risk of infection of a flock becoming infected after the first. However, given the small sample size (n=30) relative to the total number of infection events and census points recorded over the study (130) the first-event model is not considered further.
For the independent increment and conditional models there were many more events and in common with the first-event model mean monthly temperature at the month of slaughter (for both models) and the extent of pad burn for the independent increment model, were positively associated with the risk of infection. There was evidence that the effects of temperature and pad burn were not proportional in either model when analysed as monotonic covariates. Penalized models fitted with splines for mean temperature in the month of slaughter and pad burn showed that there was a significant nonlinear component to the effects of both covariates. This suggests that either a key variable was missing from the models or that the underlying assumptions of proportionality were violated. Inclusion of a quadratic term for mean monthly temperature at slaughter and pad in the independent event model showed that hazard increased to a peak at about 12·7°C and 34·9% pad burn at slaughter. This suggests that hazard of infection peaked at 12–13°C and declined above that peak. The best conditional model whilst also including a quadratic term for mean monthly temperature at slaughter did not include a quadratic term for pad burn. Regression diagnostics for the best models are shown in Table 5. Plots of time-dependent coefficients against time for each covariate and their quadratic terms (when included) in the best independent and conditional models were flat in all cases, suggesting that there was no time dependence in the covariates. In effect this meant that the hazard of a flock becoming infected as a result of increased temperature was the same over the study period. Tests for non-proportionality for all covariates (including quadratic terms where included) in both models were not significant (Table 5) indicating that the assumption of proportional hazards for both models was valid.
Table 5. Regression diagnostics for parsimonious Cox proportional hazards models for infection events from the 30 study farms
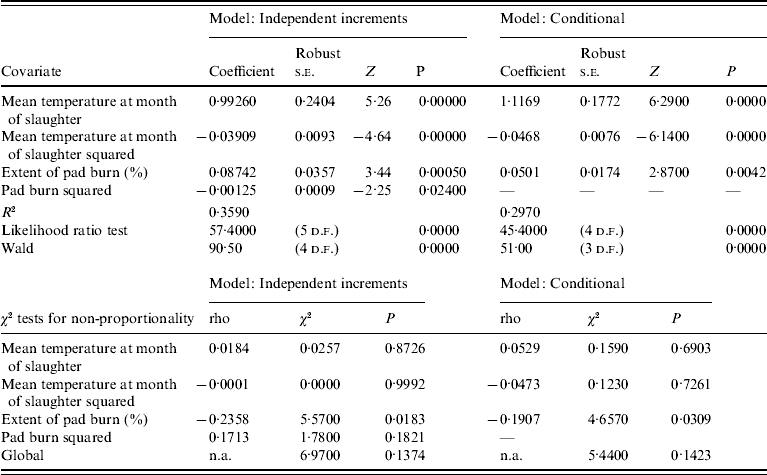
n.a., Not available.
The likelihood ratio and Wald tests for the independent increment model had higher levels of significance than for the conditional model suggesting that this was a better model for the data. This meant that the model which assumed that infection events on farms were independent of each other was a better model for the data than the one which assumed that there was serial dependency in events. In other words, infection events on farms were opportunistic rather than serially dependent.
DISCUSSION
Identifying risk factors for infection in animal production systems is a major issue in veterinary research as it provides the basis to control infection, to maximize productivity and minimize problems of animal welfare. Where the pathogen is widely distributed in the local environment and is carried by other organisms, disentangling causal relationships that link processes to infection for one host species is particularly difficult. The study presented here aimed at identifying risk factors for Campylobacter in chicken flocks using linear and mixed-effect models in combination with survival analysis to investigate prevalence patterns on farms over time. Our rationale was to undertake a longitudinal analysis on a comparatively small number of farms, where many of the risk factors would be either consistent or consistently measurable over the study period.
The mixed-effect models were undertaken at two scales, one considering prevalence of infection in a sample of 30 birds from each flock and the other considering flock infection as a simple binary yes/no response. The results of the within-flock prevalence analyses were poor, in that all models were overdispersed by at least one order of magnitude. The binomial error structure was clearly not appropriate for the data. In this context, overdispersion is likely to have arisen because the individual chicken responses – infected/not infected – were not independent samples [Reference Condon28]. It is likely that the incidence of infection in chickens is dependent on dynamic processes like contact with chickens that are themselves infected; indeed, feed, drinkers, and air become contaminated [Reference Bull29]. The overall pattern of flock prevalence across all farms was bimodal. Similar patterns of prevalence have been observed in theoretical analyses of infection dynamics in flocks of broilers [Reference Katsma30].
Analysing longitudinal data with random effects with non-normal error structures is problematic because the mathematical solution is complex [Reference Farady31] and relies on numerical approximation. This notwithstanding, the results of the farm-level models when analysed through a mixed-effect modelling approach were more clear-cut than those that included information on flock prevalence. These probably reflect the fact that by moving ‘up scale’ flock-dependent infection dynamics were factored out of the modelled response. There was a suggestion that infection events declined over the course of the study. This was manifested in terms of a significant trend in relation to time in the study from the GLMM analyses and a flattened tail for the survivorship curve, where the hazard of infection appeared to decline with time from the start. This was explained in part by differences in weather conditions (it was warmer at the beginning of the study), but it may also reflect other unmeasured farm- level risk factors, because there was a suggestion from the survival analyses that third and fourth events were less likely to occur following a first. More importantly, both the GLMM and the survival analyses showed that weather conditions during the month of slaughter were major features determining whether or not a farm had an infected flock through the sample period. Seasonality in Campylobacter infections is well known in human cases [Reference Kovats16, Reference Tam32] and has also been reported in broiler flocks in many studies [Reference Patrick17, Reference Stern33–Reference Wedderkopp38]. The organism survives best in wet conditions and at low temperatures [Reference Cook and Bolster39] and survival is poor in dry conditions [Reference Jones40]. Within-flock transmission is also higher in conditions of wet litter [Reference Line41], which is also a risk factor for pad burn ([Reference Berg, Weeks and Butterworth42], see below). The fact that weather conditions in the month of slaughter were significant predictors of whether or not a flock was infected whilst conditions in the month of placement were not probably reflects the aetiology of infection in flocks. One study noted that flocks become increasingly infected as the rearing period progressed reaching a peak around 10 days prior to slaughter [Reference Evans and Sayers20]. It is at this time when the space available for individual chickens declines, growth rate (and physiological stress) are greatest [Reference Manning, Chadd and Baines43]. Thus, one might expect that Campylobacter spread within a flock would be greatest at this time, a factor emphasized by the impact of drinkers, which would provide a rapid means of spreading infection. Ventilation varied on the farms in terms of where the air entered (sides, vent and inlets) and whether or not it was forced by fan or natural. The apparent significance of natural ventilation in enhancing disease might reflect either a magnified effect of external weather conditions – since the temperature in a fan-ventilated environment is likely to be lower than that provided by natural ventilation – or an indirect effect arising from a Campylobacter vector such as flies. In addition forced ventilation might to lead to fly mortality as flies hit fan blades, giving rise to the contrast with natural ventilation, but this could only be verified by further research. Ventilation has been cited as a risk factor in a study of Canadian broilers [Reference Guerin19], while Ekdahl et al. [Reference Ekdahl, Normann and Andersson44] argued that flies not only carry Campylobacter but can pass the bacterium onto chickens. Natural ventilation in warm temperatures, which encourage fly movement, would clearly increase the risk of the pathogen entering a broiler house. Excluding flies from broiler houses has been shown to reduce infection rates from 54% to 15% [Reference Hald, Sommer and Skovgard45].
The lack of overdispersion in the GLMM models suggests that there was no aggregation, i.e. no serial dependence in infection within farm. This coupled with the fact that the incremental independent model provided a better explanation of infection events in the survival analyses are both suggestive that infection at the flock level is to a certain extent opportunistic given the right environmental conditions. This is perhaps of little help in managing disease. One feature that differed between the results of the survival analyses and those of the GLMMs was the apparent significance of pad burn, which was identified as a significant predictor in the survival analyses but not the GLMMs. Bull et al. [Reference Bull22] noted that the closely related condition of hock burn was an important predictor of flock infection in a larger study of 760 farms (of which these data form a small subset). Indeed, the extent of pad and hock burn in flocks was significantly correlated (r=0·358, P<0·002); the relationship between survival and pad burn detected here could reflect this correlation. Whilst the relationship between hazard of infection and extent of pad burn clearly exists, it is likely that incidence of both reflects another unmeasured underlying factor associated with flock health, rather than a true causal relationship. It is possible that the underlying process is related to enhanced survival of the pathogen in the litter under the wet conditions that give rise to pad burn. Alternatively, it is possible that pad burn simply reflects a general low health status in the individual chicken, which also influences the extent to which they can become colonized by Campylobacter. Nonetheless, the existence of the relationship perhaps has an agronomic value in that it might be of use in predicting the likely risk of flocks having become infected at the point of slaughter. Of equal interest is the apparent lack of significance in our models of other previously identified risk factors, specifically, the number of people entering chicken houses, the size and number of houses present [Reference Guerin19, Reference Barrios21] and the presence of livestock, which can carry Campylobacter [Reference Guerin19, Reference Nygard46, Reference Stanley and Jones47]. It is possible that our measures of these risk factors for farms were rather crude, but one key difference between our analysis and those of the above authors is the scale and longitudinal nature of our sampling. Many previous studies have involved large-scale surveys of large numbers of farms where the range of covariates that might be potential risk factors was also large. Identifying risk factors under these circumstances is problematic whilst correlations between predictors and outcomes are easy – particularly with large samples sizes – establishing causal links less so. Studying repeated infections, on a small number of farms over a longer time period, as we did here, means that many putative covariates were effectively factored out of the infection process. In addition we might also expect that focusing study on farms belonging to one company might reduce modellable variation in some covariates because the farms are likely to have similar management procedures. The focus on mixed-effect modelling approach allowed us to focus on between-farm variations through time. Farms clearly differed in their underlying risk of flocks becoming infected – as demonstrated by the random-effect models. The fact that these effects were significantly related to the geographical position of the farm – with risk of infection greater in the wetter west suggests that this is a contributor to risk. Large-scale geographical variation in risk of Campylobacter infection has been recorded in New Zealand and it has been concluded that the infection process varies spatially, although analyses of the pattern of incidence on farms has been shown to be consistent with within-farm rather than between- farm transmission [Reference Brown48].
There are two major conclusions from our work. First, that we need considerably more research on the pattern of spread within broiler flocks. The evidence indicates that within- flock spread is highly variable and not easy to predict. The dynamics of disease spread mean that the use of simple linear models to relate flock prevalence to putative risk factors is likely to be difficult. Processes which encapsulate the dynamic and allow for the fact that disease dynamics will vary through time and across farms are needed to investigate the disease at the appropriate scale. More interestingly, the results of our farm-level mixed-effect models gave considerable insight into the factors determining incidence of disease at this scale. In fact the goodness-of-fit of model predictions to observed behaviour suggests that it may be possible to predict when flocks on farms are at risk of Campylobacter infection, both in terms of weather and possible intervention through manipulation of ventilation regimes. This is a first step towards managing disease and reducing the risks posed to the human population.
ACKNOWLEDGEMENTS
We are grateful for the participation of the poultry companies involved and thank their management, farm and technical staff, together with their contract farmers, for their cooperation. We also particularly thank Mr David Lanning for his constant help and support and for critically reading draft manuscripts. We also thank Roger Lovell for technical assistance, Alistair Thomas for original data collation, and Sue Meredith for her excellent contribution inputting data. The work was supported by the Food Standards Agency (project code B15001) and the Health Protection Agency.
DECLARATION OF INTEREST
None.










