Compliance with prescribed medication is observed in only around 50% of patients with a range of physical and mental diseases (Reference Haynes, McDonald and GargHaynes et al, 2002) and can be defined as the extent to which a treatment regime is followed. Poor compliance can reduce the efficacy of treatments, resulting in worse health outcomes for patients (World Health Organization, 2003). A number of pragmatic interventions to enhance compliance have been tested in randomised controlled trials. Compliance therapy - a pragmatic intervention based on motivational interviewing and cognitive-behavioural therapy - has shown some promise (Reference Kemp and DavidKemp & David, 1996; Reference Kemp, Kirov and EverittKemp et al, 1998; Reference O'Donnell, Donohoe and SharkeyO'Donnell et al, 2003) but to enable large numbers of clinicians to deliver compliance therapy they will require training. We hypothesised that training community mental health nurses (CMHNs) to deliver compliance therapy would improve clinical outcomes in patients with schizophrenia.
METHOD
The aim of this pragmatic trial was to investigate whether medication management training is superior to treatment as usual in improving clinical outcomes for patients with schizophrenia. The trial does not adhere explicitly to CONSORT standards.
Community mental health nurses
We sent written invitations to CMHNs working in two mental health care providers in London, inviting them to participate. The CMHNs were accepted into the trial if they were registered nurses and had at least 12 months of post-registration experience. Once accepted into the trial each CMHN identified two patients on their case-load who satisfied the inclusion/exclusion criteria. The CMHNs were aware of which group they had been allocated to when identifying appropriate patients.
Patients
Patients who were prescribed antipsychotic medication with a recorded ICD-10 diagnosis of schizophrenia or schizoaffective disorder (World Health Organization, 1992) were invited to participate in the study if they were over 18 years of age with known or suspected poor treatment compliance (reported by the CMHN) or who had, within the previous 12 months, at least one admission or relapse. Patients were excluded at screening if they had a diagnosis of moderate or severe learning disabilities or organic brain disorders concurrent with schizophrenia, were being treated by forensic psychiatric services (or posed a current or serious risk of suicide or homicide) or were in-patients at the start of the trial. Other exclusion criteria included pregnancy (or a likelihood of becoming pregnant), lactation and alcohol/substance dependence. Local ethics committees approved the study and patients gave oral and written informed consent to participate.
Study design
This was a pragmatic 26-week, randomised, single-blind controlled study conducted in London, UK. The CMHNs were organised into 12 clusters (five CMHNs per cluster) based on the geographical location of the community mental health team or general practitioner surgery where they were based. The trial was staggered over three phases with 20 CMHNs (four clusters) in each phase. Randomisation sequences were prepared prior to the start of the trial and kept in opaque sealed envelopes. Clusters were randomised, at the start of each of the three phases of the trial, to receive 80 h of medication management training or to continue with treatment as usual. Patients completed a battery of self-report and research-worker-rated outcome measures at baseline and again after 26 weeks (Fig. 1). The research workers were masked to whether the nurse was in the training or treatment as usual group. Nurses were told not to discuss any aspect of their training allocation with the rater. All patients were seen either in their own home or in an out-patient clinic.

Fig. 1 Trial CONSORT diagram. CMHNs, community mental health nurses; MM, medication management; TAU, treatment as usual.
Training and fidelity
Medication management training consisted of 80 h of teaching delivered on a day-release basis over 10 weeks. Training focused on teaching CMHNs the compliance therapy approach detailed in a treatment manual (Reference Kemp, Hayward and DavidKemp et al, 1997). Additionally, the programme included training in the use of a range of standardised measures to assess the side-effects of medication and patients' beliefs and feelings about treatments, and a psychopharmacology component that considered effective treatment strategies for schizophrenia and the management of common side-effects. A multidisciplinary team that included clinical nurse specialists, psychologists and psychiatric pharmacists provided the training. The cost of training each CMHN was estimated at £1474. A detailed training manual is available from the authors upon request.
We have reported elsewhere (Reference Gray, Wykes and GournayGray et al, 2003) that training resulted in significant improvements in clinical skills. Performance on a role-play task was rated independently using the Cognitive Therapy Scale (CTS; Reference Vallis, Shaw and DobsonVallis et al, 1986) both pre- and post-training. A score of 30 indicates satisfactory clinical skills. The mean pre-training score was 13.9. Following training CTS total scores improved significantly (mean 30.6, P<0.01). Nurses who attended training also reported a high degree of satisfaction and clinical applicability.
Outcome measures
All patient interviews were performed by one of two research workers (R.G. and Sara Dickson, see Acknowledgements) masked to the training condition. Both researchers attended a 1-day training workshop in administering and reliably rating the Positive and Negative Syndrome Scale (PANSS; Reference Kay, Opler and LindenmayerKay et al, 1989) and obtained a satisfactory level of interrater reliability.
Primary outcome measure
Positive and Negative Syndrome Scale
The PANSS is a widely used measure for evaluating the symptoms of schizophrenia in clinical trials of both pharmacological and psychological interventions. Thirty items are rated on a seven-point scale following the general format of the Brief Psychiatric Rating Scale (Reference Overall and GorhamOverall & Gorham, 1962). The PANSS has strict operational criteria for conducting a 30-40 min patient interview, thorough definitions for all 30 items and detailed rating criteria for each level of psychopathology (Reference Kay, Opler and LindenmayerKay et al, 1989). The measure has established interrater, test-retest and internal reliability, and internal, external and construct validity (Reference Kay, Opler and LindenmayerKay et al, 1989). A ten-point reduction in PANSS total scores would represent a clinically important training effect.
Secondary outcome measures
Three further scales were used to assess efficacy; the Hogan Drug Attitude Inventory (DAI-30; Reference Hogan, Awad and EastwoodHogan et al, 1983), the Clinician Rating of Compliance Scale (Reference Kemp, Kirov and EverittKemp et al, 1998) and the Liverpool University Neuroleptic Side Effect Rating Scale (LUNSERS; Reference Day, Wood and DeweyDay et al, 1995). The DAI-30 is a 30-item self-report measure predictive of compliance in people with schizophrenia. Each item is rated by the patient as being true or false and produces a total score ranging from +30 to -30. A positive score is predictive of compliance and a negative score is predictive of non-compliance. The Clinician Rating of Compliance Scale is an observer rating of compliance on a seven-point scale ranging from 1 (complete refusal) to 7 (active participation in treatment). The LUNSERS is a self-report measure of the side-effects of antipsychotic medication. Forty-one items cover psychological, neurological, autonomic, hormonal and miscellaneous side-effects. Each item is rated on a five-point scale ranging from ‘not at all’ to ‘very much’, based on how frequently the patient has experienced the side-effect in the preceding month.
Additional patient information
Patients' age, gender, ethnicity, diagnosis and duration of illness were collected from the patients' medical notes at the baseline assessment and confirmed with the patient at interview. All the medication that patients were prescribed on the day of assessment was recorded. The dose of antipsychotic medication was converted to chlorpromazine equivalents using the World Health Organization's Anatomical Therapeutic Chemical Classification (World Health Organization, 1993). We also observed for any serious or unexpected adverse events throughout the trial, including death or attempted suicide.
Nurse information
Nurses completed a brief questionnaire detailing their age, gender, ethnicity, clinical and academic experience, grade and case-load.
Statistical analyses
A sample size of 120 patients (and therefore 60 CMHNs) was chosen to determine the effect of medication management training on the clinical outcome of patients with schizophrenia. Power calculations suggested that to detect a ten-point difference in PANSS total scores, assuming a standard deviation of 12.4 (Reference GrayGray, 2001), 120 patients should be recruited to give an 80% power at a significance level of 5%, allowing for drop-out of 20%. Our power analysis did not allow for clustering of patients. Retrospectively it would have been preferable in principle to have allowed for this through the variance inflation factor. In fact, the observed intraclass correlation within clusters was very low and therefore any underestimate of power would have been negligible.
Data were analysed initially using the Statistical Package for the Social Sciences Version 11 for Windows to compare the randomised groups of nurses and patients at baseline. The distributions of the outcome variables were approximately normal at baseline. Differences between the two groups at baseline and after intervention are reported with confidence intervals (but not the estimate of the intervention effect) adjusted for the effect of clustering. This is achieved by applying the variance inflation factor based on the intraclass correlation to the within-group standard errors (Reference Donner and KlarDonner & Klar, 2000). This also allows a simple adjustment to the standard t-test and is implemented in the clttest routine in Stata version 7 for Windows. The effects of the intervention are reported as change scores. Sensitivity checks were performed on all significant findings by performing a mixed effect regression (using the xtreg-mle procedure), controlling for baseline level, age, gender and ethnic group and allowing for clustering at geographical and CMHN cluster level separately.
RESULTS
Sample of CMHNs
Sixty CMHNs were recruited in the trial and randomised (Fig. 1). Prior to recruiting the patients, eight CMHNs withdrew from the trial. Five had found alternative employment and two withdrew consent, reporting that they were too busy to attend the training. One withdrew for an unspecified reason. The demographic profile of CMHNs who entered the trial (Table 1) was similar to that described in the national census (Reference Brooker and WhiteBrooker & White, 1997), although CMHNs in this study were from more diverse ethnic backgrounds. The only baseline difference between the two groups was that nurses in the experimental group were more experienced.
Table 1 Trainee demographics
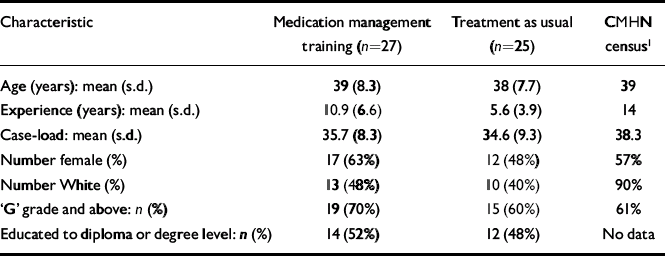
| Characteristic | Medication management training (n=27) | Treatment as usual (n=25) | CMHN census1 |
|---|---|---|---|
| Age (years): mean (s.d.) | 39 (8.3) | 38 (7.7) | 39 |
| Experience (years): mean (s.d.) | 10.9 (6.6) | 5.6 (3.9) | 14 |
| Case-load: mean (s.d.) | 35.7 (8.3) | 34.6 (9.3) | 38.3 |
| Number female (%) | 17 (63%) | 12 (48%) | 57% |
| Number White (%) | 13 (48%) | 10 (40%) | 90% |
| ‘G’ grade and above: n (%) | 19 (70%) | 15 (60%) | 61% |
| Educated to diploma or degree level: n (%) | 14 (52%) | 12 (48%) | No data |
Sample of patients
The CMHNs identified 89 patients to take part in the study (Fig. 1). At trial entry, seven were excluded because they did not satisfy the inclusion/exclusion criteria: five were not diagnosed with schizophrenia or schizoaffective disorder and two were not on the case-load of the CMHN who referred them. Of the 82 who were eligible to take part, three refused to participate, two withdrew their consent and five did not complete the baseline assessment. Seventy-two patients gave written consent and entered the study, which is a mean of 1.4 per CMHN. The patients who entered the trial (Table 2) were similar to populations of patients with schizophrenia in other trials of compliance interventions (Reference Kemp, Kirov and EverittKemp et al, 1998). The two groups were comparable in terms of demographic features, duration of illness, age at illness and number of admissions.
Table 2 Patient demographic and clinical characteristics
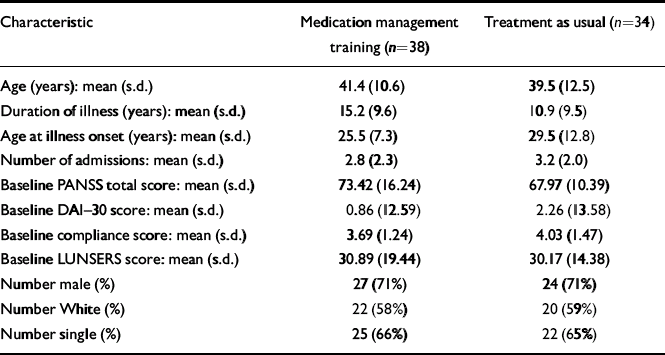
| Characteristic | Medication management training (n=38) | Treatment as usual (n=34) |
|---|---|---|
| Age (years): mean (s.d.) | 41.4 (10.6) | 39.5 (12.5) |
| Duration of illness (years): mean (s.d.) | 15.2 (9.6) | 10.9 (9.5) |
| Age at illness onset (years): mean (s.d.) | 25.5 (7.3) | 29.5 (12.8) |
| Number of admissions: mean (s.d.) | 2.8 (2.3) | 3.2 (2.0) |
| Baseline PANSS total score: mean (s.d.) | 73.42 (16.24) | 67.97 (10.39) |
| Baseline DAI-30 score: mean (s.d.) | 0.86 (12.59) | 2.26 (13.58) |
| Baseline compliance score: mean (s.d.) | 3.69 (1.24) | 4.03 (1.47) |
| Baseline LUNSERS score: mean (s.d.) | 30.89 (19.44) | 30.17 (14.38) |
| Number male (%) | 27 (71%) | 24 (71%) |
| Number White (%) | 22 (58%) | 20 (59%) |
| Number single (%) | 25 (66%) | 22 (65%) |
Follow-up assessment
All CMHNs who entered the trial completed training (i.e. attended >80% of the course). Of the 72 patients who entered the trial 53 (74%; 29 in the training group and 24 in the treatment-as-usual groups; Fig. 1) were assessed on the primary outcome measure (PANSS total) at the trial end-point. Eleven refused to be interviewed but did not withdraw consent, five were not available for interview, two had moved away and one could not be traced. There was no evidence for differential drop-out. For those who were missing at follow-up, the PANSS total baseline score was similar in both groups (training=68.5 v. treatment as usual=67.7).
Medication dosage
The dose of antipsychotic medication prescribed for the duration of the trial was stable in both groups. At baseline there was no significant difference between the two groups in the mean dose of antipsychotic medication (in chlorpromazine equivalents) prescribed (training=400 mg/day v. treatment as usual=469 mg/day). There was also no evidence for a difference in the proportion of patients prescribed atypical antipsychotics (training n=6 v. treatment as usual n=8). At the trial end-point there had been no significant changes between the groups in the dose of antipsychotic medication prescribed (training=307 mg/day v. treatment as usual=379 mg/day) or the proportion prescribed atypicals (training n=3 v. treatment as usual n=6).
Efficacy outcomes
Baseline scores (Table 2) were indicative of moderate levels of schizophrenic symptoms, and ambivalence about the need for taking-medication. The LUNSERS scores suggested that patients were experiencing a moderate number of side-effects from antipsychotic medication. Although patients in the intervention group tended to have more symptoms, lower compliance and more side-effects than those in the treatment-as-usual group, the differences were not statistically significant.
Statistically significant improvements were seen in the medication management training group compared with the treatment-as-usual group (Table 3) in overall psychopathology (PANSS total), attitudes towards antipsychotic medication (DAI-30) and compliance. No significant differences between the groups were seen in patients' antipsychotic side-effects. The sensitivity analyses adjusting for both cluster effects and confounders showed very similar results, with a slight attenuation of the effect for the PANSS total score (mean difference=16.1). Clinically significant improvements in psychopathology (defined as an improvement of at least 30%) were seen in 6 of the 29 patients in the medication management group but in none of the 24 in the treatment-as-usual group.
Table 3 Outcome measures, baseline and follow-up scores and mean change scores (95% CI) for which follow-up data were available

| Measure | Medication management training (MM) mean | Treatment as usual (TAU) mean | Difference (MM-TAU) mean (95% CI)12 | P | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Baseline scores | Follow-up scores | Change | n | Baseline scores | Follow-up scores | Change | |||
| Symptomatology | 29 | 74.93 | 58.31 | − 16.62 | 24 | 68.08 | 69.25 | 1.17 | − 17.79 (−24.12 to −11.45) | <0.001 |
| (PANSS total)3 | ||||||||||
| Attitudes (DAI-30)4 | 27 | − 0.64 | 8.03 | 8.67 | 25 | 3.20 | 3.24 | 0.04 | 8.63 (2.59 to 14.67) | 0.01 |
| Compliance4 | 28 | 3.64 | 4.43 | 0.89 | 25 | 4.20 | 4.36 | 0.16 | 0.73 (0.37 to 1.10) | 0.001 |
| Side-effects | 15 | 30.81 | 21.60 | − 9.21 | 13 | 28.08 | 13.46 | − 14.62 | 5.40 (−20.70 to 9.89) | 0.44 |
| (LUNSERS)3 | ||||||||||
Safety assessments
Relapse was defined as a 30% or more increase in PANSS total scores. None of the patients in the training group and one in the treatment-as-usual group experienced a relapse during the trial. There were no patient deaths during the trial and no attempted suicides.
DISCUSSION
The aim of this trial was to assess the effectiveness of medication management training compared with treatment as usual in improving clinical outcomes for patients with schizophrenia.
Community mental health nurses
The CMHNs provide much of the day-to-day care for people with schizophrenia and they are ideally placed to deliver compliance therapy. In this trial the demographic characteristics of CMHNs were comparable with those in the national census, suggesting that they were representative of those currently practising in the UK. The only important difference was that CMHNs were from a more diverse ethnic background. However, this would be anticipated, given that the study was carried out in south London where the population is more ethnically diverse. The training and treatment-as-usual groups were generally well matched.
Patient population
People with schizophrenia are often non-compliant with antipsychotic medication, resulting in increased levels of psychopathology or relapse. The baseline demographic and clinical data from this study underscore this observation. In an apparently stable population prescribed fairly high doses of antipsychotic medication, patients were experiencing moderately severe levels of psychopathology. Scores on the DAI-30 suggested that patients were ambivalent about taking medication and ratings on the clinician rating of compliance indicated that they questioned the need to take medication. Participants in this trial were representative of patients living in the community managed by CMHNs.
Medication management training efficacy and safety
This study demonstrated that medication management training for CMHNs is effective in improving clinical outcomes in people with schizophrenia over a 26-week period. The primary efficacy measure (PANSS total) showed statistically significant improvements compared with treatment as usual at the week 26 assessment. Significant improvements were observed also in patients' attitudes towards treatment (DAI-30) and compliance (clinician rating) compared with treatment as usual. However, there were no improvements in medication side-effects (LUNSERS total). The improvements in patients' attitudes towards treatment and compliance are consistent with the original compliance therapy trial (Reference Kemp, Kirov and EverittKemp et al, 1998) and suggest that medication management training equips CMHNs with the skills that they need to be effective in delivering compliance therapy. However, anticipated improvements in antipsychotic side-effects were not realised. Medication management training was acceptable to patients and did not result in any unexpected findings with regard to safety.
Methodological considerations
The proportion of patients for whom complete data were not available was high (26%) but below the average rate of 33% reported in a systematic review of dropout in published randomised trials with this patient population (Reference Wahlbeck, Tuunainen and AhokasWahlbeck et al, 2001). The large number of patients dropping out of the trial may be explained by the nature of the disorder: patients are chaotic, they miss appointments and are often distrustful of strangers. The study may have benefitted from a comparison with an inert training intervention that would allow for training time to be controlled. However, it would be unethical and expensive to provide training that was of no real benefit to CMHNs. In this study we used self-report (DAI-30) and clinician ratings of compliance. These measures have been criticised because they may introduce observer bias. However, direct methods such as electronic monitoring were impractical and costly and, in any case, also can be subject to bias. Patients were followed up for a relatively short (6-month) period. It would be important to examine whether the improvements observed are maintained over a longer period of time or whether the effects of training begin to degrade. Allowing CMHNs to identify patients for inclusion in the trial after randomisation may have introduced the potential for selection bias. This is suggested by the baseline differences, even though they are non-significant. Recruiting patients who satisfied the inclusion/exclusion criteria randomly from CMHNs case-loads could have addressed this.
Clinical Implications and Limitations
CLINICAL IMPLICATIONS
-
▪ Community mental health nurses (CMHNs) given medication management training can safely and effectively deliver compliance therapy to patients with schizophrenia.
-
▪ Overall, psychopathology, attitudes towards treatment and compliance can be improved in people with schizophrenia following training.
-
▪ Medication management training is a manualised package and should lend itself well to rapid dissemination.
LIMITATIONS
-
▪ The effect of training time was not controlled for.
-
▪ Complete data were not available for one-third of patients at the end of the trial.
-
▪ Choice of patients was made by CMHNs after randomisation.
Acknowledgements
We thank Sara Dickson and all the mental health nurses and patients who participated in this trial.







eLetters
No eLetters have been published for this article.