Introduction
Extracranial traumatic vertebral artery injury (eTVAI) occurs in approximately 1–2% of non-penetrating head and neck traumas.Reference Stein, Boswell, Sliker, Lui and Scalea1–Reference Biffl, Moore and Elliott4 Most patients are initially asymptomatic but have an increased risk for delayed stroke and mortality.Reference Biffl, Moore and Elliott4–Reference Friedman, Flanders, Thomas and Millar7 Guidelines for eTVAI are outdated and supported predominantly by Level 3 evidence.Reference Harrigan, Hadley and Dhall8 Furthermore, limited evidence is available to guide the management of asymptomatic patients with eTVAI.Reference Harrigan, Hadley and Dhall8
Screening for eTVAI using computed topography angiography (CTA) may be considered in cases of blunt trauma; guidelines for the management of eTVAI recommend the use of screening criteria.Reference Harrigan, Hadley and Dhall8 The modified Denver and Memphis criteria are screening criteria for blunt cerebrovascular injury (BCVI) in patients with high-risk features such as expanding cervical hematoma, cardiothoracic injuries, focal neurological deficits, concomitant traumatic brain injury, and radiographic findings such as fractures or ischemia (Table 1).Reference Biffl, Moore and Offner9–Reference Cothren, Moore, Ray, Johnson, Moore and Burch12 Despite evidence supporting their utility, these criteria may be underutilized, particularly in asymptomatic patients.Reference Biffl, Moore and Elliott4,Reference Biffl, Moore and Offner9,Reference Biffl, Ray and Moore10,Reference Ciapetti, Circelli and Zagli13,Reference Geddes, Burlew and Wagenaar14
Table 1: Criterion-based screening tools for blunt cerebrovascular injury

BCVI = blunt cerebrovascular injury; CT/CTA = computed tomography angiography; GCS = Glasgow Coma Scale; MRI = magnetic resonance imaging; TIA = transient ischemic attack.
a Cothren Burlew et al. J Trauma Acute Care Surg, 2012, 72, 330
b Ciapetti et al. Scand J Trauma Resusc Emerg Med. 2010, 18, 61
c Emmett et al. J Trauma, 2011, 70, 1058.
Regarding treatment and follow-up, the Cervical Artery Dissection in Stroke Study (CADISS) may guide medical management of patients with symptomatic cerebrovascular dissection; however, the trial included spontaneous vertebral artery injuries.Reference trial investigators, Markus and Hayter15 It did not include asymptomatic patients or elaborate on optimal dosages, treatment duration, or choice of antiplatelet or anticoagulation therapy. Guidelines for eTVAI recommend that choice of therapy should be individualized based on the vertebral artery injury, other associated injuries, and potential risk of bleeding.Reference Harrigan, Hadley and Dhall8 Digital subtraction angiography (DSA) may be considered for the diagnosis of eTVAI in select patients; however, the role of endovascular therapy in eTVAI remains undefined.Reference Harrigan, Hadley and Dhall8,Reference Burlew, Biffl and Moore16–Reference Schneidereit, Simons and Nicolaou18 Guidelines for eTVAI do not provide a recommendation regarding the use of endovascular therapy as an adjunct to antithrombotic therapy in adult patients to reduce the risk of stroke.Reference Harrigan, Hadley and Dhall8
Given the limited evidence available to guide the management of asymptomatic eTVAI, the purpose of this study was to investigate Canadian practice patterns reflecting screening, treatment, and follow-up domains. Such findings would be broadly relevant to neurosurgeons, spinal surgeons, stroke neurologists, neuro-interventionalists, and trauma specialists managing the work-up and/or treatment of eTVAI. They may also be used to inform a shared decision-making approach with patients and their families.
Methods
Study Design and Target Population
The Canadian Neurosurgery Research Collaborative (CNRC) is a group of resident neurosurgeons seeking to advance the care of patients through collaborative research. Our group conducted a self-administered, cross-sectional survey of Canadian physicians who screen, treat, and/or follow patients with eTVAI. Neurosurgeons, fellowship-trained spinal surgeons, neurologists, and neuro-interventionalists were eligible for study inclusion. We excluded respondents who self-reported they do not work-up or manage patients with eTVAI. This study adheres to the Checklist for Reporting of Survey Studies (CROSS) guidelines (Supplemental Material A).Reference Sharma, Minh Duc and Luu Lam Thang19
Survey Development and Testing
A narrative literature review was performed in order to identify eTVAI-related management domains lacking scientific support. MEDLINE, Embase, and the Cochrane Central Register of Controlled Trials were search from inception until September 2020. Free-text words relevant to presentation (asymptomatic and symptomatic), location (extracranial, neck, and cervical), mechanism of injury (non-penetrating, blunt, and traumatic), and vascular injury (vertebral artery and dissection) were included. Abstracts were reviewed by two investigators (MAM and CJT). Management domains lacking scientific support were identified and informed development of a draft 32-question survey (Supplemental Material B). The survey did not involve patients, and case descriptions did not include actual patient data. The survey draft was provided to the CNRC steering committee and was assessed in order to optimize redundancy and improve clarity. Assessment of face and content validity was conducted by fellowship-trained spinal neurosurgeons and stroke neurologists at the coordinating site.
Survey Design
Demographics (subspecialty and years in practice) were recorded. We assessed respondents perceived value of clinical and radiographic symptoms and signs in screening for eTVAI. We inquired about use of criterion-based screening tools (e.g., modified Denver or Memphis criteria).Reference Biffl, Moore and Offner9,Reference Cothren, Moore, Ray, Johnson, Moore and Burch12 Stroke and transient ischemic attack (TIA)-related symptoms were considered separate, as most respondents listed both exclusively. Two case-based scenarios were presented, involving asymptomatic patients with eTVAI, no additional injuries, and conventional arterial anatomy. Response options included “none” and “other” (specified by free text). To avoid ambiguity regarding interpretation of “asymptomatic,” we used the term “neurologically intact.”
Case 1 was described as a 35-year-old patient with a fall from standing height. Unenhanced CT demonstrated a cervical lateral mass fracture extending into the vertebral foramen. Respondents were asked if they would screen for eTVAI, and if so, their modality of choice. Subsequently, respondents were asked to assume that CTA was obtained and demonstrated nonprogressive eTVAI. Treatment and follow-up questions were then stratified according to: (a) <25% lumen diameter reduction without intimal flap and (b) >25% luminal diameter reduction with raised intimal flap. These cutoffs are in keeping with the Denver Grading Scale for BCVI, including eTVAI.Reference Biffl, Moore and Elliott4,Reference Harrigan, Hadley and Dhall8,Reference Fassett, Dailey and Vaccaro20,Reference Desouza, Crocker, Haliasos, Rennie and Saxena21
Case 2 described a 55-year-old patient in a high-speed motor vehicle collision. Unenhanced CT demonstrated an atypical hangman’s fracture (unilateral oblique C2 body and contralateral pars fracture) (Supplemental Material B). Respondents were asked if they would screen for eTVAI, and if so, their modality of choice. Respondents were subsequently asked to assume that CTA imaging was obtained and positive for pseudoaneurysm dissection eTVAI.
Survey Administration
Anonymous and voluntary survey responses were collected electronically using Survey Monkey (San Mateo, CA, USA). Participation in the survey was considered as consent for enrollment in the study. No financial incentive was provided. In order to ensure our sample was representative of the study population, we included 150 members of the Canadian Neurosurgical Society (CNSS). We also included 83 orthopedic spine surgeon members of the Canadian Spine Society (CSS). Stroke neurologists and neuro-interventionalists (n = 64) who investigate and/or manage asymptomatic eTVAI were approached by residents distributing the survey at individual sites. A follow-up reminder was sent at 5 weeks. If respondents answered more than 80% of the survey, it was considered complete. Respondent demographic data were used to ensure single survey participation.
Statistical Analysis
Responses were analyzed using descriptive statistics. Categorical data were reported as counts and percentages. Results are featured as proportions with 95% confidence intervals.
Results
Thirty-six percent (108 of 297) of clinicians responded, representing 20 academic institutions. The response rate for each question exceeded 80%. Sixty-three percent of respondents were neurosurgeons, 17% stroke neurologists, 16% orthopedic spinal surgeons, and 5% neuro-interventionalists (Figure 1a). Years in practice were evenly distributed (Figure 1b).

Figure 1: Demographic variables: (a) specialty and (b) years in practice.
Self-Confidence and Evidence-Based Supporting Decision-Making
Fifty-six percent of respondents agree or strongly agree they are confident in their ability to manage asymptomatic eTVAI, whereas 30% were neutral (Figure 2a). Respondents were asked if their decision-making regarding screening, treatment, and follow-up is evidence-based, as opposed to expert opinion (Figure 2b-d; Supplemental Material C). The majority of respondents neither agree nor disagree regarding screening (53%), treatment (47%), and follow-up (56%).

Figure 2: (a) Self-confidence and (b) evidence-based supporting decision-making in eTVAI.
Clinical and radiological symptoms associated with eTVAI
Respondents reported their perceived top three clinical signs of eTVAI (Figure 3a; Table 2). The three most frequent responses were “posterior circulation/brainstem stroke-related symptoms” (76%), “neck pain” (56%), and vertigo or dizziness (24%).

Figure 3: (a) Clinical and (b) radiological signs ranked by respondents according to perceived association with eTVAI; (c) criterion-based screening tools utilized in eTVAI; and (d) reasons for not using screening tools in eTVAI.
Table 2: Respondents top perceived clinical and radiographic signs of eTVAI

CTA = computed tomography angiography; DSA = digital subtraction angiography; MRA = magnetic resonance imaging angiography; NOS = not otherwise specified.
Respondents reported their perceived top three radiographic signs of eTVAI (Figure 3b; Table 2). The three most frequent responses were “cervical fracture or dislocation” (40%), “vessel luminal narrowing” (36%), and “vessel occlusions” (36%).
Screening criteria
Seventy-one percent of respondents do not use a criterion-based tool to diagnose eTVAI (Figure 3c). Modified Denver and Memphis criteria were used by 17 and 8%, respectively. The three most frequent reasons for not using a criterion-based screening tool included “difficult to remember” (34%), “low yield” (15%), and “not aware of a criterion-based screening tool” (12%) (Figure 3d; Supplemental Material C).
Scenario-Based Questions:
Table 3 presents a summary of respondent’s screening, treatment, and follow-up practices for both cases of eTVAI. Expanded data are available in Supplemental Material C.
Table 3: Clinical case details* and respondents preferred screening, management, and follow-up

CTA = computed tomography angiography; F/U = follow-up; MVC = motor vehicle collision.
* For complete response data, including expanded “other” categories, please refer to tables found in Supplemental material C.
** (n, %)
For Case 1 (i.e., low-energy injury mechanism and uncomplicated fracture pattern), the majority of respondents would screen with CTA (Figure 4a), immediately (Figure 4b). Regardless of luminal diameter reduction of presence of intimal flap, most respondents would start treatment with ASA (Figures 5a and b), continue medical therapy for 3–6 months (Figure 5c), follow-up clinically every 1–3 months (Figures 6a and b), and follow-up radiographically every 1–3 or 3–6 months (Figures 6a and b). The overall duration of clinical and radiographic follow-up varied from 3–6 months to 1–3 years (Figure 7a). A minority of respondents would follow radiologically “until complete regression.”
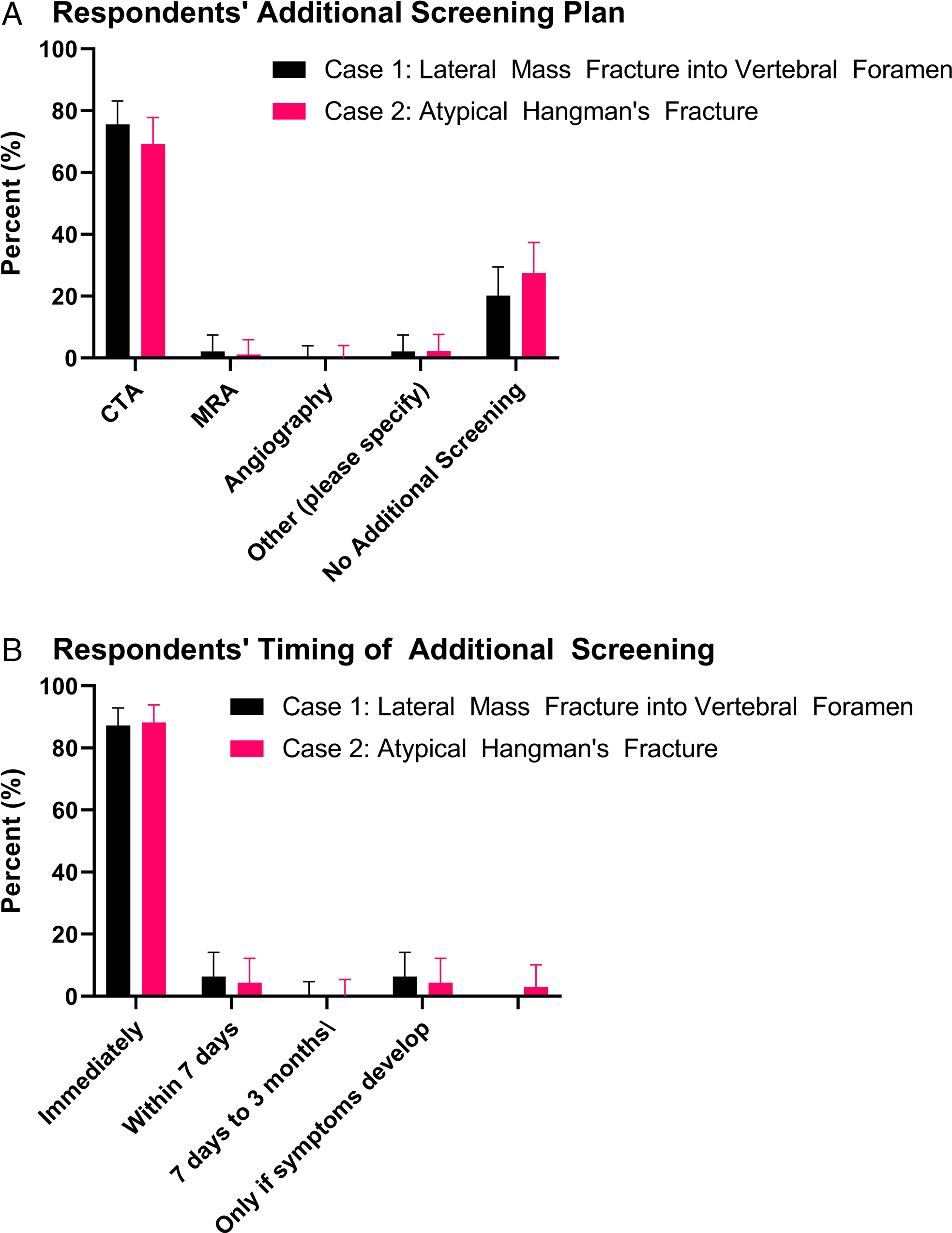
Figure 4: Respondents (a) screening plan and (b) timing of additional screening for Cases 1 and 2.

Figure 5: Case 1: (a) treatment of choice for eTVAI with <25% luminal diameter reduction and (b) >25% luminal diameter reduction; (c) overall duration of medical treatment. Case 2: (a) timing of endovascular therapy (if applicable); (b) treatment of choice for eTVAI with pseudoaneurysm dissection; and (c) duration of medical treatment in eTVAI with pseudoaneurysm dissection.

Figure 6: Frequency of follow-up for eTVAI with: (a) <25% luminal diameter reduction; (b) >25% luminal diameter reduction; and (c) pseudoaneurysm dissection.
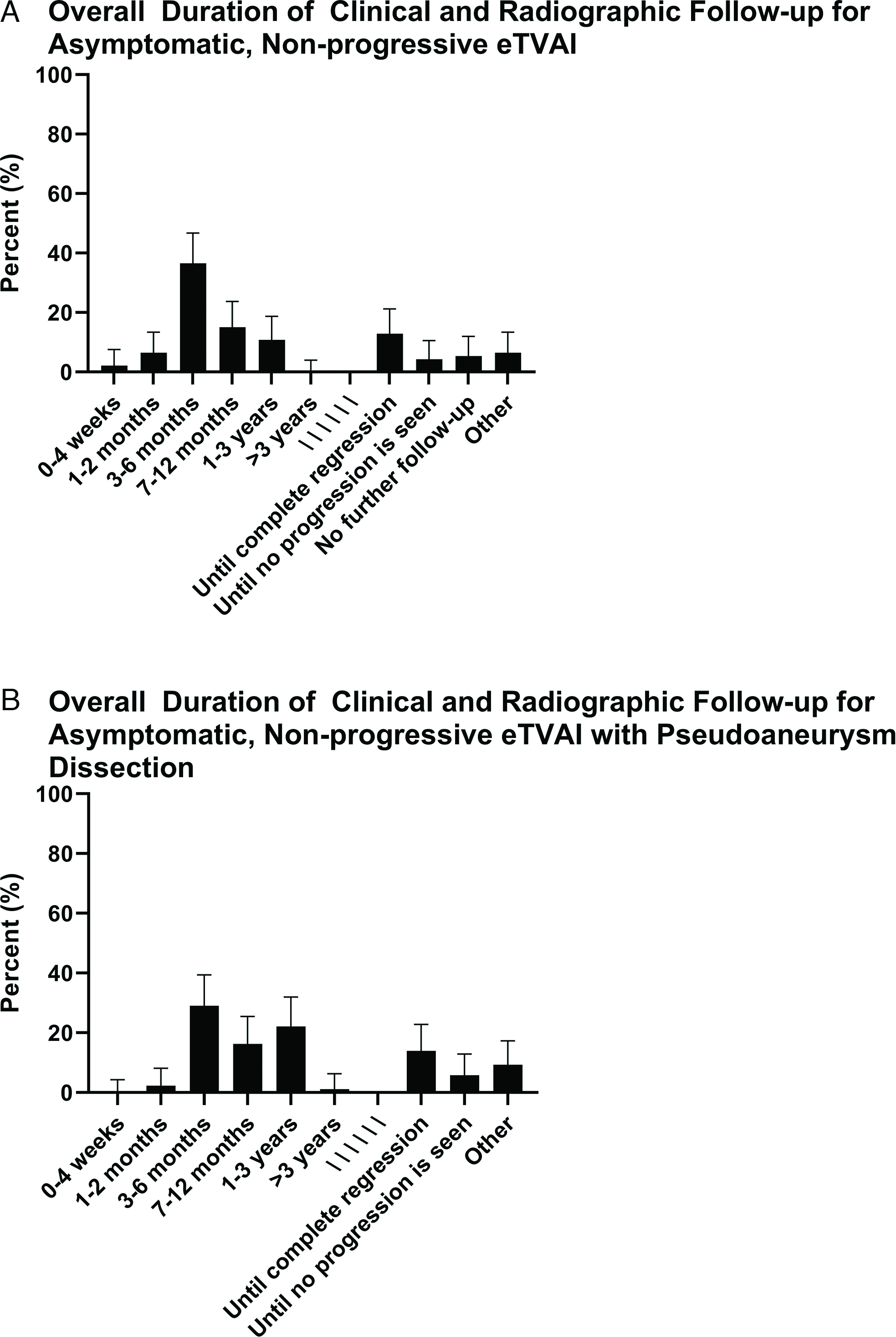
Figure 7: Overall duration of follow-up for eTVAI in (a) Case 1 and (b) Case 2.
For Case 2 (i.e., high-energy injury mechanism and complicated fracture pattern), the majority of respondents would screen with CTA (Figure 4a), immediately (Figure 4b). Most respondents would treat eTVAI-related pseudoaneurysm dissection with ASA or endovascular surgery (Figure 5e); the duration for medical therapy varied, but 3–6 months was most frequently reported (Figure 5f). Most respondents would follow up clinically every 1–4 weeks or 1–3 months, and radiologically every 1–3 or 3–6 months (Figure 6c). The overall duration of clinical and radiological follow-up ranged from 3–6 months to 1–3 years (Figure 7b). A minority of respondents would follow radiologically “until complete regression.”
Discussion
Unrecognized eTVAI is associated with high rates of stroke and mortality.Reference Stein, Boswell, Sliker, Lui and Scalea1,Reference Biffl, Moore and Elliott4,Reference Desouza, Crocker, Haliasos, Rennie and Saxena21 Comprehensive screening and timely initiation of treatment is associated with increased detection of BCVI and decreased risk of stroke and mortality.Reference Geddes, Burlew and Wagenaar14 Current recommendations for the work-up and management of eTVAI pertain primarily to symptomatic cases.Reference Harrigan, Hadley and Dhall8 Given the paucity of evidence pertaining to asymptomatic eTVAI, the CNRC investigated national practice patterns across 20 academic institutions. We presented two clinical scenarios of blunt trauma resulting in asymptomatic eTVAI, stratified based on trauma mechanism, fracture complexity, and degree of vessel injury. In both cases, the majority of respondents opted to screen for eTVAI with CTA, initiate aspirin therapy for 3–6 months, and follow-up clinically and radiographically within 1–3 months, respectively (Table 3). These findings are relevant to neurosurgeons, spinal surgeons, stroke neurologists, and neuro-interventionalists caring for patients with eTVAI. Furthermore, they may inform a shared decision-making approach with patients and their families. A summary of potential management consideration for the screening, treatment, and follow-up of asymptomatic eTVAI is highlighted in Table 4.
Table 4: Potential management considerations for the screening, treatment, and follow-up of asymptomatic eTVAI

CT = computed tomography; eTVAI = extracranial traumatic vertebral artery injury.
Clinical and Radiographic Findings in eTVAI
Patients with eTVAI are often asymptomatic.Reference Biffl, Moore and Elliott4,Reference Fassett, Dailey and Vaccaro20 Immediate symptoms may be explained by the high rate of associated injuries (93% of patients).Reference Desouza, Crocker, Haliasos, Rennie and Saxena21 Cervical fractures are prevalent (70–75% of patients).Reference Stein, Boswell, Sliker, Lui and Scalea1,Reference Biffl, Moore and Elliott4,Reference Fassett, Dailey and Vaccaro20 Delayed symptoms typically develop within 10–72 hoursReference Biffl, Ray and Moore10,Reference Fassett, Dailey and Vaccaro20,Reference Tulyapronchote, Selhorst, Malkoff and Gomez22,Reference Cothren, Biffl, Moore, Kashuk and Johnson23 and may be explained by infarction secondary to thromboembolism, or vessel luminal narrowing that progresses to occlusion.Reference Fassett, Dailey and Vaccaro20 Vessel occlusion may not manifest symptoms in patients with adequate collateral circulation.Reference Desouza, Crocker, Haliasos, Rennie and Saxena21 Symptomatic patients typically present with headache, neck pain, and neurological deficits related to the posterior circulation territory infarcted (e.g., long-tract signs, gait disturbance, vertigo, and Horner’s syndrome).Reference Desouza, Crocker, Haliasos, Rennie and Saxena21,Reference Kim and Schulman24 The most common vertebral artery findings on radiographic imaging are luminal narrowing, dilatation, occlusion, intimal flap, and pseudoaneurysm.Reference Gottesman, Sharma and Robinson25 Herein, the most frequently reported clinical and radiographic symptoms and signs of eTVAI considered by respondents were similar to those most frequently reported in the literature (Table 2).Reference Biffl, Moore and Offner9,Reference Geddes, Burlew and Wagenaar14,Reference Fassett, Dailey and Vaccaro20,Reference Desouza, Crocker, Haliasos, Rennie and Saxena21,Reference Emmett, Fabian, DiCocco, Zarzaur and Croce26
Screening for eTVAI
Screening protocols for eTVAI were implemented in the 1990s upon recognition of an early asymptomatic period and specific patterns of associated injuries.Reference Burlew, Biffl, Moore, Barnett, Johnson and Bensard27 Prior to this, stroke and mortality rates were 80 and 40%, respectively.Reference Biffl, Moore and Offner9,Reference Fabian, Patton, Croce, Minard, Kudsk and Pritchard28,Reference Bromberg, Collier and Diebel29 Despite an increase in the identification of BCVI, including asymptomatic eTVAI,Reference Emmett, Fabian, DiCocco, Zarzaur and Croce26 in centers applying such criteria, 20–30% of cases remained unrecognized until patients progressed and became symptomatic.Reference Emmett, Fabian, DiCocco, Zarzaur and Croce26 The modified DenverReference Burlew, Biffl, Moore, Barnett, Johnson and Bensard27 and Memphis criteriaReference Emmett, Fabian, DiCocco, Zarzaur and Croce26 were subsequently proposed (Table 1) and led to a reduction in missed injuries, allowing for earlier initiation of treatment and prevention of stroke and stroke-related mortality.Reference Ciapetti, Circelli and Zagli13,Reference Geddes, Burlew and Wagenaar14 Multiple studies have since highlighted the utility of comprehensive screening protocols, including the use of expanded screening criteria;Reference Stein, Boswell, Sliker, Lui and Scalea1,Reference Geddes, Burlew and Wagenaar14,Reference Cothren, Biffl, Moore, Kashuk and Johnson23,Reference Emmett, Fabian, DiCocco, Zarzaur and Croce26,Reference Burlew, Biffl, Moore, Barnett, Johnson and Bensard27 stroke in asymptomatic patients can be almost universally avoided with early detection and appropriate treatment.Reference Burlew, Biffl, Moore, Barnett, Johnson and Bensard27
Despite the aforementioned evidence supporting the use of screening criteria, including recommendation in eTVAI practice guidelines,Reference Harrigan, Hadley and Dhall8 the majority of respondents in this study indicated they do not use a criterion-based screening tool (Figure 3c). Reasons for not using a tool included “difficulty remembering,” “low yield,” “time-consuming," or “lack of evidence or applicability.” The majority of respondents in this study were neurosurgeons. Use of criterion-based screening tools may vary with the specialty of the physician performing the trauma assessment. Trauma leaders responsible for decision-making may have background training in general surgery, anesthesiology, emergency medicine, or neurosurgery. Our study draws attention to the apparent need for more Canadian physicians managing eTVAI to recognize the value of screening criteria and employ them in routine practice. Targeted education around the utility of these criteria, awareness of guideline recommendations, and use of diagnostic algorithms could potentially facilitate increased awareness and use. Level I evidence pertaining to eTVAI is limited to screening modality;Reference Harrigan, Hadley and Dhall8 in patients who meet the Modified Denver Screening Criteria for BCVI, CTA is recommended.Reference Biffl, Moore and Offner9,Reference Cothren, Moore, Ray, Johnson, Moore and Burch12,Reference Burlew, Biffl, Moore, Barnett, Johnson and Bensard27 As such, it is not surprising that the majority of respondents herein would screen for eTVAI (Case 1, 78%; Case 2, 73%) with CTA (Case 1 and 2, 96%, respectively) (Table 3). Thin-slice, high-resolution CTA provides similar sensitivity and specificity compared to DSA for diagnosing eTVAI; it also is associated with fewer complications.Reference Chen, Tseng, Lee, Hsu and See30 CTA is useful for detection of commonly associated vertebral fractures, subluxation, and facet dislocations.Reference Rodriguez, Tyberghien and Matgé5 Most respondents would not screen with magnetic resonance imaging (MRI)/MRA. This may be explained by the fact that CTA offers reduced duration of scanning and improved detection of arterial injury compared to MRI/MRA.Reference Vertinsky, Schwartz, Fischbein, Rosenberg, Albers and Zaharchuk31 However, MRI/MRA may be warranted in cases of suspected spinal cord injury, vertebral ligamentous injury, or posterior circulation ischemia. Adjunct use of DSA is largely reserved for indeterminate cases and can assess collateral circulation or facilitate endovascular repair.
Treatment and Outcomes
Treatment of eTVAI is associated with a reduction in stroke and mortality.Reference Biffl, Moore and Elliott4,Reference Desouza, Crocker, Haliasos, Rennie and Saxena21,Reference Cothren, Biffl, Moore, Kashuk and Johnson23,Reference Fabian, Patton, Croce, Minard, Kudsk and Pritchard28,Reference Miller, Fabian and Bee32 The risk reduction of anticoagulation or antiplatelet therapy in both symptomatic and asymptomatic eTVAI remain unknown.Reference Desouza, Crocker, Haliasos, Rennie and Saxena21 Reported treatment effects pertaining to stroke rates vary across studies. Guideline recommendations for the treatment of eTVAI include consideration of antiplatelet or anticoagulation (versus no therapy) based on characteristics of the vertebral artery injury, associated injuries, and risk of bleeding.Reference Harrigan, Hadley and Dhall8 Particularly in the setting of concomitant traumatic brain injury or solid organ injuries, multidisciplinary discussion may guide determination of safe timing for the initiation of antithrombotic therapy. Cothren et al. reported equivalent stroke risk and vessel healing rates for both antiplatelet and anticoagulation therapies.Reference Cothren, Biffl, Moore, Kashuk and Johnson23 The CADISS study demonstrated anticoagulant therapy is not superior to antiplatelet therapy for preventing stroke or death, in symptomatic patients with cervical (carotid or vertebral) artery dissection.Reference Biffl, Moore and Elliott4 However, the CADISS study included mostly spontaneous dissections and was likely underpowered to detect a difference in outcome.Reference Kasner33
Respondents in our study demonstrated a preference for antiplatelet therapy in both cases. A recent Canadian retrospective review of the British Columbia Trauma Registry found similar findings. They identified 186 patients with BCVI and 88.9% were treated with ASA monotherapy.Reference D’Souza, Birnie, Ko, Evans, Field and Joos34 Few patients in their study received a loading dose of ASA, or dual antiplatelet therapy. Although evidence supports these practices for nontraumatic stroke,Reference Su, Chan and Lee35,Reference Wang, Wang and Zhao36 their role remains unclear for stroke prevention in BCVI.
Endovascular Therapies
The utility of endovascular treatment of eTVAI remains relatively undefined compared to spontaneous vertebral artery dissections; recommendations regarding stenting, occlusion, and pseudoaneurysm coil embolization are based on low-quality evidence.Reference Stein, Boswell, Sliker, Lui and Scalea1 A systematic review and meta-analysis found insufficient data for the assessment of efficacy of thrombolysis or stenting in the treatment of symptomatic TVAI.Reference Weber, Lefering and Kobbe2 However, complication rates in retrospective and non-randomized studies appear similar to those reported for thrombolysis in ischemic stroke or carotid artery stenting in cases of stenosis related to atherosclerosis.Reference Weber, Lefering and Kobbe2,Reference Friedman, Flanders, Thomas and Millar7 Choice of technique may depend on eTVAI grade, injury site, and collateral circulation.Reference Desouza, Crocker, Haliasos, Rennie and Saxena21 Few of our respondents would employ endovascular therapy in the absence of pseudoaneurysm dissection. The obligatory use of dual antiplatelet therapy following stenting represents a potential deterrent in trauma patients with risk of hemorrhage,Reference Stein, Boswell, Sliker, Lui and Scalea1 or concomitant traumatic brain injury (41% in one series).Reference Biffl, Moore and Elliott4
Follow-up for TVAI
Limited evidence is available to guide clinicians with regard to clinical and radiographic follow-up of eTVAI; routine follow-up may be recommended.Reference Desouza, Crocker, Haliasos, Rennie and Saxena21,Reference Brommeland, Helseth and Aarhus37 Given the potential for initial false-positive interpretations, to examine for vessel healing which has been shown to vary by injury grade,Reference Biffl, Ray and Moore10 low-quality evidence supports follow-up CTA at 7 days and 3 months following TVAI.Reference Brommeland, Helseth and Aarhus37 In keeping with published guidelines,Reference Brommeland, Helseth and Aarhus37 most respondents in our study would follow up clinically and radiographically in 1–3 months. Respondents emphasized a lack of scientific evidence in this domain. What Canadian clinicians do in actual practice may differ, as a recent study of Canadian patients with BCVI found only 35.7% received repeat imaging within 7 days.Reference D’Souza, Birnie, Ko, Evans, Field and Joos34 The authors highlight a potential benefit to consistent follow-up imaging, including an influence on the duration of antithrombotic therapy, with reduction of late bleeding complications associated with prolonged therapy.Reference Biffl, Ray and Moore10 Reasons for suboptimal repeat imaging rates included a lack of appropriate documentation pertaining to their BCVI treatment algorithm in a third of discharge summaries, as well as potential difficulty in accessing neuroimaging and outpatient services in remote communities. They propose a routine pathway for arranging clinical follow-up and radiographic imaging, including clear communication with general practitioners responsible for follow-up care of patients with trauma.
Limitations
Limitations of this study relate to its survey design. However, we attained a high response rate and identified consensus across all survey domains. Furthermore, current management guidelines pertain to symptomatic eTVAI and are supported predominantly by Level 3 evidence. Responses obtained in this study pertain to eTVAI and are not generalizable to patients with spontaneous dissections. We did not attempt to define or compare stroke rates.
Future Work
Further research may seek to determine the optimal dosage, treatment period, choice of medical therapy, and treatment effect for asymptomatic patients with eTVAI. Prospective studies should further define subgroups of asymptomatic eTVAI at risk of progression and potentially require additional therapy. Additionally, the role of endovascular therapy should be clarified. National consensus guidelines for the management of eTVAI may be useful. Prospective validation of screening tools for eTVAI in the context of current trauma practices may facilitate increased early recognition of eTVAI to improve clinical outcomes.
Conclusion
We identified consistency in national practice patterns across 20 academic institutions for the screening, treatment, and follow-up of asymptomatic eTVAI. We presented two clinical scenarios featuring asymptomatic eTVAI, stratified based on trauma mechanism, fracture complexity, and degree of vessel injury. In both cases, the majority of respondents opted to screen for eTVAI with CTA, initiate antithrombotic therapy for 3–6 months, and follow-up clinically and radiographically within 1–3 months, respectively. The findings herein are limited by the survey design but may be useful to neurosurgeons, spinal surgeons, stroke neurologists, and neuro-interventionalists, facilitating a shared decision-making approach with patients and their families.
Supplementary Material
To view supplementary material for this article, please visit https://doi.org/10.1017/cjn.2022.292.
Acknowledgments
The authors thank Majid Aljoghaiman for their contribution during manuscript preparation. The authors would like to thank the Canadian Neurosurgical Society and Canadian Spine Society for their assistance in the distribution and participation in this survey.
Conflicts of Interest/Disclosures
The authors have no conflicts to disclose. This study was not funded.
Statement of Authorship
MAM, CJT, JA, and SDC were involved in study conceptualization and design. MAM, CJT, TD, AA, DB, MKS, ARLP, and NS were involved in data collection, analysis, and manuscript preparation. All authors reviewed and edited the manuscript prior to submission.
Study Quality Guidelines
This study adheres to CROSS guidelines for reporting survey studies.













