Osteoarthritis (OA) is a common chronic joint disease associated with increased morbidity and disability risk and contributing to an enormous financial burden on healthcare systems worldwide( Reference Vos, Flaxman and Naghavi 1 ). In recent years, the pathophysiologic concept of OA has been changed from a degenerative joint disorder to a more complex concept involving multiple aetiologies and pathogeneses( Reference Man and Mologhianu 2 ). Inflammation is intricately linked to the aetiology of OA and has been implicated in the pathogenesis of OA( Reference Wang, Hunter and Xu 3 ). Experimental and observational studies have demonstrated that inflammatory and/or metabolic biomarkers are mediators of the inflammatory process of OA( Reference Sokolove and Lepus 4 ). In addition, there is increasing evidence for a potential role of vitamin D deficiency in OA. Vitamin D receptor (VDR) is expressed in chondrocytes, osteoclasts and osteoblasts( Reference Mabey and Honsawek 5 ), and vitamin D can reduce bone turnover and cartilage degradation, and thus it has the potential to delay the development and progression of OA( Reference Cao, Winzenberg and Nguo 6 ).
Interestingly, several experimental studies have reported that vitamin D may reduce the inflammatory response by modulating human monocyte function or VDR signalling( Reference Calton, Keane and Newsholme 7 , Reference Szeto, Sun and Kong 8 ). Observational studies have shown that vitamin D deficiency is associated with increased inflammation in chronic conditions, including asthma, inflammatory bowel disease and rheumatoid arthritis (RA)( Reference Hong, Xu and Xu 9 , Reference Yin and Agrawal 10 ). Such evidence suggests that increased inflammation may be a key underlying mechanism linking vitamin D deficiency to OA, and vitamin D could modify OA disease progression through inhibition of inflammation. Previous studies have examined the effect of vitamin D supplementation on inflammatory biomarkers in older adults and patients with some chronic diseases, and have shown inconsistent results( Reference de Medeiros Cavalcante, Silva and Costa 11 – Reference Schleithoff, Zittermann and Tenderich 14 ), but no study has reported whether vitamin D supplementation has effects on inflammatory and metabolic biomarkers in OA patients.
Recently we reported that, compared with placebo, vitamin D supplementation had no significant effects on MRI-measured tibial cartilage volume or the Western Ontario and McMaster Universities Arthritis Index (WOMAC) assessed knee pain( Reference Jin, Jones and Cicuttini 15 ), but significantly reduced MRI-measured joint effusion–synovitis in patients with symptomatic knee OA( Reference Wang, Cicuttini and Jin 16 ). This suggests that vitamin D supplementation could have anti-inflammatory effects by regulating serum levels of inflammatory or metabolic biomarkers in knee OA patients. The aim of the current study was, therefore, to determine whether vitamin D supplementation affected serum inflammatory and metabolic biomarkers and whether variation in vitamin D status over 2 years was associated with change in biomarkers in patients with knee OA and vitamin D deficiency.
Methods
Study design and participants
This study was a post hoc analysis of the Vitamin D Effect on Osteoarthritis (VIDEO) study, which was a multicentre randomised, double-blind, placebo-controlled trial in knee OA patients with vitamin D deficiency. The method and protocol of the trial were described previously( Reference Cao, Jones and Cicuttini 17 ). The trial was conducted from June 2010 to December 2013.
In brief, eligible participants who had knee symptomatic OA (assessed using American College of Rheumatology criteria)( Reference Altman, Asch and Bloch 18 ) at least for 6 months and had pain of >20 mm on a 100-mm visual analogue scale (VAS) with low levels of 25-hydroxyvitamin D (25(OH) D, between 12·5 and 60 nmol/l) were enrolled in Tasmania and Victoria, Australia. Participants with the following conditions were excluded: severe radiographic changes (grade 3 of Altman and Gold Atlas)( Reference Altman and Gold 19 ), severe knee pain on standing (>80 mm on a 100-mm VAS), contraindications to MRI, RA or psoriatic arthritis, lupus, cancer, severe cardiac or renal impairment, hypersensitivity to vitamin D, conditions affecting oral drug absorption, anticipated knee or hip surgery within the next 2 years, history of significant trauma of knees (e.g. arthroscopy or injury to ligaments or menisci within 1 year preceding the study) and history of taking vitamin D or other investigational drugs, such as some compound drugs including vitamin D that affected serum vitamin D levels, within the last 30 d( Reference Cao, Jones and Cicuttini 17 ).
After the trial had been completed, 200 participants were randomly selected for the measurements of inflammatory and metabolic biomarkers from Tasmania.
Ethics approval was received from Tasmania Health and Human Medical Research Ethics Committee (reference number H1040) and Monash University Human Research Ethics Committee (reference no. CF10/1182-2010000616).
Randomisation and treatment
Participants were allocated to either vitamin D or placebo arm at a ratio of 1:1 based on computer-generated random numbers. Allocation concealment was ensured by a centrally automated allocation procedure with security in place to ensure that allocation data cannot be accessed or influenced by any person from the investigative team. Participants received oral vitamin D capsules at a dose of 1·25 mg vitamin D3 (cholecalciferol) per month for 24 months in the treatment group. Participants received an identical inert placebo capsule in the placebo group( Reference Cao, Jones and Cicuttini 17 ).
Serum inflammatory and metabolic biomarker level measurement
All fasting blood samples were collected at baseline and at 24 months. The measurements were performed according to the manufacturer’s instruction. Serum levels of high-sensitive C-reactive protein (hs-CRP), IL-6, IL-8, IL-10, resistin, leptin, adiponectin, adipsin and apelin-36 were measured. Serum hs-CRP was measured by enzyme immunoassays (IBL Inc.). Serum leptin and apelin-36 were measured by ELISA (Phoenix Pharmaceuticals Inc.). Serum adiponectin, adipsin and resistin were measured by ELISA (Millipore Inc.). Serum IL-6, IL-8 and IL-10 were measured by Bio-plex Luminex assay kits (Bio-Rad Laboratories Inc.). The inter-assay and intra-assay CV were <10 and <15 % for all inflammatory biomarkers.
Serum vitamin D level measurement
Serum 25-hydroxyvitamin D (25(OH)D) was measured at baseline, and at months 3 and 24 using direct competitive chemiluminescent immunoassays (DiaSorin Inc.). The intra-assay and inter-assay CV were 3·2 and 6·0 %, respectively( Reference Jin, Jones and Cicuttini 15 ). In addition, the season of blood sample was recorded. In this study, we defined serum 25-(OH)D below than 50 nmol/l as vitamin D deficiency.
Variation in vitamin D status
Participants were classified into two groups according to the levels of 25(OH)D at months 3 and 24 as follows: not consistently sufficient (serum 25(OH)D≤50 nmol/l at either month 3 or 24), and consistently sufficient (serum 25(OH)D>50 nmol/l at both months 3 and 24).
Assessment of effusion–synovitis and cartilage volume
MRI scans of the study knee were obtained according to a standardised protocol using a 1·5 T whole-body MRI unit with a commercial transmit-receive extremity coil at baseline and 2 years.
Effusion–synovitis was assessed using T2-weighted fast-spin echo sequences at four regions (suprapatellar pouch, central portion, posterior femoral recess and subpoploteal recess). Effusion–synovitis in each subregion was scored individually according to Whole-Organ Magnetic Resonance Imaging Score, grading collectively from 0 to 3 in terms of the estimated maximal distention of the synovial cavity: 0 refers to normal; 1 to <33 % of maximum potential distention; 2 to 33–66 % of maximum potential distention; and 3 to >66 % of maximum potential distention. The presence of effusion–synovitis of the whole joint was defined as a score of ≥2 in any subregion( Reference Wang, Cicuttini and Jin 16 ).
Effusion–synovitis volumes at 4 regions were isolated from the total volume selecting each region of interest according to the intra-articular fluid-equivalent signal on a section-by-section basis and then resampled by means of bilinear and cubic interpolation for final 3D rendering using OsiriX imaging software (32-bit version 5.9; Pixmeo SARL). The intra-class correlation coefficients were from 0·96 to 0·97( Reference Zheng, Jin and Cicuttini 20 ).
Cartilage volume was determined using the previously described image processing techniques( Reference Cao, Jones and Cicuttini 17 ). The volumes of individual cartilage plates (medial tibial and lateral tibial) were isolated by manually drawing disarticulation contours around the cartilage boundaries on a section-by-section basis and then resampled using bilinear and cubic interpolation for final three-dimensional rendering using OsiriX imaging software. The CV was 2·1 to 2·2 %( Reference Zheng, Jin and Cicuttini 20 ). Total tibial cartilage volume was calculated as the sum of the medial tibia and lateral tibial cartilage plates.
Change in cartilage volume and effusion–synovitis volume was calculated as follows: Absolute change (ml)=(follow-up volume)−(baseline volume).
Anthropometrics
Height was measured to the nearest 0·1 cm (with shoes removed) using a stadiometer (Leicester Height Measure; Invicta Plastics Ltd). Weight was measured to the nearest 0·1 kg (with shoes and bulky clothing removed) using electronic scales (Heine S-7307; Heine). BMI (kg/m2) was calculated.
Data analyses
Very few studies have examined the effect of vitamin D supplementation on inflammatory biomarkers; therefore, there was limited information to inform our sample size calculation. On the basis of a systematic review, the absolute difference in serum hs-CRP between people with OA and healthy controls is estimated as 1·19 mg/l( Reference Jin, Beguerie and Zhang 21 ). Data from the Tasmanian Older Adult Cohort Study provided a SD estimate of 2·78 mg/l. With these estimates, a sample size of eighty-seven in each group would give 80 % power with a 5 % probability of type 1 error (α=0·05, β=0·8)( Reference Kadam and Bhalerao 22 ) to detect this effect size for hs-CRP.
Baseline characteristic differences between the vitamin D supplementation and placebo groups were compared using Student’s t tests or χ 2 tests as appropriate. Box–Cox transformation was used, when variables were not normally distributed, and transformed variables were used in the following analyses. The differences in changes of inflammatory biomarkers between treatment and placebo groups were analysed using linear mixed effects model with adjustment for age, sex, BMI and seasonal change of blood sampling. The within-subject correlation between the repeated measures, including baseline and follow-up data, was taken into account using the individual participant identification as a random effect. The effect of vitamin D supplementation on biomarkers was evaluated by the interaction between treatment and time (i.e. month). The differences in changes of inflammatory biomarkers between not consistently sufficient and consistently sufficient groups were analysed using linear mixed effects model with adjustment for age, sex, BMI and seasonal change of blood sampling. Subgroup analyses were performed in participants with or without effusion–synovitis at baseline. We also have taken the weight change as confounder into analyses to make sure that the weight change did not play a role in this study. Further adjustment for changes in cartilage volume and effusion–synovitis volume was performed to account for the effect of disease progression on serum biomarker change. We used Stata 12.0 for Windows (StataCorp LP) for all analyses. A P value<0·05 (two-tailed) was regarded as statistically significant.
Results
Baseline characteristics of participants
A total of 599 participants were screened for eligibility, 413 participants were enrolled and randomly assigned to vitamin D or placebo group (261 participants in Hobart and 152 participants in Melbourne) and 340 participants (82·0 % retention rate in Hobart and 83·6 % retention rate in Melbourne) completed the study (Fig. 1). A total of 200 participants from Hobart were randomly selected for the inflammatory biomarker measurements: ninety-four participants from the placebo group and 106 from the vitamin D treatment group. The mean age of participants was 63·1 years: 107 (53·5 %) were women and mean BMI was 29·5 kg/m2. There were no significant differences in baseline characteristics (age, sex, BMI, serum 25(OH)D level, serum inflammatory biomarkers levels and season of blood sample) between the placebo and vitamin D groups (Table 1). There were no significant differences in baseline characteristics between those included and not included in this study (data not shown). Significant differences in baseline characteristics, inflammatory and metabolic biomarkers between consistently sufficient and not consistently sufficient groups were not found (data not shown).

Fig. 1 Flowchart of the study.
Table 1 Baseline characteristics of participants (Mean values and standard deviations; numbers and percentages; medians and interquartile ranges (IQR))
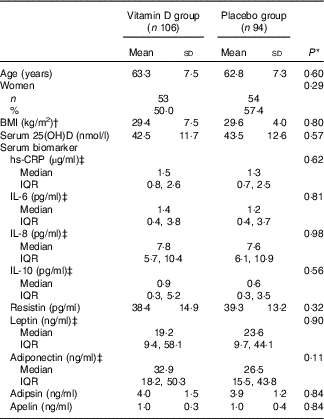
25(OH)D, 25-hydroxyvitamin D; hs-CRP, high-sensitivity C-reactive protein.
* Student’s t tests or χ 2 tests.
† BMI was calculated as weight in kg divided by height in m2.
‡ Skewed distribution.
Vitamin D supplementation and inflammatory and metabolic biomarkers
The mean serum 25(OH)D level increased significantly in the vitamin D treatment group (44·9 nmol/l) compared with the placebo group (7·0 nmol/l) over 2 years. The effect of vitamin D treatment on cytokines and adipokines is shown in Table 2. Vitamin D supplementation had no significant effect on change in serum inflammatory and metabolic biomarkers. There were no statistically significant differences in changes in any biomarkers between the placebo and vitamin D groups. After further adjustment for potential confounders including the seasonal change of blood sample, the differences between groups remained non-significant in the mixed effect model (Table 2). Within the group, serum resistin increased by 1·9 pg/ml (95 % CI 0·1, 3·8) in the placebo group and by 4·4 pg/ml (95 % CI 2·7, 6·2) in the vitamin D group from baseline to month 24. Difference in change in serum resistin between two groups was of borderline statistical significance (P=0·05). Serum adipsin increased by 0·2 ng/ml (95 % CI 0·1, 0·4) in the vitamin D group, but did not increase in the placebo group from baseline to month 24. Serum hs-CRP, IL-6, IL-8, IL-10, leptin, adiponectin and apelin did not change significantly over 24 months in either group.
Table 2 Comparisons of change in inflammatory biomarkers between vitamin D and placebo groups over 24 months (Mean values and 95 % confidence intervals)
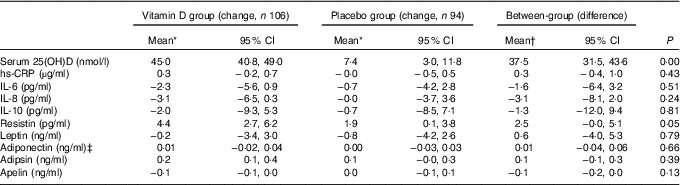
25(OH)D, 25-hydroxyvitamin D; hs-CRP, high-sensitivity C-reactive protein.
* Changes in inflammatory biomarkers are generated from mixed models adjusted for age, sex, BMI and change in season of blood sampling.
† Between-group difference was calculated using vitamin D group values minus placebo group values.
‡ Box–Cox transformation.
Further analyses were performed in participants who had baseline effusion–synovitis or not. Results remained largely unchanged (data not shown). The results remained unchanged after further adjustment for change in weight, and changes in cartilage volume and effusion–synovitis volume (data not shown).
Vitamin D status and change in biomarkers
There were no statistically significant differences in changes of these biomarkers between consistently sufficient and not consistently sufficient groups (Table 3). In the consistently vitamin D sufficient group, there were significant increases in serum resistin and adipsin (3·8 pg/ml and 0·3 ng/ml, respectively) and a significant decrease in serum IL-8 (3·0 pg/ml) from baseline to month 24 (Table 3). In contrast, there was no significant change over the study period in the not consistently sufficient group. Serum IL-6, IL-10, CRP, leptin, adiponectin and apelin did not change significantly from baseline to month 24 in either group.
Table 3 Comparison of change in inflammatory biomarkers between different vitamin D status over 24 months (Mean values and 95 % confidence intervals)
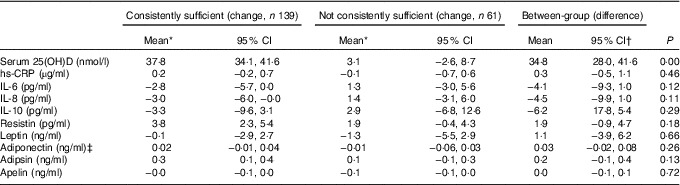
25(OH)D, 25-hydroxyvitamin D; hs-CRP, high-sensitivity C-reactive protein.
* Changes in inflammatory biomarkers are generated from mixed models adjusted for vitamin D treatment, age, sex, BMI and change in season of blood sampling.
† Between-group difference was calculated using consistently sufficient group values minus not consistently sufficient group values.
‡ Box–Cox transformation.
The results remained largely unchanged if patients with and without baseline effusion–synovitis were separated for analyses (data not shown). Results remained largely unchanged after further adjustment for changes in weight, cartilage volume and effusion–synovitis volume (data not shown). In addition, no significant associations were found between the changes in inflammatory/metabolic biomarkers and change in 25(OH)D over 24 months, and the results remained largely unchanged after further adjustment for the baseline serum 25(OH)D level (except for change in resistin, P=0·04) (data not shown).
Discussion
To the best of our knowledge, this study is the first to explore the effect of vitamin D supplementation on inflammatory and metabolic biomarkers in patients with knee OA and to compare the effect of vitamin D sufficiency on levels of serum inflammatory and metabolic biomarkers in OA patients. Vitamin D supplementation had no significant effects on serum inflammatory and metabolic biomarkers in patients with knee OA. Furthermore, there were no significant differences in serum inflammatory and metabolic biomarkers between those who were consistently sufficient and those who were not over 24 months. These results suggest that vitamin D supplementation and maintaining sufficient vitamin D status may not have effects on systemic inflammation in knee OA patients.
Low-grade systemic inflammation triggered by abnormally inflammatory or metabolic biomarkers has been implicated in the OA pathogenesis. There is a vast amount of evidence linking inflammatory biomarkers and OA, as well as the association between vitamin D and inflammation. Serum hs-CRP levels were statistically significantly higher in the OA group than in the control group and were associated with increased pain and decreased physical function( Reference Jin, Beguerie and Zhang 21 ). Serum IL-6 was correlated with radiographic OA, knee cartilage loss and increased knee pain over time( Reference Stannus, Jones and Cicuttini 23 , Reference Stannus, Jones and Blizzard 24 ). Adipokines such as leptin and resistin may disrupt cartilage homoeostasis by directly inducing joint structural degradation or regulating local inflammatory processes and are regarded as metabolic biomarkers in the inflammatory process of OA( Reference de Boer, van Spil and Huisman 25 , Reference Martel-Pelletier, Raynauld and Dorais 26 ). Serum leptin was associated with reduced knee cartilage volume and increased loss of cartilage thickness( Reference Stannus, Cao and Antony 27 ). Furthermore, high serum leptin and IL-6 were associated with reduced 25(OH)D levels over time( Reference Ding, Parameswaran and Blizzard 28 ). These suggest that systemic inflammation triggered by inflammatory or metabolic biomarkers may be a key underlying mechanism linking vitamin D deficiency to OA.
Although there has been no previous RCT examining the effect of vitamin D supplementation on inflammatory and metabolic biomarkers in knee OA patients, some RCT have examined the effect in healthy individuals, older adults or patients with other chronic diseases such as obesity, asthma, diabetes and chronic kidney disease, and reported inconsistent results( Reference Ticinesi, Meschi and Lauretani 29 ). The inconsistency between these study findings may be caused by small sample sizes, diverse characteristics of participants, treatment with different doses and measurements of different inflammatory and metabolic biomarkers for the different clinical trials( Reference Chen, Wan and Han 30 ). For example, two RCT with small sample size were conducted in patients with diabetes. One study reported that supplementation with 1·25 mg vitamin D per 2 weeks for 12 weeks in 60 patients significantly reduced serum hs-CRP level compared with the placebo group( Reference Razzaghi, Pourbagheri and Momen-Heravi 31 ), but another study reported that treatment with 1·25 mg/week vitamin D and/or 1000 mg Ca/d twice for 8 weeks did not result in significant difference in change in serum CRP and leptin, but did result in significant reduction in serum IL-6 and TNF-α compared with the placebo group in 118 diabetic patients( Reference Tabesh, Azadbakht and Faghihimani 12 ).
Our results are consistent with findings from RCT in healthy or older adults without specific health conditions as vitamin D supplementation in these groups has shown no effect on inflammatory and metabolic biomarkers( Reference Pittas, Harris and Stark 32 – Reference Wood, Secombes and Thies 35 ). A randomised placebo-controlled trial was conducted in elderly adults without a specific disease in Australia, aiming to examine the effects of vitamin D supplementation for 12 months on hs-CRP, leptin, adiponectin, IL-6 and IL-10( Reference Waterhouse, Tran and Ebeling 36 ). IL-6 was numerically higher in those participants supplemented with 1500 µg/month vitamin D compared with those supplemented with 750 µg/month, although this was not significant. In our current study, we found that vitamin D supplementation had no significant effect on serum inflammatory and metabolic biomarkers, with the exception of a possible effect on serum resistin, in patients with knee OA. Serum resistin increased twofold in the vitamin D group than in the placebo group, but the difference was of borderline significance. Although evidence shows that serum resistin is a pro-inflammatory cytokine and is positively associated with severity of OA( Reference Li, Tian and Wang 37 ), the clinical relevance of this finding is unknown. Subgroup analyses were performed in participants who had baseline effusion–synovitis or not, and results remained unchanged. We previously reported that vitamin D supplementation relieved the progression of effusion–synovitis in patients with an inflammatory OA phenotype( Reference Wang, Cicuttini and Jin 16 ). These indicated that vitamin D supplementation would have effects on local rather than systemic inflammation. The underlying mechanisms are unclear and need to be explored by further studies.
A high proportion of participants in the placebo group achieved sufficient vitamin D level in month 24 (62 % participants >50 nmol/l) in the VIDEO study, which may have masked the effect of vitamin D supplementation on inflammatory and metabolic biomarkers. Therefore, we performed further post hoc analyses to examine whether maintaining sufficient vitamin D level over the treatment period had effects on the biomarkers. Although serum resistin and adipsin increased, and serum IL-8 decreased from baseline to month 24 in the consistently vitamin D sufficient group, changes in serum resistin, adipsin and IL-8 were not significantly different between the patients with different vitamin D status. Until now, only one cross-sectional study compared the level of inflammatory cytokines (IL-1β, 2, 4, 5, 6, 8, 10, 12, 13 and hs-CRP) in different serum vitamin D status (deficient, insufficient or sufficient) in patients with knee OA( Reference Barker, Henriksen and Rogers 38 ), and did not find that vitamin D status was associated with circulating inflammatory biomarkers. These results were generally consistent with the findings in our current study. Maintaining serum vitamin D sufficiency may not have effects on systemic inflammation in knee OA patients.
We used the direct competitive chemiluminescent immunoassays (Diasorin Liaison assay) to measure the serum 25(OH)D, which may not be as sensitive and specific as HPLC methodology; however, it showed good correlation with HPLC, and would also be specific for both 25(OH)D2 and 25(OH)D3 ( Reference Wootton 39 , Reference Holick 40 ). The vitamin D status, measured via serum 25(OH)D concentrations, can be affected by factors such as obesity. In addition, expression of vitamin D-dependent genes could be served as a marker of vitamin D status, and this may also influence the effect of vitamin D supplementation on inflammatory markers( Reference Vukic, Neme and Seuter 41 , Reference Carlberg, Seuter and de Mello 42 ). These need to be explored in future studies.
There were some limitations of the current study. First, it was a post hoc analysis within a subsample of an RCT, which was not designed to examine the effect of vitamin D supplementation on inflammatory and metabolic biomarkers in patients with knee OA. The findings need to be confirmed by further RCT using these biomarkers as the primary end points. Second, this study had reduced sample size than what was designed for the original study. However, the sample size is sufficient to detect a significant difference for hs-CRP. Third, there was a high proportion of participants in the placebo group who achieved sufficient vitamin D levels at months 3 and 24 (62 % participants >50 nmol/l) in the RCT study, which could have diluted the effect of vitamin D supplementation. Thus, we performed further post hoc analyses using variations in vitamin D status over the treatment period and the results were consistent. Fourth, three time points (baseline, 3 months and 24 months) may not be adequate to define the ‘consistently or inconsistently vitamin D sufficient’, and the inflammatory and metabolic markers were not measured at an intermediate time point. It was unknown whether vitamin D supplementation had intermediate effects on inflammatory markers in knee OA patients. Therefore, further studies are required.
Conclusion
Vitamin D supplementation and maintaining vitamin D sufficiency did not alter serum levels of inflammatory and metabolic biomarkers over 2 years in knee OA patients who were vitamin D insufficient, suggesting that they may not affect systemic inflammation in knee OA patients.
Acknowledgements
The authors specially thank the participants who made this study possible, and the authors gratefully acknowledge the role of Vitamin D Effect on Osteoarthritis Study staff and volunteers in collecting the data. The authors thank the research assistants Jodi Barling, Kay Nguo, Judy Hankin and Alice Noon who were involved in the coordination of this study.
This work was supported by the Australian National Health and Medical Research Council Grant (Project Code 605501).
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. S. Z. had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. S. Z. and C. D. designed the study, collected data, carried out data analyses, interpreted the results and drafted the manuscript. B. W., W. H., Z. Z., X. W., X. J., B. A., F. C., A. W., T. W., D. A., L. B. and G. J. were involved in collecting the data, helping the data analyses, interpreting the results and revising the manuscript.
The authors declare that there are no conflicts of interest.






