1. Introduction
One of the most important and daunting roles of the early academic is the pursuit of National Institutes of Health (NIH) grant funding. Although NIH funding allows for great autonomy and comes with validation and prestige, the process can feel overwhelming even for the most seasoned investigators. Therefore, being armed with information is crucial.
Most importantly, it is vital to keep in mind that applying for NIH funding is much more of a marathon than a sprint. Only, it is a marathon where there is no planned route, where you often realize you have been going in the wrong direction and have to double-back with few signs to assure you. In addition, it is a run in which everyone else also struggles at one time or another, but most are much more eager to talk of their success than their struggles. You will be questioned and second-guessed at every step by those evaluating your performance as well as your supporters, and you will be guaranteed to feel like you are stumbling across the finish line no matter how confident you were at the start.
With those caveats in place, it is a marathon with some tangible positives for those who are successful, including resources to do your research in the best way possible with an opportunity to build a research team of pre- and post-doctoral trainees and support staff, as well as better visibility in the research community and a big boost in the promotion and tenure process. Moreover, these scientific benefits also often come with financial support which may serve as the basis for your salary in an academic medical setting or allow you more time to devote to research through course buy-outs or summer salary support in a Psychology Department. Clearly, the pursuit of an NIH grant is a high-risk/high-reward venture that should not be entered into lightly, but also should be an option for anyone who is willing.
Aiming to provide a guide to NIH grants with the early stage investigator in mind, this chapter outlines many of the key issues you will tackle throughout the process. These include: (a) Developing Your Idea; (b) Finding the Right Mechanism for You and Your Idea; (c) Preparing Your Application; d) Submission and Receipt of Your Application; (e) The Review Process; and (f) Post-Review Strategies. We will address these issues in light of the recent changes in the NIH grant submission and review process to provide an objective source, complemented by our favorite tips for your consideration.
2. Developing Your Idea
A lot must go into moving from the first spark of an idea to the completion of a fully formed grant. A viable grant should begin with an idea that is well suited to your background and focused on a topic you know well. It is important to select research questions that will allow you to maximize your professional development and provide a chance to make your own “mark” on the field. Therefore, it is critical to consider how you can strategically develop your research to be programmatic in nature so that it will be sustaining and long-lasting, making numerous cumulative contributions to the field. While it is imperative to select a topic that fits with your expertise and interests, a successful NIH grant also must have clear public health relevance, a place within the scientific literature in that field, and potential to significantly advance the existing knowledge base.
Based on the review criteria we will discuss in detail later, key questions to consider when generating ideas include: How will this study be significant, exciting, or new? Is there a compelling rationale? Is there potential for high impact? How will aims be focused, clear, feasible, and not overly ambitious? How will the study clearly link to future directions? Have I demonstrated expertise or publications in line with the approach? Do I have collaborators who offer expertise to the proposed research? Do I have the necessary institutional support?
Once you get a bit further along in developing your idea, it can be helpful to talk to NIH staff, particularly staff who have a portfolio that includes similar types of grants. One way to see funded grants to ensure your research idea is reasonable (and also not already being done!) is NIH REPORTER (http://projectreporter.nih.gov/reporter.cfm). This electronic database provides information on NIH-funded research including titles, principal investigators, and abstracts. REPORTER is a means to get a snapshot of one’s field including possible collaborators and competitors.
3. Finding the Right Mechanism for You and Your Idea
A critical component of the idea development process is selecting the right grant mechanism. Similar to getting advice on your grant idea as noted above, you should consider checking with a program official from the institute you are targeting with your application to assess fit between your idea and programmatic priorities, your career trajectory and goals, and a particular mechanism. As an early career psychologist, the choice will likely be between a career development award (“K award”) or an investigator-initiated research award (“R grant”).
In the following sections we provide a detailed description of the K award and the R01 grant including a direct comparison of the two. Although we will not discuss it here, you should also be aware that NIH also offers post-doctoral fellowship awards called F32’s that may be a useful option to consider (see https://grants.nih.gov/grants/guide/pa-files/PA-20-242.html for details). Moreover, there are some exciting newer mechanisms that provide high levels of flexible funding opportunities for unusually creative early stage investigators. You should consider these especially if your work is particularly interdisciplinary and/or novel in ways that do not fit well into existing funding niches. We will not address these here but more information can be found at the NIH Office of Extramural Research (https://researchtraining.nih.gov/).
3.1 K Awards
There are a number of types of K awards (for more details see: https://researchtraining.nih.gov/programs/career-development). The most relevant for early career psychologists are the K01 (Mentored Research Scientist Development Award for career development in a new area of research or for a minority candidate), K08 (Mentored Clinical Scientist Development Award for development of the independent clinical research scientist), and K23 (Mentored Patient-Oriented Research Career Development Award for development of the independent research scientist in the clinical arena). There also are mid-career and even later career development awards that provide resources for investigators to develop new areas of expertise – and provide mentorship to junior investigators. The K award usually requires that at least 75 percent of your effort (9 calendar months in NIH terms) be devoted to the research project and to career development for 3–5 years. These awards are evaluated as training mechanisms. Applications require not only a research plan but also a training plan for career development activities under the guidance of a research mentor, local collaborators, and external consultants. The university must usually agree to release the PI from most teaching, clinical, and administrative duties. In return, NIH will pay the PI’s salary, up to certain limits. There is a great deal of variation among the different NIH institutes as to which Career Awards are available, what PI qualifications they expect, the dollar limits for salary and research expenses that they will award, their application deadlines, and their supplemental proposal instructions. It is best to contact the relevant institute prior to preparing your proposal to be sure you understand that institute’s guidelines for a K award.
3.2 R Grants
The R grants most relevant to the early academic include the R03, R21, R34, and R01 (for more details see: http://grants.nih.gov/grants/funding/funding_program.htm#RSeries). The R03, small grant program, provides limited funding for a short period of time. Funding is available for two years with a budget up to $50,000 per year. Some institutes (e.g., NIDA) also offer rapid transition awards called a B/Start (i.e., Behavioral Science Track Award for Rapid Transition), which consists of one year of funding for $75,000. Because reviewers submit reviews without a full review meeting, this mechanism often includes a shorter lag time to completion of the review process (i.e., funding occurs within approximately 6 months of the date of receipt of the application).
The R21 is considered to be an exploratory/developmental research grant used to support the early stages of project development (e.g., pilot or feasibility studies). Funding is available for two years and the budget cannot exceed $275,000. Extensive preliminary data are not expected, but applications must make clear that the proposed research is sound and that the investigators and available resources are appropriate to the task. While the R21 mechanism can sometimes be considered to be most relevant for previously funded senior investigators undertaking high-risk/high-reward research and/or a new area of research, it is our experience that first-time investigators can be successful seeking R21s if their idea is novel and has potential for transformative impact in their field of study.
The R34 is a clinical trial planning grant intended to support development of a clinical trial. This program may support: establishment of the research team, development of tools for data management, development of a trial design, finalization of the protocol, and preparation of a manual. For example, NIDA offers this mechanism exclusively for treatment development and some initial testing. The R34 lasts for 3 years with a budget of $450,000, with no more than $225,000 in direct costs allowed in any single year.
The R01 is NIH’s most commonly used grant program which is generally awarded for 3–5 years. There is no specific budget limit, but budgets under a particular amount can be submitted with less detail than more expensive R01s (called modular and typically $250,000 direct costs each year). Budgets over a particular amount (typically $500,000 direct costs each year) must obtain institute approval before being submitted. Although you should request the budget you need to conduct your project, an extremely large scope and budget in an application from a new investigator may raise red flags for reviewers. Interestingly, it is our experience that some early career investigators avoid the R01 mechanism because of their junior status; however, as outlined below, NIH has taken some steps to encourage early career investigators with a “big” idea and adequate pilot data to consider an R01.
3.3 K/R Hybrids
Of note, there is an additional mechanism that serves as a bridge between a K award and an R grant called a Pathway to Independence Award (K99/R00, nicknamed kangaroo). This mechanism provides up to five years of support consisting of two phases. The first phase provides 1–2 years of mentored support as a postdoctoral fellow. The second phase is up to 3 years of independent support (contingent on securing an independent research position). Recipients are expected to compete for independent R01 support during the second phase to allow for continued funding once the K99/R00 support has ended. Eligible principal investigators must have no more than 5 years of postdoctoral research training.
3.4 Advantages and Disadvantages of K Awards and R Grants
K awards and R grant mechanisms each have a number of advantages and disadvantages. A K award can provide 50–100 percent of your salary (depending on the type of K and branch of NIH) for up to 5 years. This allows for a more highly stable period of funding than the typical R01, which usually funds only 20–40 percent of the principal investigator’s (PI) salary for a period of 3–5 years. This allows investigators to concentrate on their specified research efforts without the concerns or distractions of needing to constantly be pursuing additional sources of support or fulfilling extensive clinical or teaching responsibilities at their university. Other advantages of the K award are the opportunities for mentorship, training, and thoughtful development of a programmatic line of research in the PI’s chosen area. The K will provide funding (typically $50,000 in addition to salary support) specifically to support these critical opportunities, which include: time and funds for focused coursework, study materials, access to consultants and mentors – and funds to travel to meet with off-site mentors at their research labs or attend professional conferences. These resources are paired with a highly personalized training plan that is developed as a part of the grant application. Because career development and training is a central aspect of K awards, the expectation of research is different and more modest than that for an R grant that will have a much more highly specified research project (and no training component).
For all of those reasons, the K award is very well suited for the needs of junior investigators who may have only limited pilot data of their own and require additional training experiences before attempting the larger-scale R grant projects. Also, the fact that a K award covers most if not all of one’s salary can be very helpful in environments that require a large percentage or even all of one’s salary to be covered on grants, which often include most medical school positions. It is unusual for more than 33 percent of one’s salary to be covered by an R01, even though the latter is a larger grant because it is less focused on training/support of a junior investigator and more focused on supporting the research project. More recently, even psychology departments and other similar environments that are more associated with hard salary funding have begun creating positions or providing greater flexibility in existing positions for those with a K Award, expanding the scope of environments in which they have great value. Nevertheless, the K award is not necessarily the best mechanism for some junior investigators. Some are discouraged by the prospect of an ongoing role as “trainee.” Others are deterred by the lack of flexibility in the mechanism itself. For example, a K can be transferred to other institutions, but it can take some time and specific evidence that the new environment can support the research and that relevant and willing mentors are on-site. They also do not provide sufficient funding to implement large-scale research projects (e.g., a randomized clinical trial). Moreover, they require significant institutional support documented within the application that is not always proffered or feasible for budgetary reasons or instructional needs. Mentors on Ks do not receive financial support from the grant, which can create challenges getting engagement and sufficient time devotion. K awards also pay a vastly lower indirect cost rate (8 percent) than R grants (typically in the 50–65 percent range). Indirect costs are funds provided to the applicant’s institution to cover the costs of administering and supporting the applicant’s research. This amount is above and beyond the funds provided to the applicant for the research (called direct costs), but is calculated as a percentage of the direct costs. Although this should not lead you to apply for an R grant over a K award if the latter is a better choice for you and your research, you should be aware that the disparity in indirect costs of a K award may leave junior faculty investigators at a disadvantage in terms of obtaining additional institutional support once the application is funded and the research begins. Finally, there has been considerable chatter in recent years that while a K award can really jump-start a career for some, they might counterintuitively slow progress to an R grant for others. Because K awards cover so much of one’s salary it is actually challenging to show one has the available effort to devote to other projects, particularly as the PI, and there is always some confusion about when someone with a K is “allowed” to start submitting R-level grants. As such, K awardees may be slower to write their first R than those without a K award. We should be clear, this is not an argument against a K award per se, as they are indeed great for one’s career, but this unexpected potential impediment to future development of an independently funded research portfolio is important to be aware of for those who pursue a K. These timing concerns need to be balanced with preliminary evidence that researchers who have received a K award compared to those who did not have great success getting their first NIH award (https://nexus.od.nih.gov/all/2019/04/02/association-between-receiving-an-individual-mentored-career-development-k-award-and-subsequent-research-support/).
A major advantage of the conventional R01 award (and to a lesser extent other R grants) is the significantly larger project budgets, dictated by the specific requirements of the scientific protocol. However, new investigators applying for any R grant must be prepared to demonstrate to the review committee that they have the appropriate background, expertise, and skills to implement and complete an independent research project. There are a number of ways to successfully demonstrate these qualities. They include the availability of relevant scientific pilot data, a “track record” of publications in your area of research, and a thorough, well-conceived, and convincingly argued research plan (i.e., scientific protocol). Applications for R funding are evaluated almost exclusively on their scientific merit, significance, and innovation. R01 grants are quite competitive, but there is a tangible advantage in the evaluation process if you are a new investigator defined as not previously or currently holding R01 support (previous R01 submissions do not affect this status until one is funded). Specifically, in many cases your application will be considered in a separate pool of applications devoted to only new investigators. This “levels the playing field” and prevents your application from competing directly with applications from more seasoned investigators. While not a significant disadvantage per se, as noted above, even large-scale R grants rarely cover all or even most of one’s salary. This is unlikely to be a problem in environments where other “hard” or “soft” funding is available, but should be a consideration where one is expected to cover a large portion of their salary as an R grant alone will not be sufficient in these cases.
3.5 Application Types
A large percentage of applications are investigator-initiated (often called “unsolicited”). Investigator-initiated applications can be submitted according to published submission deadlines, most often in February, June, and October. Applications that fall under special interest areas such as HIV/AIDS have different deadlines that accommodate a faster review, so you are encouraged to check these deadlines closely (see https://grants.nih.gov/grants/how-to-apply-application-guide/due-dates-and-submission-policies/due-dates.htm).
Another option is to submit in response to a Request for Applications (RFA). RFAs are meant to stimulate research activity to address NIH-identified high priority issues and areas. They do not utilize regular deadlines and are announced with a specified deadline (often less than 4 months from the announcement). As such, researchers most interested and immersed in these areas of research have a decided advantage because they are likely to have already thought through some of the key issues and in some cases already have available pilot data that could serve as the base for the RFA submission. Of note, these applications typically are reviewed by specially convened panels that are selected based on the specific RFA and are therefore likely to have significant relevant expertise. As one might guess this can be an advantage in that one is getting a review from individuals who are most qualified to evaluate that application. However, an expert also may have particular expectations about how things should be done and may be more likely to focus on esoteric aspects of the application that might go unnoticed by reviewers with less expertise in that area.
One source of confusion can be Program Announcements. PAs are similar to RFAs in that they are issued by one or many Institutes and outline topics that are of particular interest. Like an RFA, PAs provide a level of assurance that the type of research you are proposing will be of interest to the institute that issued the PA. More recently, NIH has begun phasing out PAs and opting to implement Notice of Special Interest (NOSI). A NOSI is a new format for NIH Institutes/Centers to share and update their research priorities. NOSIs are intended to replace PAs that do not have special review criteria or set-aside funds. Each NOSI describes aims in a specific scientific area(s) and points to Funding Opportunity Announcements (FOAs) through which investigators can apply for support.
4. Preparing Your Application
The following paragraphs outline each section of a typical research grant. We also provide practical guidance regarding a few things to do and not do. Please note that in addition to this information, you can find helpful information on preparing your application at: http://grants.nih.gov/grants/writing_application.htm and information on page limits can be found at: http://grants.nih.gov/grants/forms_page_limits.htm. Moreover, we also encourage you to utilize the following link which provide more general tips in a video format: https://public.csr.nih.gov/FAQs/ApplicantsFAQs.
4.1 Project Summary
The project summary is a two-part overview of your proposed project. The first part is the abstract in which you have 30 lines to describe succinctly every major aspect of the proposed project, including a brief background, specific aims, objectives, and/or hypotheses, public health significance, innovative aspects, methodology proposed, expected results, and implications. The second component of the Project Summary is the Project Narrative, which provides a plain-language 2–3 sentence description of your application.
4.2 Aims
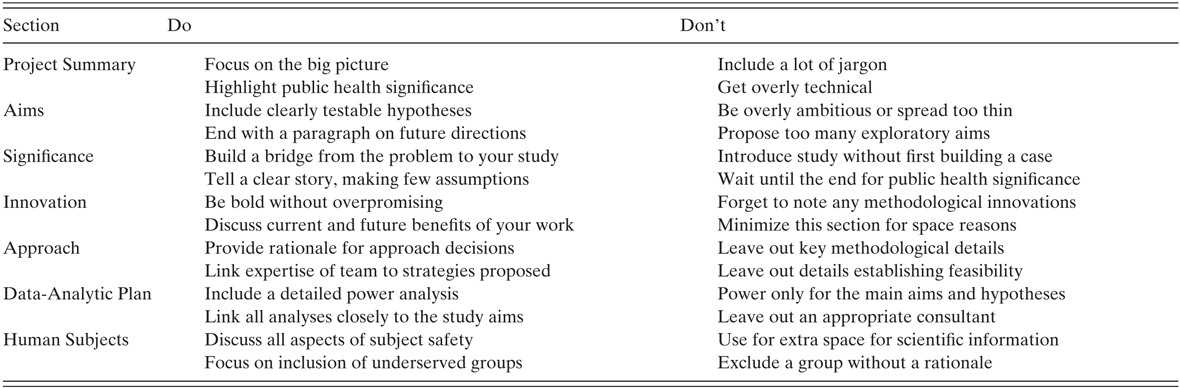
Aims provide a one-page statement of your goal, objectives, and expected outcomes and implications. The aims should start with a brief statement of the problem and its public health impact, followed by what is known, and then the gap between what is known and how your project will address this gap. The most important part is the statement of your specific aims and the hypotheses you have for each aim. These statements should be concise and include clear, testable hypotheses. Occasionally, you may include an exploratory aim that addresses an important question but for which enough information is not available to draw a hypothesis; however, these should be used sparingly. You then should conclude with a summary paragraph that also suggests the research directions and implications that this work will spawn. NIH wants long-term not short-term relationships with its applicants. As such, your ability to discuss how this work will not be a single effort but the start of an effective line of research is crucial. A handy template of possible steps to follow in arranging your aim statement is provided in Table 15.1 and we provide guidance on things to consider doing and to avoid doing at the end of this section in Table 15.2.
Table 15.1 Possible template of steps in your aims section
| Step 1: Identify the important societal/health problem of focus |
| Step 2: Review work that has been done towards solving this problem |
| Step 3: Identify the gap in the literature (e.g., what is missing, next, or even wrong with existing work) |
| Step 4: Articulate how you intend to address this gap with basic details of your intended approach/method |
| Step 5: Specify your aims for this research (i.e., specific aims) and what you expect to find (i.e., hypotheses) |
| Step 6: Highlight the potential implication of this research and how it sets up future studies (and your long-term independent line of research if possible) to make short- and long-term progress towards solving the problem outlined in Step 1 |
Table 15.2 Tips by grant section
| Section | Do | Don’t |
|---|---|---|
| Project Summary |
|
|
| Aims |
|
|
| Significance |
|
|
| Innovation |
|
|
| Approach |
|
|
| Data-Analytic Plan |
|
|
| Human Subjects |
|
|
4.3 Research Strategy
4.3.1 Significance
This section explains the importance of the problem or critical barrier to progress in the field that the proposed project addresses, and how the project will advance the application of scientific knowledge. In doing so, this section outlines the relevant literature and how this project directly addresses relevant gaps.
4.3.2 Innovation
This section explains how this work takes a new perspective, develops/utilizes a new approach, and/or moves the field in new directions. It is important in this section to emphasize that the novelty is not simply for the sake of being new, but holds important strengths over existing approaches – and sometimes novelty involves nothing new per se but creative use of existing methods or samples. You also should note that innovation can be a slow process and your work can be innovative if it sets the stage for future work. However, in this case it is especially up to you to be clear how your work can be the start of a fruitful and impactful line of research and why that makes the current work innovative. This may be especially true for those conducting pre-clinical or other forms of basic research.
4.3.3 Approach
This section describes the overall strategy, scientific methodology, and analyses to be used to accomplish the specific aims of the project. It is useful to link the approach as clearly as possible to the specific aims and hypotheses. Although there is a human subjects section below, human subjects issues that have important scientific bearing are addressed here. These might include an empirical justification for including only one gender or a theoretical reason to focus on a narrow developmental period in adolescence. Within Approach you also are encouraged to include two subsections. One subsection is Preliminary Studies, which outlines the previous work of you and other members of your research team that support your aims and hypotheses, and establishes that you are qualified to undertake and successfully complete the project. The other subsection is Potential Problems, Alternative Strategies, and Benchmarks for Success, which provides you with the opportunity to anticipate and address the questions that reviewers are likely to ask themselves as they read your application. We discuss the importance of these subsections and strategies to make the most of them below in “Tips.”
4.3.4 Data-Analytic Plan
This section outlines your statistical approach. Here it is crucial to address issues of statistical power and sample size calculation and preliminary analyses before outlining the primary analyses. The readability of this section and the overall flow of the application will be greatly enhanced if the plan is presented in the context of the specific aims and hypotheses.
4.4 Human Subjects
Although it is not placed in the body of the research plan, the section on the protection of human subjects and the inclusion of both genders, children, and underserved members of minority groups is an important part of your application. It should carefully describe aspects of the grant related to the risk–benefit ratio and demonstrate that all necessary precautions are in place to protect the rights and safety of human subject participants. In most R grants this section includes virtually all of the information expected in an application for Institutional Review Board (IRB) approval. This should include strategies to ensure adequate recruitment of underserved groups and a clear statement for why certain groups aren’t included, especially if for methodological reason (which also should be noted in the section on Approach). This section also should include a data and safety monitoring plan as it is now required for all clinical trials (phases I, II, or III) and a monitoring board for larger-scale trials, multi-site trials, and those including vulnerable populations (e.g., prisoner populations).
4.5 Additional Sections
The following sections also need to be included in your grant application: Appendix Materials, Bibliography & References Cited, Care and Use of Vertebrate Animals in Research, Consortium/Contractual Arrangements, Consultants, Facilities & Other Resources, Resource Sharing Plan(s), Select Agents, Multiple PD/PI, and Use of Internet Sites. See http://grants.nih.gov/grants/writing_application.htm for additional details. Additional content sections specific to K award applications include the Candidate’s Background, Career Goals and Objectives, Career Development/Training Activities during Award Period, Training in the Responsible Conduct of Research, Statements by Mentor, Co-mentor(s), Consultants, Contributors, Description of Institutional Environment, and Institutional Commitment to the Candidate’s Research Career Development.
5. Submission and Receipt of Your Application
All applications are submitted through an electronic portal called grants.gov. You should note that your application must be submitted and free of errors by the due date. Therefore, be sure to closely follow all of the rules and regulations governing each aspect of the application to prevent your application from being withdrawn from the review process. Given these warnings, the actual submission process might seem daunting in its own right. However, your research office should have numerous tutorials and provide support to ensure that you complete this part on time and accurately.
Once you have worked with your research office to submit your application on grants.gov, an NIH referral officer will typically assign the application to the most appropriate institute. Although this includes a review of the entire application, decisions are driven by the title, abstract, and to a lesser extent the aims. This process also can be influenced by a cover letter you can prepare with your application indicating which institute you believe is the best fit for the application. The most common institute for psychologists to submit applications is the National Institute of Mental Health. However, it is important for you to develop your idea and then consider the most appropriate institute, which often means branching out to other institutes (for a list of institutes see www.nih.gov/icd/). Once directed to a particular institute, it will be assigned to an Integrated Review Group (IRG) and then ultimately a study section within that IRG. These study sections keep a regular roster of reviewers that rotates every four years. You can get an idea of the study section based on the roster and you may choose to request a particular section in the previously motioned cover letter.
Once your application has received an assignment to a NIH institute and study section, it is given a unique grant number. Shortly thereafter, you will receive a notice documenting this information and providing you with the name and contact information for the Scientific Review Officer (SRO) who organizes the work of the review committee (e.g., distributing applications; assigning specific reviewers; coordinating dates and sites for the three review committee meetings each year). There is a lengthy interval between the time you submit your application and the time it is actually reviewed; for example, applications received on June 1 are typically reviewed in October or November. For this reason, many study sections will accept supplementary materials in the 3–4 weeks prior to review. For example, if you have collected additional pilot data since submitting your application, you may want to provide a brief report about these research activities and results. Such supplemental materials should be brief (e.g., 1–2 pages). To determine whether and when you might submit a supplement, contact your SRO. Supplemental material can be helpful, especially when a new paper is accepted for publication or if new data become available that were not expected at the time of the submission. With that said, we do not recommend relying on supplementary material as “extra time” to add to your application after the deadline. Although often accommodated, supplemental material is not always accepted and more importantly there is no guarantee that reviewers will consider this additional material, especially given that they already will have plenty to cover in the original application.
6. The Review Process
Approximately 6 weeks prior to the review meeting, members of the study section receive copies of all of the applications being reviewed in that cycle. Typically, three members (designated as primary, secondary, and tertiary) are assigned to each application, based on the fit between their research expertise and the content of the grant. Reviewers provide written critiques of the application, organized according to the NIH review criteria: significance, approach, innovation, investigator, and research environment (see Table 15.3 for more detail about the criteria and how best to address them). If sufficient expertise is not available from the standing membership of the committee, the SRO can invite ad-hoc reviewers to participate. However, do not assume everyone or even anyone will be an expert in your particular topic, and be sure that your application does not rely on jargon or make assumptions about reviewer familiarity regarding idiosyncrasies or convention approaches in a particular area of research.
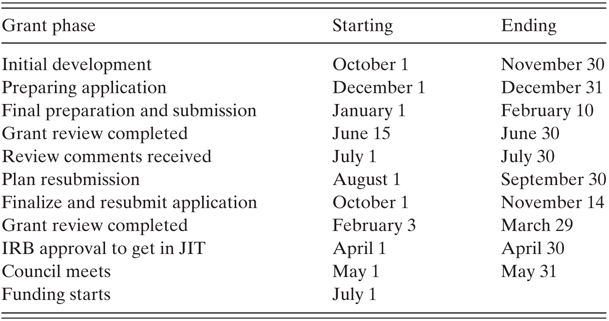
Table 15.3 Hypothetical grant timeline
| Grant phase | Starting | Ending |
|---|---|---|
| Initial development | October 1 | November 30 |
| Preparing application | December 1 | December 31 |
| Final preparation and submission | January 1 | February 10 |
| Grant review completed | June 15 | June 30 |
| Review comments received | July 1 | July 30 |
| Plan resubmission | August 1 | September 30 |
| Finalize and resubmit application | October 1 | November 14 |
| Grant review completed | February 3 | March 29 |
| IRB approval to get in JIT | April 1 | April 30 |
| Council meets | May 1 | May 31 |
| Funding starts | July 1 |
As the meeting approaches, the SRO will solicit feedback about which grants are ranked in the bottom half of the current group and will not be formally discussed at the meeting (referred to as streamlining). A final consensus about streamlining is usually made at the beginning of each review meeting. Although they will not be discussed at the meeting and will not receive a score, the PI will receive the feedback prepared by each of the three reviewers for the meeting. The rationale for streamlining is to allow greater time for discussion about those applications perceived to be ready for support and thus to maximize the value of the review for both applicants and NIH program staff.
About the top half of applications are discussed at the review meeting. The primary reviewer provides a description of the application and then outlines strengths and weakness in the domains listed above. Each additional reviewer adds any further information and can add new points or issues where they disagree with a previous reviewer. At this point the other panel members can ask questions and raise additional points (although they are not required to have read the application). The group then has a discussion. The goal is consensus, but this is not a requirement and sometimes there can be significant disagreement among the reviewers. After discussion, the reviewers provide scores again. Reviewers may shift scores after the discussion to support consensus but are under no obligation. The remaining committee members then provide their votes anonymously; however, if they are outside of the low and high score by a predetermined range, they are asked to provide a written explanation.
6.1 Core Review Criteria
Your application is evaluated on the following five core review criteria: (1) Significance, (2) Investigator(s), (3) Innovation, (4) Approach, and (5) Environment. For a detailed outline of these criteria with a comparison with previous criteria, see http://grants.nih.gov/grants/peer/guidelines_general/comparison_of_review_criteria.pdf. As we have covered Innovation and Approach thoroughly in “Preparing Your Application,” we focus only on the key features of the other three criteria here.
Significance. Is this work addressing an important question and will have an impact on the field in terms of knowledge, application, or in the best case scenario both? It is not crucial that the application be immediately addressed in a submission (especially in more basic research projects), but reviewers will want to see evidence of how this work ultimately could have such impact.
Investigator(s). Are you qualified to conduct this project and how well does your team of collaborators (or mentors for career awards) provide specific support in areas where your experience and expertise could be supplemented? For evaluating your credentials, reviewers often will focus on training and specific research productivity. Also, evidence that there is a specific role for the collaborators/mentors is crucial, as is some evidence of past work together or future plans to ensure their participation. This can be best represented in a letter of support and clearly articulated in “personnel justification,” which is an additional administrative section of the grant not covered here.
Environment. Can the work be carried out with adequate institutional support and resources? Additionally, are there unique features of the scientific environment, subject populations, or collaborative arrangements that are evident at the research site? These strengths should be clearly articulated in “facilities,” which is an additional administrative section of the grant not covered here.
6.2 Overall Impact/Priority Score
For each of the five core review criteria, reviewers evaluate your application and provide a score from 1 (exceptional) to 9 (poor). Each reviewer then also provides an overall score, also from 1 to 9. There are no clear guidelines to reviewers in how to develop the overall score from the scores for the core areas, and it is not meant to be an average or median score. Moreover, your score can be influenced by several other additional criteria including human (or animal) subject issues. Reviewers can make recommendations about your budget, but these recommendations should not affect your score.
From the overall scores of each reviewer as well as the other committee members, a normalized average is calculated and multiplied by 10 to provide a final priority score from 10 to 90, where 10 is the best score possible. As much as we’d like to indicate a range of likely fundable scores, there just simply aren’t hard rules that apply in all cases across all institutes (but for some guidance see: www.nlm.nih.gov/ep/FAQScores.html). With that said, many PIs would be quite pleased with a score under 30.
7. Post-Review Strategies
Often within a week of the review meeting, you will be informed via eRA Commons about whether your application was scored, and if so, the priority score. The written critiques are organized into “summary statements” (still called “pink sheets” by some older investigators because of the color of the paper originally used). Approximately 4–8 weeks later, you will receive this summary statement, which includes a brief account of the committee discussion as well as the written comments provided by separate reviewers. A new feature is that reviewers can now make additional comments that will be made available to the PI. Sometimes it is difficult to read between the lines of reviews and these comments are an opportunity to provide direct recommendations about the overall viability of the project and particular methodological issues.
At this point several things can happen. If your application was scored it will go to a “Council” meeting (the second level of review) where the quality of the SRG review is assessed, recommendations to Institute staff on funding are made, and the program priorities and relevance of the applications are evaluated and considered. If your application was unscored or it went to Council, but was not recommended for funding (or it was recommended, but for one reason or another such as budget issues ultimately wasn’t funded), then you can consider resubmitting. Of note, before the Council meeting you will receive a request from a member of Grants Management staff for the following additional documentation, referred to as “Just-in-time information” (JIT): updated “other support” for key participants; the status of IRB action on your proposal; certification that key personnel have received training in the protection of human subjects. This request for additional information is not an award notice, although it is encouraging because it represents a critical step prior to the notice of grant award (NGA).
It can be difficult to decide on what to do next if the original submission is unscored. The first thing to do is to avoid the very real feeling that you and your grant have been rejected. Without question your grant being scored is better than it being unscored and in some case reviews will indicate serious problems that might not be addressable without considerable reformulation or even at all, but in most cases the eventual fate of this research project is in no way doomed by an initial submission being unscored.
An unscored grant may be revised, resubmitted, and eventually funded, but you should read the reviews carefully and with an open mind to help your decision. There is no simple formula to determine whether you should resubmit. Ask yourself several questions: Do the reviewers acknowledge the importance and innovation of the proposed research? Do they credit you, the PI, with having the appropriate background and abilities to accomplish the work in the area? Are their scientific concerns ones that you can effectively address? If the answer to each of these questions is “yes,” then you should strongly consider resubmission of a revised application. Many of us have had the experience of going from an unscored application to a funded grant award upon resubmission. However, it’s important to be honest with yourself about what is realistic. Talking with a relevant program officer also may be helpful to discuss next steps, especially if they were in attendance at the review committee when your application was discussed and can offer insights from the discussion.
We don’t mean to suggest that it is easy to objectively consider reviews of your grant. Particularly on a first read it is easy to jump to assume a reviewer (or all of the reviewers) clearly didn’t understand the grant and that their points are all wrong. This is a natural reaction and probably is rooted in important self-preservation in other important ways. However, an honest assessment requires stepping away from the grant and the review for a few days, considering that the reviews probably have a lot to offer. Moreover, the first set of reviews will always have an impact on the review of your resubmitted grant, and resubmitted grants that do not heed critical feedback almost never succeed. It is also important to read between the lines. In most cases, a poor score with few accompanying comments that aren’t really addressable is worse than a poor score with many comments that are addressable. Unless you are sure you understand why the grant was unscored, and what you can do to meaningfully improve it, you may want to consider that it may be hard to change reviewers’ minds and that it may not be best to resubmit.
If you do decide to resubmit, possibly the most important part of your revised application is the single page you are given to address all reviewer comments, called the Introduction to the Revised Application. The success of your application will be greatly influenced by the thoughtfulness of your response to the reviewers outlined in this page. Although your revisions will be reflected in the application (we recommend doing so with underlining as opposed to bolding to save space if needed), it is crucial to show that you understand and have addressed the reviewer points. And in rare cases where you disagree with the reviewer point, it is crucial here to address the spirit of the point, and make a clear theoretical, empirical, or practical argument to defend your choice. Although mindlessly agreeing with reviewers or other empty attempts at pandering will certainly not help your case, declining reviewer suggestions should not be undertaken lightly. Also be sensitive to the “tone” of your response, because the reviewers most certainly will be!
Finally, if your application was unscored (or in some unusual cases scored) and you are not optimistic about your likelihood of significantly improving your chances for funding in a resubmission, then you can consider going back to the drawing board and developing a new application. By new it can be entirely different with a new focus and aims, but it also can be similar in rationale and goals but also meaningfully distinct from the original application with these differences possibly manifesting in the focus on the question, the methodology used, or the specific way the larger question is addressed. Although there is no official connection between these applications, the good news is that the new application often benefits from your experiences in preparation and review of the original application.
8. Tips
8.1 Respect Deadlines
For many individuals deadlines are crucial to setting goals, staying on task, and not losing motivation. Be aware of the deadlines and what goes into getting things done in a timely manner. Be more conservative with things that rely on others, such as letters of support or analytic sections prepared by a statistician. With that said, deadlines can have their drawbacks, because they can lead to procrastination and a burst of work near the deadline, without ample time to run ideas past others and have a sufficient pre-review of the application from collaborators and potentially helpful colleagues. For this reason it can be useful to utilize a timeline for each step along the way to submitting your application. As can be seen in Table 15.3 illustrating a mock timeline, it can take nearly two years from the start of idea development for a grant to actual funding should resubmission be needed (as it most often is). This timeline illustrates the previously stated notion that grant funding is more of a marathon than a sprint.
8.2 Ensure Feasibility
While you want your application to be methodologically rigorous and have high impact on your field, you cannot lose sight of feasibility. The reviewer code word used when there are doubts about feasibility is “over-ambitious,” and it is a clear kiss of death when this term is used to describe an application. Therefore, keep your specific aims focused, and make hypotheses that you can clearly tie back to theory and/or pre-existing data. Consider the necessity of multiple studies within a single grant. Although these can be quite elegant, the connection between studies can provide many pitfalls, especially if subsequent studies rely on particular results from initial studies. Remember, although your passion for your research area may be strong and your intellectual curiosity high, each grant application represents only one small step in a research career that may last for several decades. Try not to be ruled by emotions (especially when receiving and responding to critical feedback) and keep a clear eye on your long-term goals. Persistence, patience, and creative problem solving are usually critical ingredients in the career of a successful independently funded investigator.
8.3 Be Clear
NIH clearly states that you cannot have any contact with reviewers before, during, or after your review. Therefore, the only way you can get your point across is the extent to which you communicate with them in the application. Within the section on Approach, the subsections on Preliminary Studies as well as Potential Problems, Alternative Strategies, and Benchmarks for Success provide a great opportunity for this. For the subsection on Preliminary Studies, you can make your case that you have sufficient background (and pilot data especially for an R01) to conduct this work and that it marks a logical next step in this line of research, both for you and for the field in general. For the subsections on Potential Problems and Alternative Strategies (previously referred to as design considerations), this is your chance to walk reviewers through the highly complex discussions you and your collaborators had when you determined the best decisions for the application. This is an interesting section and presents a real opportunity because some applicants largely ignore it and at best tell the reviewers essentially “don’t worry we know what we are doing” or “we’ve got it covered.” As a new investigator, it is up to you to ensure that the reviewers understand the decisions you made. This section also increases the odds that the primary reviewer can best present your application and that others reading can quickly understand some of the key features of your application. Think of it as giving reviewers access to all the critical thought that went into the strategies you ultimately chose (as well as those you didn’t choose). Finally, your Benchmarks for Success show a level of sophistication and often can help ameliorate any fears about feasibility. This section would benefit greatly from a table that outlines the planned activities of the grant and the deliverables at each time point.
8.4 Show You Know the Literature and Your Work is Adding to it
Especially as a young investigator, your research team is crucial and it is important for you to clearly highlight their role in your application. For K awards, mentors are especially key elements of the successful application. It is critical to tell a clear story of each person’s role in your training, with as much detail as possible. Explicitly, it is not enough to simply list the “right” people. It is necessary to explain who they are and why they were chosen, show that you will have the right training experience with them, and describe how each mentor will contribute to your career development.
For R grants and the young investigator, the role of collaborators can be a bit more ambiguous. In some academic settings, you may experience a tension between the traditional value placed on independence and the emerging growth of team-based or multidisciplinary science where it’s no longer expected (or even possible) for one individual to master all elements of a complex research project. In fact, at NIH it is usually expected that applications will include a team of experts representing different domains. For example, in applications related to mental health and addictions it is common to see psychologists, psychiatrists, statisticians, anthropologists, epidemiologists, neuroscientists, economists, etc. collaborating together. A true research team will involve well-selected experts that can work well together, each contributing unique and relevant expertise to the proposed project. It is crucial to clearly articulate the key parts of the application and the role that each collaborator plays in those parts.
8.5 Trust the System and Put Your Best Work Forward
As mentioned above, there are strategies to increase the odds of funding such as trying to steer your application to the most appropriate committee, “guessing” what likely reviewers might want, and talking to program staff to avoid making mistakes or proceeding in a negative direction. However, you should be careful about these efforts becoming more about gaming the system than developing the best application for you. It is important to note that for every great game player, there is a straight-shooting scientist who has a strong sense of their interests, is willing to find a mechanism in NIH that accommodates that interest, makes efforts to align their interests with that of NIH including RFAs and PAs but does not let this betray their own actual interests, and simply allows the process to play out. This is not to say that some strategizing is not warranted, but when the strategies approach more of a game-like level, they hold as much likelihood of backfiring (or simply being irrelevant) than actually helping.
9. Final Words
In conclusion, the NIH grants process can be frightening and exhausting, and sometimes the secrets to securing them can feel quite elusive. However, your biggest weapon in this battle is knowledge to give you both the direction you need to be most effective in developing your application as well as the confidence to endure the ups and downs of the process. This is simply one of many available resources and we encourage you to utilize as many as possible as you begin to develop your own style and secrets to your success!