Introduction
Solid acid catalysts have been applied to acid-catalyzed reactions as a replacement for liquid acid catalysts due to their easy recyclability, low corrosivity, and small degree of environmental pollution (Palani & Pandurangan, Reference Palani and Pandurangan2005; Sejidov et al., Reference Sejidov, Mansoori and Goodarzi2005; Testa et al., Reference Testa, Parola and Venezia2010; Pileidis et al., Reference Pileidis, Tabassum, Coutts and Ttitirici2014). Sulfonated carbon catalyst is a novel solid acid catalyst derived from biomaterials and their products (Ngaosuwan et al., Reference Ngaosuwan, Goodwin and Prasertdham2016; Toda et al., Reference Toda, Takagaki, Okamura, Kondo, Hayashi, Domen and Hara2005). Sulfonated carbon catalysts exhibit high catalytic performance owing to their high acid density and good lipophilicity (Ballotin et al., Reference Ballotin, da Silva, Lago and de Carvalho Teixeir2020; Shu et al., Reference Shu, Gao, Nawaz, Liao, Wang and Wang2010). However, their catalytic activities are often limited due to small surface area (Dehkhoda et al., Reference Dehkhoda, West and Ellis2010; Rao et al., Reference Rao, Chandra Mouli, Rambabu, Dalai and Prasad2011). Hence, the introduction of a porous structure to the interiors of sulfonated carbon materials (Konwar et al., Reference Konwar, Das, Thakur, Salminen, Mäki-Arvela, Kumar, Mikkola and Deka2014) or the loading of sulfonated carbon materials on the surface of porous materials (Van de Vyver et al., Reference Van de Vyver, Peng, Geboers, Schepers, de Clippel, Gommes, Goderis, Jacobs and Sels2010) can increase the catalyst’s surface area, resulting in better catalytic performance. The preparation of sulfonated carbon catalysts often requires concentrated sulfuric acid as the sulfonation agent, which causes larger quantities of wastewater and consequently environmental pollution (Konwar et al., Reference Konwar, Das, Thakur, Salminen, Mäki-Arvela, Kumar, Mikkola and Deka2014). Sulfonated carbon catalysts have also been prepared by simultaneous carbonization-sulfonation of glucose and p-toluenesulfonic acid (TsOH) (Wang et al., Reference Wang, Xu, Ren, Liu, Lu and Wang2011). Reports described this approach as simple and eco-friendly, where TsOH replaces concentrated sulfuric acid. The sulfonated carbon catalyst exhibits excellent catalytic performance in the dehydration of fructose into 5-hydroxymethylfurfural for high acid density.
In addition, sulfonated carbon catalysts with a small particle size were used to enhance the catalytic performance of a liquid-phase reaction system (Ngaosuwan et al., Reference Ngaosuwan, Goodwin and Prasertdham2016; Shu et al., Reference Shu, Gao, Nawaz, Liao, Wang and Wang2010). The separation and recovery of the catalysts from the liquid phase remain a challenge, therefore. The magnetic separation technique has been utilized to separate nanocatalysts effectively from the liquid phase (Mansoori & Mansoori, Reference Mansoori and Mansoori2018; Polshettiwar et al., Reference Polshettiwar, Luque, Fihri, Zhu, Bouhrara and Basset2011). Magnetic heterogeneous catalysts also exhibit high catalytic activity and stability for the esterification reaction (Xie et al., Reference Xie, Han and Wang2018; Xie & Wang, Reference Xie and Wang2021). Magnetic materials, such as Fe3O4 and Fe nanoparticles, were introduced into sulfonated carbon catalysts by loading and encapsulation (Kasprzak et al., Reference Kasprzak, Bystrzejewski and Poplawska2018; Wang et al., Reference Wang, Yang, Xu, Wang, Wang, Zhang, Wang and Liang2019; Yang et al., Reference Yang, Ao, Wu, Zhang, Chi, Hou and Qian2020). The resulting sulfonated carbon catalysts inherited good magnetic properties from magnetic materials and also retained good catalytic performance.
Palygorskite (Plg) is a natural clay mineral that has been used as a functional carrier, such as for magnetic materials and carbon, due to its porous structure and nanofibrous morphology (Middea et al., Reference Middea, Spinelli, Souza Junior, Neumann, da FM Gomes, Fernandes, de Lima, Barthem and de Carvalho2015; Murray, Reference Murray2000; Sarkar et al., Reference Sarkar, Liu, McClure, Sundaramurthy, Srinivasan and Naidu2015). Sulfonated carbon was loaded onto the surface of Plg nanorods via carbonization of sucrose, followed by sulfonation using concentrated sulfuric acid as the sulfonating agent. The resulting composite exhibited high catalytic performance for the synthesis of n-butyl acetate (Jiang, Xu, et al., Reference Jiang, Xu, Duanmu, Gu and Chen2012b). Recently, sulfonated carbon/Plg has been synthesized (via one-step carbonization-sulfonation using Plg as the support, sucrose as the carbon source, and TsOH as the sulfonating agent) and exhibited high catalytic performance for the esterification of oleic acid with methanol (Zhang et al., Reference Zhang, Jiang, Jiang, Wu, Shi, Mao and Deng2021). Furthermore, magnetic carbon/Plg composites have been prepared via hydrothermal treatment or impregnation-reduction (Tang et al., Reference Tang, Mu, Zong, Zheng and Wang2017; Zhang et al., Reference Zhang, Zhong, Yang, Tang and Zuo2020). The composite magnetic nanoparticles were well dispersed on the surface of rod-shaped Plg carriers. In addition, the magnetic carbon/Plg composite not only had good magnetic properties but also had significant adsorption capacities for pollutants.
The current study aimed to develop an environmentally friendly and economical preparation method of magnetic sulfonated carbon/Fe3O4/Plg composite catalyst, while obtaining the catalyst by the simultaneous calcination and sulfonation of the mixture of Fe3O4/Plg, TsOH, and sucrose. A further objective was to investigate the catalytic performance, magnetic property, and recyclability of the catalyst.
Materials and Methods
Materials
Plg from Gaojiawa (Xuyi, Jiangsu Province, China) was used. The sucrose, FeSO4·7H2O, and NaCl were supplied by Tianjin Kemiou Chemical Reagent Co., Ltd., (Tianjin, China). TsOH and NaOH were purchased from Nanjing Chemical Reagent Co., Ltd., (Nanjing, China). The methanol, oleic acid, FeCl3·6H2O, and HNO3 were provided by Sinopharm Chemical Reagent Co., Ltd., (Shanghai, China). All reagents were of analytical grade and used directly without further purification.
Materials Preparation
Plg was milled using a ball grinder and then treated with HNO3 solution (5 wt.%) for 24 h under magnetic stirring. The Plg obtained was centrifuged, washed with distilled water, and then dried at 80°C in an oven. Subsequently, pretreated Plg (5 g) was added to 100 mL of H2O and ultrasonicated for 20 min. The mixture was added to 4.56 g of FeSO4·7H2O and 0.8 g of FeCl3·6H2O and stirred at 75°C for 10 min. The pH value of the mixture was adjusted to 12 using 1 mol/L NaOH solution. The magnetic Fe3O4/Plg composite was obtained after filtering, rinsing, and drying the solid precipitate.
The Fe3O4/Plg composite (5 g) was added to a solution composed of 15 mL of H2O, 5 g of sucrose, and 1 g of TsOH. The mixture was stirred at room temperature for 1 h and dried under vacuum at 40°C. The dried solid was milled in a ceramic mortar and then transferred to alumina combustion, which was placed in a horizontal tube furnace and heated at 200°C for 3 h under an N2 atmosphere. The product was labeled SC-F-Plg-n, where n denotes the mass of the added sucrose, such as SC-F-Plg-1, SC-F-Plg-3, SC-F-Plg-5, and SC-F-Plg-7 at sucrose dosages of 1, 3, 5, and 7 g, respectively. As contrasting samples, sulfonated carbon/Plg (SC-Plg) and sulfonated carbon (SC) were prepared from a mixture of sucrose and TsOH under the same conditions with/without Plg, respectively.
Characterization
The sample morphologies were obtained using an S-3000N scanning electron microscope (Hitachi Corporation, Tokyo, Japan). The X-ray diffraction (XRD) patterns of the samples were recorded using a D8 Advance X-ray powder diffractometer (Bruker AXS, Karlsruhe, Germany) with CuKα radiation from 5 to 80°2θ at a scanning rate of 2°2θ/min. Transmission electron microscope (TEM) images and the corresponding TEM-EDS mapping were performed using a JEM 2100F transmission electronic microscope (JEOL Corporation, Tokyo, Japan) operating at 200 kV. Fourier-transform infrared (FTIR) spectra were recorded using a ThermoFisher Nicolet 5700 spectrometer (ThermoFisher Nicolet Corporation, Waltham, Massachusetts, USA). Elemental analysis of the catalysts was performed using an energy-dispersive X-ray spectrometer (EDX, EX-250, Horiba Corporation, Kyoto, Japan) and Elementar Vario EL III element analyzer (Elementar Corporation, Hannah, Germany), respectively. N2 adsorption isotherms of the catalysts were obtained using a Micromeritics TriStar II 3020 apparatus (Micromeritics Instruments Corporation, Norcross, Georgia, USA) at –196°C (77 K). Before each measurement, the samples were degassed for 12 h at 150°C under N2 atmosphere. The magnetic properties of the magnetic samples were determined using a superconducting quantum interference device (SQUID, Quantum Design, San Diego, California, USA). Chemical analyses of samples were determined using an X-ray fluorescence (XRF) spectrometer (S4 PIONEER, Bruker AXS, Karlsruhe, Germany).
The amount of –SO3 groups on the surfaces of the prepared catalysts was determined by titration (Jiang, Xu, et al., Reference Jiang, Xu, Duanmu, Gu and Chen2012b). The catalyst (0.1 g) was dispersed in 20 mL of NaCl solution (2 mol/L) and then stirred mechanically for 24 h. The filtrate was titrated with 6 mmol/L of NaOH solution and phenolphthalein was used as an indicator.
Catalytic Performance
The esterification of oleic acid with methanol was used to investigate the catalytic properties of the prepared catalysts. In a typical experiment, 1 g of oleic acid, 8 mL of methanol, and 0.05 g of catalyst were added to a 25 mL three-neck round-bottomed flask equipped with a reflux condenser and a mechanical agitator. The mixture was stirred at 65°C for 4 h. The reaction solution was separated from the mixture using a magnet. The catalyst was recycled by washing with ethanol. The product (0.5 mL) was diluted with 10 mL of n-hexane and determined by the GC method (Agilent 6820 GC with a DB-1 capillary column, Agilent Technologies, Santa Clara, California, USA). Heptadecane was used as the internal standard. The catalytic experiment was repeated five times.
Recycling experiments were performed to evaluate the catalyst’s stability. At the end of each reaction cycle, the catalyst was recycled using a magnet, washed with ethanol, and dried at 80°C.
Results and Discussion
Preparation and Characterization of Catalyst
XRF analysis of Plg and acid-treated Plg (Table 1) revealed that the component contents of Plg all increased except for CaO, MgO, and MnO after acid treatment. The proportion of CaO (wt.%) decreased visibly from 8.12 to 2.26 wt.%, which suggested that the carbonate minerals existing in Plg were dissolved in the HNO3 solution. The carbonate mineral in Plg was often dolomite, CaMg(CO3)2, and so the CaO and MgO contents decreased after the HNO3 treatment. The loss of MgO was much less than that of CaO, however, because Mg also existed in Plg crystals. The Fe2O3 content in Fe3O4/Plg increased significantly from 11.33 to 40.01 wt.% after loading of Fe3O4 (Table 1) while the amounts of other components decreased. The results indicated that Fe3O4 was loaded successfully onto the surface of Plg nanorods, which probably provides the magnetism of the SC-F-Plg catalyst. SO3 was observed in the acid-treated Plg and Fe3O4/Plg, which was derived from the reagents used in the preparation process of acid-treated Plg and Fe3O4. A new component of Na2O appeared in the Fe3O4/Plg, which was introduced during the process of forming Fe3O4. The amounts of other components were reduced except for SO3 after the sulfonation and carbonation of Fe3O4/Plg, and the amount of SO3 increased from 4.48 to 16.95%, which suggested that SC existed in the SC-F-Plg catalyst.
Table 1 Chemical compositions (wt.%) of Plg, acid-treated Plg, and Fe3O4/Plg

The XRD pattern of Plg (Fig. 1a) showed two diffraction peaks at 8.3 and 19.9°2θ, which were ascribed to the characteristic diffraction peaks of Plg (Suárez Barrios et al., Reference Suárez Barrios, Flores González, Vicente Rodríguez and Martín Pozas1995). In addition, characteristic diffraction peaks of the quartz impurity (21.2 and 26.5°2θ) also existed in the XRD pattern of Plg after removal of the carbonates using HNO3 solution (Jiang, Feng, et al., Reference Jiang, Feng, Gu, Qian, Gu and Duanmu2012a; Suárez Barrios et al., Reference Suárez Barrios, Flores González, Vicente Rodríguez and Martín Pozas1995). No other type of mineral except for quartz was found in the XRD pattern of Plg. A weak and broad diffraction peak from 15 to 30°2θ was observed in the SC patterns (Jiang, Xu, et al., Reference Jiang, Xu, Duanmu, Gu and Chen2012b), suggesting the presence of an amorphous carbon material (Fig. 1b). The intensities of the diffraction peaks of Plg and quartz were significantly reduced after sucrose carbonization, which may be due to the loading of amorphous carbon materials (Fig. 1c). Their intensities were further reduced after loading of Fe3O4 and subsequent carbonization of sucrose (Fig. 1d). The characteristic diffraction peaks located at 30.2 and 35.5°2θ were observed in the XRD pattern of SC-F-Plg, indicating the presence of Fe3O4 in the catalyst (Bae et al., Reference Bae, Han, Cho and Choi1998). In addition, two weak diffraction peaks at 6.9 and 18.9°2θ, ascribed to smectite (Golden & Dixon, Reference Golden and Dixon1990; Whitney, Reference Whitney1990), could be observed in the XRD pattern of SC-Plg-3. Two diffraction peaks of smectite at 6.9 and 18.9°2θ were also present in the XRD pattern of SC-F-Plg-3 and their peak intensities were greatly increased after the introduction of Fe3O4. The intensity of the diffraction peak of smectite at 6.9°2θ varied significantly with acidity (Cui et al., Reference Cui, Zhang and Han2020). The diffraction peak of smectite broadened gradually with increase in the pH and its intensity was greatest at pH = 1 because smectite was gradually disordered with increasing pH value (Cui et al., Reference Cui, Zhang and Han2020). In addtion, due to the introduction of Fe3O4 nanoparticles into the interlamellar spaces of smectite, the disordered-layers structure of smectite was reorganized to the ordered-layers, resulting in the appearance of a (001) diffraction peak (Zeynizadeh et al., Reference Zeynizadeh, Rahmani and Tizhoush2020). Therefore, smectite may have existed in the original Plg in this study, and then the iron species and high acidity collectively promoted the disordered-layers structure of smectite to the ordered-layers, which caused the appearance of the diffraction peaks of smectite. The results also illustrated that sucrose carbonization at 200°C did not change the Fe3O4 phase, which determined the magnetic property of the catalyst.

Fig. 1 XRD patterns of a Plg, b SC, c SC-Plg-3, and d SC-F-Plg-3
The SEM image of Plg showed that Plg possessed typical rod-like morphology (Fig. 2a). Sulfonated carbon comprised solid particles with a smoother surface (Fig. 2b) (Jiang, Xu, et al., Reference Jiang, Xu, Duanmu, Gu and Chen2012b). The spaces between Plg nanorods were filled by carbon material and the surface of the SC-Plg-3 was smoother than that of Plg though dispersed Plg nanorods were clearly observed in SC-Plg-3 (Fig. 2c), indicating that carbon material was formed on the surface of Plg nanorods after sucrose carbonization. The morphology of SC-F-Plg-3 was similar to that of the SC-Plg-3, though some solid particles appeared in the SC-F-Plg-3 (Fig. 2d), which correspond to Fe3O4 nanoparticles according to XRD analysis.

Fig. 2 SEM images of a Plg, b SC, c SC-Plg-3, and d SC-F-Plg-3
The TEM image of Plg crystals displayed a rod-shaped morphology with lengths of 10–30 nm (Fig. 3a). Some nanosized spherical particles, ascribed to quartz, were attached to these Plg nanorods (Jiang et al., Reference Jiang, Chen, Duanmu, Gu, Chen and Ni2014). The Plg nanorods and spherical nanoparticles were wrapped in carbon materials after sucrose carbonization (Fig. 3b). The TEM images of SC-F-Plg-3 (Fig. 3c) revealed the appearance of numerous nanoparticles on the Plg surfaces, which was a mixture of SC and Plg nanorods. The magnified TEM images of SC-F-Plg-3 (Fig. 3d) further indicated that these nanoparticles were loaded on rod-shaped Plg crystals and then were coated with a carbon nanolayer. The black nanoparticles were identified as Fe3O4 according to the XRD analysis results of SC-F-Plg-3.
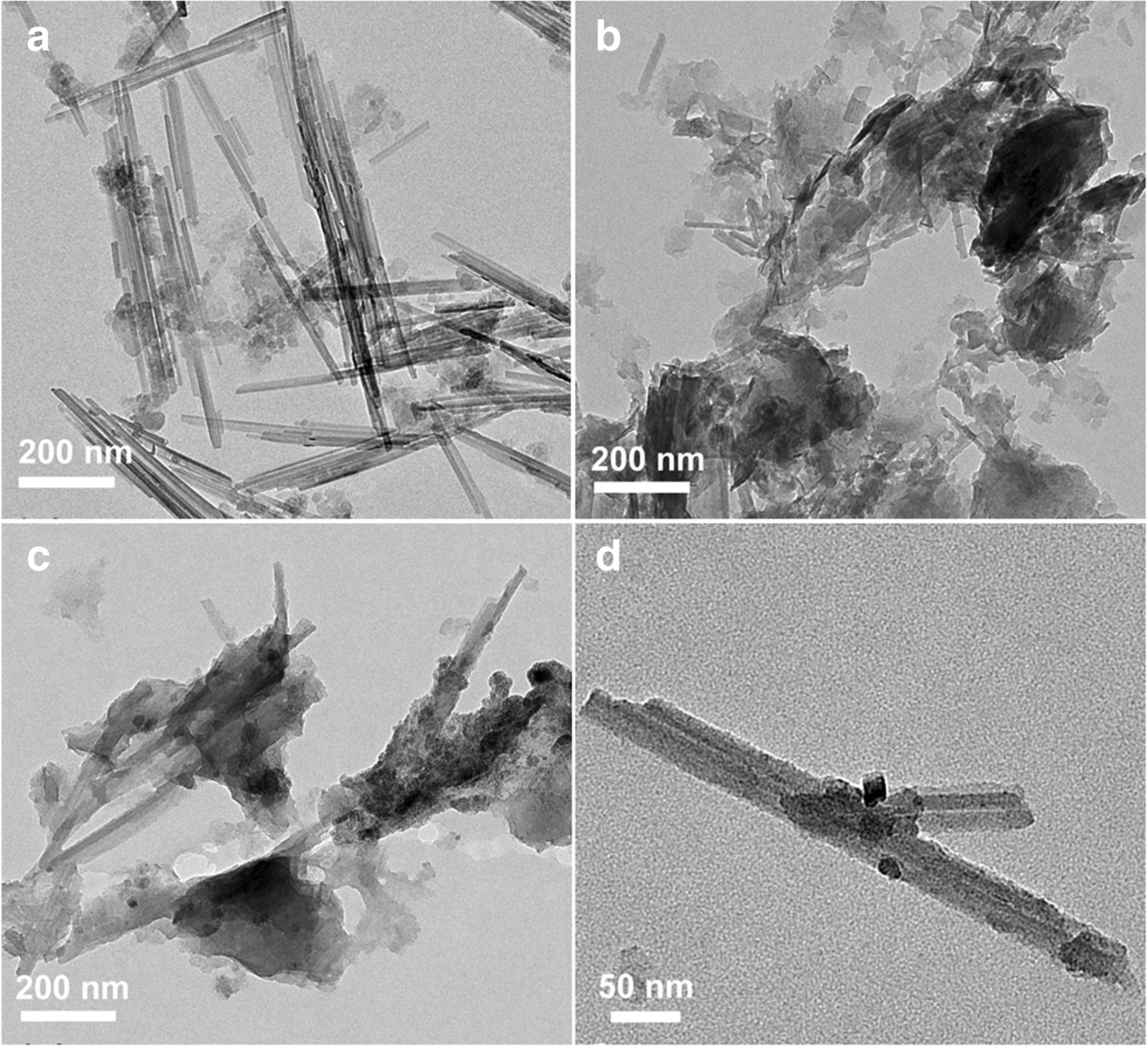
Fig. 3 TEM images of a Plg, b SC, c SC-Plg-3, and d SC-F-Plg-3
The EDX spectra of Plg revealed that silicon (Si), magnesium (Mg), aluminum (Al), iron (Fe), and oxygen (O) were the major elements of Plg (Fig. 4), while trace carbon was also present (Zhang et al., Reference Zhang, Zhong, Yang, Tang and Zuo2020). As shown by the EDX spectra of Plg and SC-F-Plg-3, the peak intensities of Si, Mg, Al, and O decreased after loading of Fe3O4 and sucrose carbonization. Furthermore, the peak intensities of Fe and carbon increased significantly. Sulfur derived from TsOH appeared in the EDX spectrum of SC-F-Plg-3. The TEM-EDX images of SC-F-Plg-3 indicated that O, Si, and Fe were distributed uniformly in the same area of SC-F-Plg-3 (Fig. 4). The distribution densities of O and Si were much greater than that of Fe because O and Si were the main elements in Plg. However, the distribution of S in SC-F-Plg-3 was relatively sparse compared to these elements derived from Plg. Note that the distribution densities of Fe and S were close to that of Si in the left section of the SC-F-Plg-3 nanorod, which illustrated the presence of Fe3O4 and sulfonated carbon. The results further indicated that Fe3O4 and sulfur-containing carbon material were loaded onto the surface of the Plg nanorods.

Fig. 4 EDX spectra of Plg and SC-F-Plg-3 and TEM-EDX element mapping images of SC-F-Plg-3
The FTIR spectra of Plg and SC-F-Plg-3 were used to identify the presence of –SO3H groups (Fig. 5). Compared to Plg, five new bands at 1008, 1040, 1125, 1380, and 1620 cm–1 were observed in the FTIR spectrum of SC-F-Plg-3 (Fig. 5). The vibrational bands at 1008 and 1040 cm–1 corresponded to S=O asymmetric stretching of –SO3H groups (Geng et al., Reference Geng, Wang, Yu and Zhu2011; Iroh & Williams, Reference Iroh and Williams1999), while the bands at 1125 and 1380 cm–1 corresponded to the asymmetric SO2 stretching of –SO3H groups (Geng et al., Reference Geng, Wang, Yu and Zhu2011; Peng et al., Reference Peng, Philippaerts, Ke, Noyen, Clippel, Tendeloo, Jacobs and Sels2010). The band at 1620 cm–1 is related to C=C skeletal vibrations (Laohapornchaiphan et al., Reference Laohapornchaiphan, Smith and Smith2017; Xiao et al., Reference Xiao, Guo and Liang2010). These results showed that –SO3H groups were introduced into the carbonaceous material via the addition of TsOH.

Fig. 5 FTIR spectra of Plg and SC-F-Plg-3
The S 2p XPS spectrum of SC-F-Plg-3 (Fig. 6a) revealed a single S 2p peak at 168 eV, which further indicated that all S atoms of SC-F-Plg-3 were in the form of the –SO3H groups (Suganuma et al., Reference Suganuma, Nakajima, Kitano, Yamaguchi, Kato, Hayashi and Hara2010; Zhan et al., Reference Zhan, Tao, Cai, Liu and Liu2014). As shown in the Fe 2p XPS spectrum of SC-F-Plg-3 (Fig. 6b), the two peaks observed at 710 and 724 eV corresponded to Fe 2p3/2 and Fe 2p1/2, which confirmed the presence of Fe3O4 (Yamashita & Hayes, Reference Yamashita and Hayes2008). The results further illustrated that the primary acid groups and magnetic material of SC-F-Plg-3 were –SO3H group and Fe3O4, respectively.

Fig. 6 a S 2p and b Fe 2p XPS spectra of SC-F-Plg-3
The SEM images of SC-F-Plg prepared using various sucrose dosages revealed that the space between Fe3O4 nanoparticles and Plg nanorods filled gradually with increasing amounts of sucrose (Fig. 7).The rod-shaped morphology of Plg crystals was still observed in SC-F-Plg catalysts, however. The corresponding XRD patterns all revealed the presence of smectite, an Fe3O4 phase, and Plg crystals, while the intensity of these characteristic diffraction peaks was not reduced (Fig. 8), although the sucrose dosage increased. The results indicated that the carbon layer on the surface of Fe3O4 nanoparticles and Plg nanorods was very thin, in agreement with the TEM image of SC-F-Plg-3 (Fig. 3).
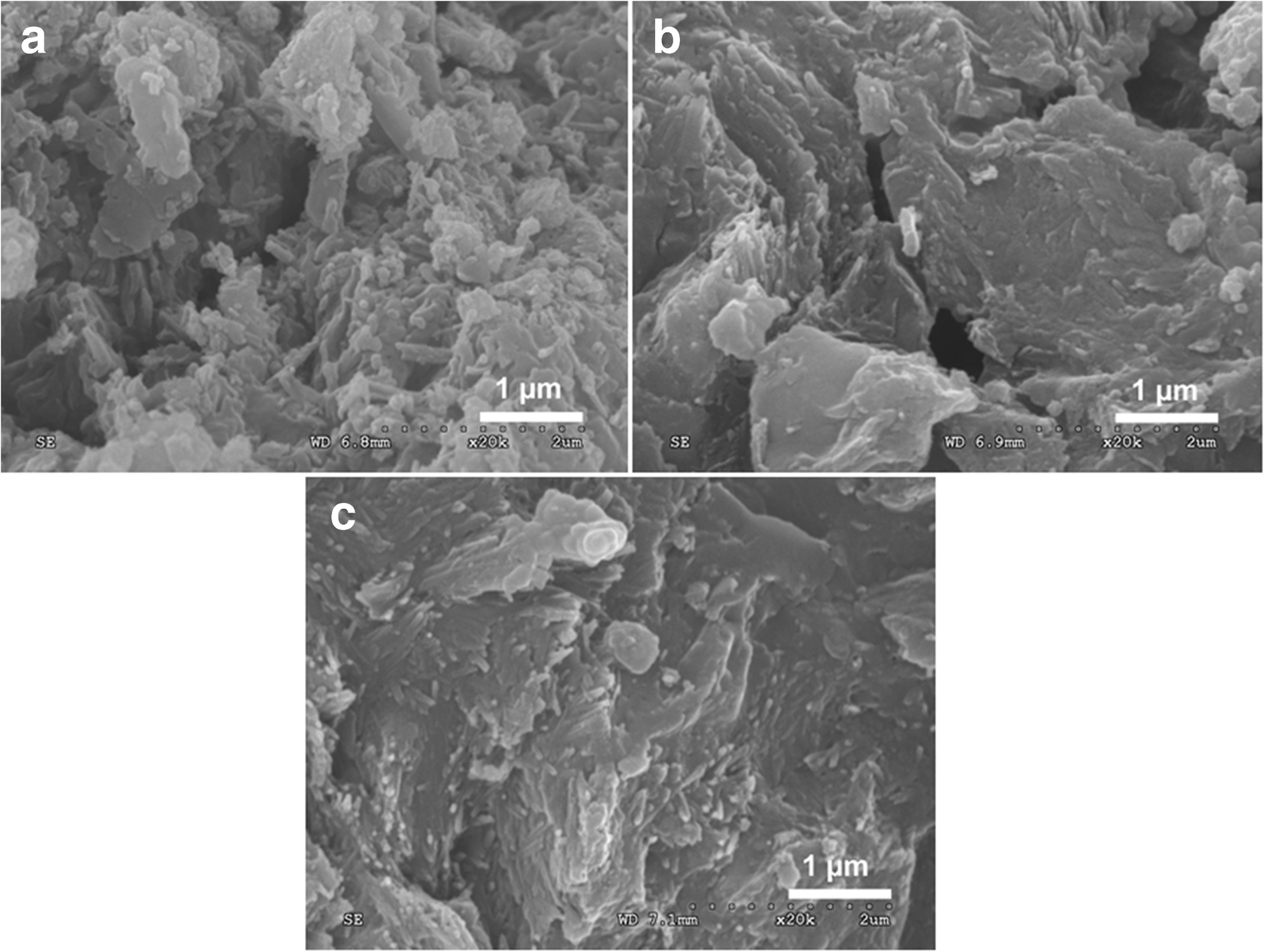
Fig. 7 SEM images of a SC-F-Plg-1, b SC-F-Plg-5, and c SC-F-Plg-7

Fig. 8 XRD patterns of a SC-F-Plg-1, b SC-F-Plg-3, c SC-F-Plg-5, and d SC-F-Plg-7
The magnetic performance of SC-F-Plg was derived from Fe3O4 nanoparticles. The XRF analysis results of Fe3O4/Plg (Table 1) revealed that the loading of Fe3O4 was ~27.7 wt.% while the amount of Plg was 72.3 wt.%. The saturation magnetization (M S ) value of Fe3O4/Plg was measured to be 20.82 emu/g. Hence, the M S value of Fe3O4 was calculated as 75.18 emu/g. The M S values of SC-F-Plg were 5.28, 5.21, 4.96, and 4.67 emu/g with increasing sucrose dosage (Table 2). The corresponding Fe3O4 amounts were 6.97, 6.93, 6.59, and 6.21 wt.%, respectively. The magnetic curve of SC-F-Plg-3 exhibited ferromagnetic behavior and the M S value was 5.21 emu/g (Fig. 9), indicating that SC-F-Plg-3 possessed an excellent magnetic property, which was greater than the saturation magnetization of ATP/C@NiFe-LDH (2.01 emu/g) (Tang et al., Reference Tang, Mu, Zong and Wang2018). Furthermore, SC-F-Plg-3 was removed easily from the liquid reaction system under an external magnetic field after the reaction. The saturation magnetization value of the recycled SC-F-Plg-3 was reduced slightly to 5.15 emg/g after five cycles, indicating that the magnetic property of SC-F-Plg-3 was very stable. The results further confirmed that Fe3O4 nanoparticles were wrapped in carbon material, which protected them from oxidation and fall-out from the catalyst.
Table 2 Elemental analysis of SC and SC-F-Plg (wt.%) and magnetic performance of SC-F-Plg
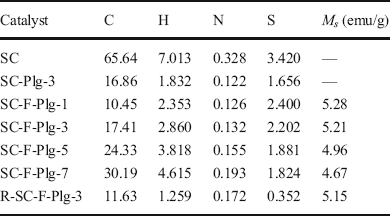
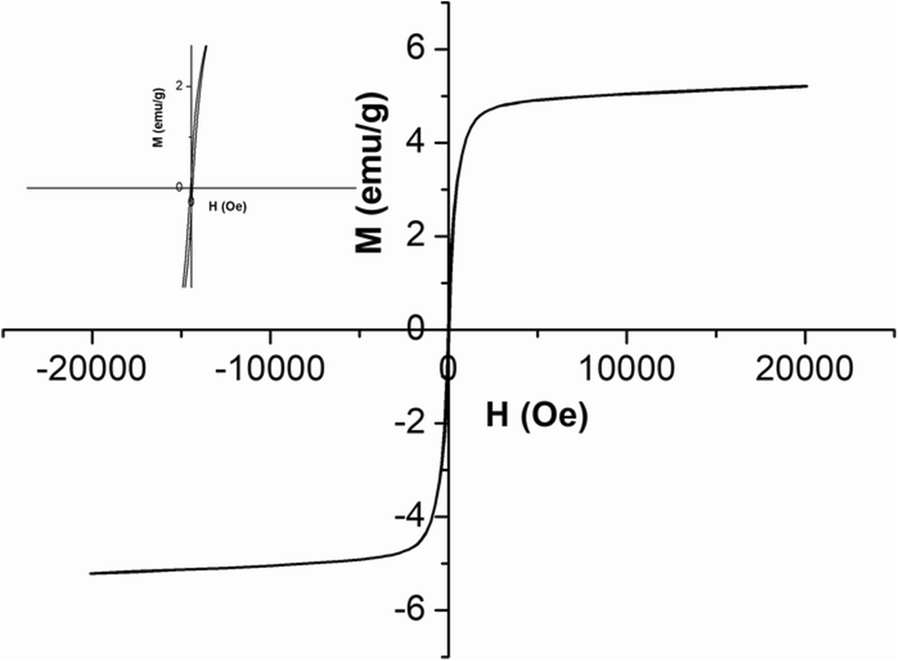
Fig. 9 Magnetic hysteresis loop of SC-F-Plg-3
The textural properties of Plg, SC, SC-Plg-3, and SC-F-Plg catalysts were compared (Table 3). The BET surface area and pore volume of Plg were 169 m2/g and 0.324 cm3/g, respectively. Owing to its non-porous structure, the BET surface area and pore volume of SC were not recorded. The BET surface area and pore volume of SC-Plg-3 increased to 62 m2/g and 0.162 cm3/g, respectively, due to the introduction of Plg nanorods with a porous structure and the dispersion of SC particles on the Plg surfaces. Compared to SC-Plg-3, the BET surface area and pore volume of SC-F-Plg decreased in the presence of Fe3O4 nanoparticles. The BET surface area and pore volume of SC-F-Plg further decreased with increasing amount of sucrose, which were approximately zero when the dosage of sucrose was 7 g. The results indicated that carbon was deposited successfully in the mesopores of Plg and on the external Plg surfaces and Fe3O4 nanoparticles, which decreased the BET surface area and pore volume of the catalysts.The BET surface area of SC increased, however, during the loading of Plg and Fe3O4 nanoparticles.
Table 3 Textural properties, composition analysis, acid density, and catalytic properties of catalysts
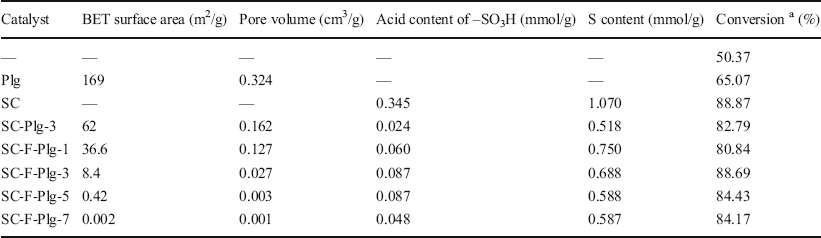
a conversion of oleic acid
Elemental analysis of SC and SC-F-Plg (Table 2) revealed the presence of sulfur, hydrogen, nitrogen, and carbon derived from sucrose and TsOH. The SC contained the most carbon and sulfur (3.420 wt.%). The sulfur and carbon contents of SC-Plg-3 decreased significantly after the introduction of Plg nanorods. The sulfur content of SC-F-Plg-1 (2.400 wt.%) was greater than that of SC-Plg-3 (1.656 wt.%) despite reducing carbon materials. SC-F-Plg-3 also contained more carbon and sulfur than SC-Plg-3. The sulfonation reaction appeared to occur at the interface between carbon materials and TsOH. This phenomenon may be caused by dispersed Fe3O4 nanoparticles which favored the deposition and dispersion of carbon material compared with Plg nanorods, increasing sulfur and carbon contents. The results indicated that the carbon layer on the surfaces of Fe3O4 nanoparticles and Plg may thicken with increasing sucrose dosage, which may hinder the sulfonation reaction. Thus, SC-F-Plg's carbon, hydrogen, and nitrogen contents increased with increasing sucrose dosage, whereas its sulfur content decreased gradually.
The catalytic properties of carbon-based solid acid catalysts are usually determined by their free acid groups (Ngaosuwan et al., Reference Ngaosuwan, Goodwin and Prasertdham2016). The numbers of acidic –SO3H groups on the surface of the catalysts were obtained by neutralization titration with NaOH (Table 3). By comparison, the units for sulfur content (wt.%) were converted to mmol/g and listed in Table 3. The acid content of SC was 0.345 mmol/g and this was due to greater sulfur content (1.070 mmol/g), indicating that SC possessed abundant surface –SO3H groups and some sulfur atoms were sealed within the carbon materials. Compared to SC, the Plg was weakly acidic (Araújo Melo et al., Reference Araújo Melo, Ruiz, Melo, Sobrinbo and Martinelli2002). The acid content in SC-Plg-3 decreased from 0.345 to 0.024 mmol/g after the introduction of Plg.
On the contrary, the acid content of SC-F-Plg-1 increased to 0.060 mmol/g after the addition of Fe3O4 nanoparticles, which further illustrated that Fe3O4 nanoparticles may benefit the dispersion of carbon material compared with Plg. These dispersed carbon materials contributed to the loading and release of –SO3H groups. The acid content of SC-F-Plg increased slowly with sucrose but decreased at a sucrose dosage of 7 g. The results indicated that the increase in layer thickness of the carbon on the surfaces of Fe3O4 nanoparticles and Plg may inhibit the release of –SO3H groups. Hence, the acid content of SC-F-Plg increased first and then decreased. Therefore, the dispersed and thin carbon layer on the surface of Fe3O4 nanoparticles benefited the free –SO3H group despite the relatively small sulfur content, which meant that the catalyst had greater catalytic properties.
Catalytic Performance of Catalyst
The esterification of oleic acid with methanol was used to evaluate the catalytic activity of SC-F-Plg (Table 3). The conversion of oleic acid was 50.37% in the absence of a catalyst but increased to 65.07% after the addition of the Plg catalyst. Because of a few acid sites in Plg, the improvement in conversion of oleic acid was only slight (Araújo Melo et al., Reference Araújo Melo, Ruiz, Melo, Sobrinbo and Martinelli2002). The –SO3H groups on the surface of SC promoted the esterification of oleic acid with methanol. The SC improved oleic acid conversion to 88.87% for a larger amount of acid, therefore. Compared to SC, the introduction of Plg maintained a conversion rate of 82.79% due to larger surface area of SC-Plg-3 despite a very small amount of acid, which increased the contact of reactants with catalytic sites and subsequently enhanced the catalytic efficiency. The conversion of oleic acid over SC-F-Plg-3 increased to 88.69% even though a significant loss of surface area was observed with the introduction of Fe3O4 nanoparticles to SC-Plg-3 catalyst. Although this catalyst had a larger surface area, oleic acid conversion over SC-F-Plg-1 showed a slight decline and was 80.84%, which stemmed from the reduced acid content when the amount of sucrose was decreased from 3 to 1 g. The loss of surface area or acid content promoted reduction of oleic acid conversion on SC-F-Plg with increasing sucrose dosage from 5 to 7 g. Hence, SC-F-Plg possessed excellent catalytic properties, owing to the surface area and acid content of the catalyst. The role of Plg nanorods was, thus, primarily as a porous carrier of SC and it increased the surface area of the catalyst despite fewer acid sites on Plg. On the other hand, Plg also contributed to the dispersion of Fe3O4 nanoparticles. The carbon layer was uniform and thin, which was well suited to the introduction of Fe3O4 nanoparticles and Plg nanorods, resulting in the increased acid content and catalytic properties of SC-F-Plg.
The catalytic activities of various sulfonated carbon catalysts were compared using the esterification of oleic acid with methanol (Table 4). The conversion of oleic acid over SC-F-Plg-3 was greater than that of mesoporous SO3H-carbon and SBA-15-C-SO3H prepared using sulfuric acid as the sulfonating agent despite the higher reaction temperature used for the latter catalysts (Peng et al., Reference Peng, Philippaerts, Ke, Noyen, Clippel, Tendeloo, Jacobs and Sels2010; Wang et al., Reference Wang, Wang, Tan, Jiang, Zheng, Tsubaki and Wu2015). Oleic acid conversion was slightly lower than sulfonated carbon (90%) (Flores et al., Reference Flores, Omega, Cabatingan, Go, Agapay and Ju2019). The results illustrated that natural clay Plg could replace expensive SBA-15 mesoporous molecular sieves as a catalyst carrier.
Table 4 Comparison of esterification of oleic acid with methanol over various catalysts

a Conversion of oleic acid
The catalyst stability and reusability were tested using the SC-F-Plg-3 sample. The SC-F-Plg-3 catalyst led to oleic-acid conversion of up to 70.31% after the fifth cycle (Fig. 10). This was greater than the recycled sugarcane bagasse-derived sulfonated carbon (66.6%) (Flores et al., Reference Flores, Omega, Cabatingan, Go, Agapay and Ju2019). The results indicated that SC-F-Plg-3 possessed good catalytic activity and stability. Its sulfur content decreased from 2.202 to 0.352 wt.% after the fifth cycle (Table 2). The carbon content of the recycled SC-F-Plg-3 (R-SC-F-Plg-3) also decreased to 11.48 wt.% (Table 2), indicating loss of carbon material from SC-F-Plg-3 catalyst during the reaction process. The decreased catalytic activity of SC-F-Plg-3 may be attributed primarily to the loss of –SO3H groups from the carbonaceous material (Jiang, Xu, et al., Reference Jiang, Xu, Duanmu, Gu and Chen2012b). On the other hand, a small loss of SC from the catalyst also led to diminished catalytic activity of the SC-F-Plg-3.

Fig. 10 Reusability of SC-F-Plg-3
Conclusions
The SC-F-Plg catalyst was prepared from a mixture of sucrose, TsOH, and Fe3O4/Plg in an environmentally friendly and low-cost approach. Plg nanorods were mainly used as the carriers to replace sucrose-derived carbon, to disperse Fe3O4 nanoparticles, and to increase the surface area of the catalyst. The introduction of Fe3O4 nanoparticles provided the catalyst with suitable magnetism, which helped with the formation of a uniform and thin carbon layer on the catalyst's surface, resulting in a larger acid content in the SC-F-Plg. The SC-F-Plg possessed good catalytic activity and stability. The magnetic property of SC-F-Plg was also stable for the protection of Fe3O4 nanoparticles in the carbon material. Hence, SC-F-Plg is a promising, cost-effective, and easily-recyclable, carbon-based solid acid composite catalyst.
Acknowledgments
This work was supported by the Six Talent Peaks Project in Jiangsu Province (No. 2018-JNHB-009), Natural Science Key Project of the Jiangsu Higher Education Institutions (19KJA430015), the Foundation of Key Laboratory for Palygorskite Science and Applied Technology of Jiangsu Province (HPZ202001), and the National & Local Joint Engineering Research Center for Mineral Salt Deep Utilization (SF201906, SF202103). The first author is grateful to Dr Nisar Ali for his help in completing the paper.
Code Availability
Not Applicable.
Authors' Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Mei Wu, Xingjun Yao, Jing Ouyang and Jinlong Jiang. All authors read and approved the final manuscript.
Funding
Funding sources are as stated in the Acknowledgements.
Data Availability
All data generated or analysed during this study are included in the manuscript.
Declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethics Approval
Not Applicable.
Consent to Participate
Not Applicable.
Consent for Publication
Not Applicable.
















