Introduction
Transport of solutes and water across the plasmalemma is vital for homoeostasis and growth of all organisms. For many of these solutes, and perhaps water in some cases, the movement of the ions and molecules is in the direction opposite to that dictated by the free energy difference across the plasmalemma for that ion or compound: this is the strict definition of active transport. For neutral solutes and water, the free energy difference is the difference in chemical activity (concentration times activity coefficient). For ions there is the additional component of electrical potential difference in the overall free energy difference. Active transport necessitates an input of free energy per molecule or ion transported in excess of the free energy difference per molecule or ion. We follow here the distinction made by Mitchell (Reference Mitchell1979) between primary and secondary active transport (see also Saier, Reference Saier2000; Saier et al., Reference Saier, Reddy, Tsu, Ahmed, Li and Moreno-Hagelsieb2015). In primary active transport the energy input to the transporter is an exergonic scalar chemical reaction (e.g. oxidation of NAD(P)H by O2; hydrolysis of ATP to produce ADP and Pi) or photons absorbed by a chromophore component of the transporter (e.g. the retinal component of ion-pumping rhodopsins). In secondary active transport the energy input to the transporter is from the energetically downhill transmembrane flux of a driving ion or ions, with re-energization by primary active transport of those driving ions.
The three major ions involved in primary active transport at the plasmalemma of Archaea, Bacteria and Eukarya are H+, Na+ and Cl−. Table 1 shows what is known of the distribution of these pumps among the three higher taxa and the immediate energy sources. In marine photosynthetic (including photoheterotrophic) organisms there are examples of all of the pumps in Table 1 located in the plasmalemma. It is these ions that are the focus of the subsequent discussion in this paper. Other important ions subject to primary active transport are Ca2+ and Mg2+, transported out of cells by P-type ATPases, that also pump, in various organisms, Na+ out:K+ in, K+ in, Na+ out, H+ out and H+ out:K+ in (Kuhlbrandt, Reference Kuhlbrandt2004; Bublitz et al., Reference Bublitz, Morth and Nissen2011; Chan et al., Reference Chan, Reyes-Prieto and Bhattacharya2011, Reference Chan, Zäuner, Wheeler, Grossman, Prochnik, Blouin, Zhuang, Benning, Berg, Yarish, Ekiksen, Klein, Lin, Levine, Brawley and Bhattachaya2012; Palmgren & Nissen, Reference Palmgren and Nissen2011; Søndergaard & Pedersen, Reference Søndergaard and Pedersen2015; Palmgren et al., Reference Palmgren, Sorenson, Hallström, Säll and Broberg2020). As well as variants pumping H+, Na+, K+, Mg2+ and Ca2+ (topological type II: Thever & Saier, Reference Thever and Saier2009, in prokaryotes and eukaryotes), there are also P-type ATPases (topological type I, Thever & Saier, Reference Thever and Saier2009, not considered further here) that pump out metal cations such as Cu(I), Ag(I), Zn(II), Cd(II) and Pb(II) (Kuhlbrandt, Reference Kuhlbrandt2004; Thever & Saier, Reference Thever and Saier2009; Bublitz et al., Reference Bublitz, Morth and Nissen2011; Palmgren & Nissen, Reference Palmgren and Nissen2011; Søndergaard & Pedersen, Reference Søndergaard and Pedersen2015). There is also an F-type Cl− influx ATPase in the plasmalemma of some marine algae, as well as P-type Cl−-ATPases as found in metazoans (Gerencser & Zhang, Reference Gerencser and Zhang2003; Raven, Reference Raven2017). There are also ATP (adenosine triphosphate) binding cassette proteins (sometimes referred to as ABC transporters) involved in active solute influx at the plasmalemma, although there is little evidence of these in marine photosynthetic organisms (Chan et al., Reference Chan, Reyes-Prieto and Bhattacharya2011, Reference Chan, Zäuner, Wheeler, Grossman, Prochnik, Blouin, Zhuang, Benning, Berg, Yarish, Ekiksen, Klein, Lin, Levine, Brawley and Bhattachaya2012; but see Badger & Price, Reference Badger and Price2003 for HCO3− accumulation in freshwater and marine cyanobacteria).
Table 1. Energy sources for primary active transporters of H+, Na+ and Cl− in the Archaea, Bacteria and Eukarya

See also Kuhlbrandt (Reference Kuhlbrandt2004), Bublitz et al. (Reference Bublitz, Morth and Nissen2011), Palmgren & Nissen (Reference Palmgren and Nissen2011), Søndergaard & Pedersen (Reference Søndergaard and Pedersen2015).
Possible original functions of primary active H+, Na+ and Cl− pumps at the plasmalemma
An argument for the early origin and functions of primary active H+ pumps (ATP-driven; light-driven via rhodopsins) is intracellular acid-base regulation in early cells related, for example, to fermentation of neutral substrates (e.g. sugars) to organic acids (e.g. lactic acid) (Raven & Smith, Reference Raven and Smith1981, Reference Raven and Smith1982) in anoxic environments. Occurrence of the redox-driven or light-driven H+ pumps and ATP-driven H+ pumps in the plasmalemma of the same cell permits the use of redox or light energy to phosphorylate ADP to ATP, granted appropriate free energy differences and stoichiometries (Raven & Smith, Reference Raven and Smith1981, Reference Raven and Smith1982). Early chemolithotrophy could generate the H+ free energy difference across the plasmalemma, and thence phosphorylate ADP (Russell & Hall, Reference Russell and Hall1997; Martin & Russell, Reference Martin and Russell2007; Mulkidjanian et al., Reference Mulkidjanian, Dibrov and Galperin2008; Duchuzeau et al., Reference Duchuzeau, Schoepp-Cothenet, Baymann, Russell and Nitscke2014; but see Jackson, Reference Jackson2016). In seawater at the present pH, and with intracellular (cytosolic) pH about 0.5 units lower than seawater (Raven & Smith, Reference Raven and Smith1981, Reference Raven and Smith1982), the electrical potential difference across the membrane must be more negative than is found in most marine eukaryotes to definitely need active H+ efflux (see below). The requirement for intracellular pH regulation in oxygenic photolithotrophic marine cells involves several metabolic reactions assimilating inorganic nutrient solutes and is shown in Table 2. Volume regulation of wall-less marine cells by active Na+ efflux would, in the case of primary active H+ transport, involve H+:Na+ antiport (Raven & Smith, Reference Raven and Smith1982; Katz et al., Reference Katz, Bental, Degani and Avron1991; Gimmler, Reference Gimmler2000).
Table 2. Intracellular H+ production and consumption in redox reactions, and calcification, in marine phytoplankton

Elemental atomic ratios based on the Redfield ratio, with a upper limit on reduced S from Ksionzek et al. (Reference Ksionzek, Lechtenfeld, McAllister, Schmitt-Kopplin, Geuer, Geibert and Koch2016) since values are for total S and not all S in the cell is reduced to the −SH level. For intracellular calcification in coccolithophores (Taylor et al., Reference Taylor, Brownlee and Wheeler2017) and some dinoflagellates (Van de Waal et al., Reference Van de Waal, John, Ziveri, Hoins, Sluija and Röst2013), a particulate inorganic C: particulate organic C ratio of 1.0 is assumed. Other references for H+:N are assimilated from Brewer & Goldman (Reference Brewer and Goldman1976), Smith & Raven (Reference Smith and Raven1979) and Raven (Reference Raven2013).
Well characterized in the, mainly freshwater, green algal macrophytes of the Characeae (Walker et al., Reference Walker, Smith and Cathers1980), localized active efflux of H+ across the plasmalemma causes a localized acidification in the cell wall and diffusion boundary layer. This shifts the CO2:HCO3– equilibrium in favour of CO2, increasing the rate of uncatalysed HCO3− conversion to CO2 and thereby improving cellular CO2 supply (Raven & Beardall, Reference Raven and Beardall2016). A similar mechanism is thought to occur in many marine macrophytes as a mechanism of using external HCO3− (Raven & Hurd, Reference Raven and Hurd2012). However, the evidence for this in marine macroalgae is indirect and based on effects of buffers on HCO3− use, inhibition of external carbonic anhydrase using membrane-impermeant inhibitors or a lack of effects of inhibitors of HCO3− transport (Raven & Hurd, Reference Raven and Hurd2012; Raven & Beardall, Reference Raven and Beardall2016). It is considered unlikely that this process occurs in microalgae, however, as their size would mean they have a thinner diffusive boundary layer and greater proton leakage (Flynn et al., Reference Flynn, Blackford, Baird, Raven, Clark, Beardall, Brownlee, Fabian and Wheeler2012; Raven & Beardall, Reference Raven and Beardall2016).
For primary active Na+ efflux at the plasmalemma of wall-less cells, the higher intracellular K+:Na+ ratio in the cytosol than in a high-salinity medium such as seawater, driven by the primary active Na+ efflux, could be involved in cell volume regulation, countering the Donnan effect of negative charge in the cytosol (Raven & Smith, Reference Raven and Smith1982). The high K+:Na+ in the cytosol is attributed by Dibrova et al. (Reference Dibrova, Galperin, Koonin and Mulkidjanian2015) to the origin of life at inland hot springs with high K+:Na+, resulting in a requirement for high (about 100 mol m−3) K+ for activity of many enzymes. When life invaded the much more widespread freshwater and marine habitats with low K+:Na+ ratios, active Na+ efflux maintained high internal K+:Na+ ratios despite a finite Na+ permeability of the plasmalemma and, in some bacteria, significant energy storage as a Na+ electrochemical potential gradient (Na+ motive force) (Skulachev, Reference Skulachev1984; Skulachev, Reference Skulachev1989; Mulkidjanian et al., Reference Mulkidjanian, Dibrov and Galperin2008; Dibrova et al., Reference Dibrova, Galperin, Koonin and Mulkidjanian2015). Primary active Na+ efflux in the marine species of the wall-less chlorophycean Dunaliella spp. is involved in osmoregulation with varying external osmolarities (Ehrenfeld & Cousin, Reference Ehrenfeld and Cousin1984; Gimmler, Reference Gimmler2000; Popova et al., Reference Popova, Shumkova, Andreev and Balnokin2005; Popova & Balnokin, Reference Popova and Balnokin2013). The Na+ electrochemical potential gradient is widely used in marine (and some other) photosynthetic organisms to energize the influx of nutrients and osmolytes by Na+ co-transport (Raven, Reference Raven1984; Chan et al., Reference Chan, Reyes-Prieto and Bhattacharya2011, Reference Chan, Zäuner, Wheeler, Grossman, Prochnik, Blouin, Zhuang, Benning, Berg, Yarish, Ekiksen, Klein, Lin, Levine, Brawley and Bhattachaya2012). A further function of the Na+ electrochemical potential difference is in the action potentials in marine diatoms (Taylor, Reference Taylor2009; Helliwell et al., Reference Helliwell, Chrachri, Koester, Wharam, Verret, Taylor, Wheeler and Brownlee2019) and, from genomic evidence, the marine phaeophycean Ectocarpus and the marine prasinophyceans Micromonas and Ostreococcus, as well as freshwater chlorophyceans (Fux et al., Reference Fux, Mehta, Moffat and Spafford2018). Recovery of the resting state after an action potential requires active Na+ efflux, either by primary active Na+ efflux, or by Na+:H+ antiport following primary active H+ efflux.
Primary active transport of Cl− at the plasmalemma of walled marine ulvophycean algal cells is involved in turgor generation, and in action potentials (Bisson et al., Reference Bisson, Beilby and Shepherd2006; Raven, Reference Raven2017). At least the turgor generation role could be performed by Cl− influx coupled to H+ or Na+ influx and primary active H+ or Na+ efflux. The roles of Cl− as an essential micronutrient in photosynthesis (Raven, Reference Raven2017) can be satisfied by passive Cl− distribution between seawater and the cytosol even with an inside-negative electrical potential of 150 mV, but not the role established in terrestrial flowering plants as a beneficial nutrient (Raven, Reference Raven2017).
Not considered in detail here is active Ca2+ efflux at the plasmalemma that maintains the very low free Ca2+ concentration in the cytosol (100–200 μmol m−3) used in preventing damage to proteins and in signalling (Roberts et al., Reference Roberts, Illot and Brownlee1994; Thompson et al., Reference Thompson, Callow, Callow, Wheeler, Taylor and Brownlee2007; Wheeler et al., Reference Wheeler, Helliwell and Brownlee2019). Ca2+-H+ antiporters and Ca2+ P-ATPases that are involved in Ca2+ efflux from the cytosol are known, on genomic evidence, to be very widespread among algae (Emery et al., Reference Emery, Whelan, Hirschi and Pittman2012; Palmgren et al., Reference Palmgren, Sorenson, Hallström, Säll and Broberg2020).
Energetics of primary active transport
An important aspect of analysing primary active ion transport at the plasmalemma is the electrochemical potential difference for ions across that membrane. The electrochemical potential difference is defined by equation (1) (Mitchell, Reference Mitchell1979; Raven, Reference Raven1984; Nobel, Reference Nobel2009; Nichols & Ferguson, Reference Nichols and Ferguson2013).
 $$\Delta \lpar \bar{\mu }\rpar _{\,j^{ +{/}-}{\rm NP}} = zF\Psi _{{\rm NP}} + {\rm RT}\ln \displaystyle{{{\lsqb {\,j^{ +{/}-}} \rsqb }_{\rm N}} \over {{\lsqb {\,j^{ +{/}-}} \rsqb }_{\rm P}}}$$
$$\Delta \lpar \bar{\mu }\rpar _{\,j^{ +{/}-}{\rm NP}} = zF\Psi _{{\rm NP}} + {\rm RT}\ln \displaystyle{{{\lsqb {\,j^{ +{/}-}} \rsqb }_{\rm N}} \over {{\lsqb {\,j^{ +{/}-}} \rsqb }_{\rm P}}}$$where 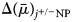 $\Delta \lpar \bar{\mu }\rpar _{j^{ +{/}-}{\rm NP}}$ = electrochemical potential of ion j +/− in phase N relative to that in phase P; N = electrically negative phase (cytosol for the plasmalemma); P = electrically positive phase (aqueous medium for the plasmalemma); j +/− = ion under consideration, either a cation (j +) or anion (j −); z = numerical charge on the ion (+1 for H+ or Na+, +2 for Ca2+, −1 for Cl−); F = Faraday constant (96,485 Joule V−1 mol−1); Ψ NP = electrical potential of phase N relative to phase P; R = gas constant (8.314 Joule mol−1 °K); T = temperature (°K); ln = natural logarithm; [j +]N = concentration of j + in phase N (mol m−3); [j −]P = concentration of j − in phase P (mol m−3).
$\Delta \lpar \bar{\mu }\rpar _{j^{ +{/}-}{\rm NP}}$ = electrochemical potential of ion j +/− in phase N relative to that in phase P; N = electrically negative phase (cytosol for the plasmalemma); P = electrically positive phase (aqueous medium for the plasmalemma); j +/− = ion under consideration, either a cation (j +) or anion (j −); z = numerical charge on the ion (+1 for H+ or Na+, +2 for Ca2+, −1 for Cl−); F = Faraday constant (96,485 Joule V−1 mol−1); Ψ NP = electrical potential of phase N relative to phase P; R = gas constant (8.314 Joule mol−1 °K); T = temperature (°K); ln = natural logarithm; [j +]N = concentration of j + in phase N (mol m−3); [j −]P = concentration of j − in phase P (mol m−3).
The sign of the electrochemical potential difference defines the direction of active transport of the ion, i.e. in the energetically uphill direction, requiring an input of energy from coupling to photons, exergonic redox reactions, or ATP conversion to ADP and phosphate in primary active transport, or coupling to exergonic ion fluxes in secondary active transport. The magnitude of the electrochemical potential difference defines the minimum energy input to primary or secondary active transport. As will be seen in the rest of the paper, Na+ invariably, and H+ very widely, are actively transported by marine photosynthetic organisms from the cytosol to the medium. If only one of these ion species, e.g. H+, is subject to primary active transport, then antiport coupling exergonic H+ re-entry to secondary active Na+ efflux must have a H+:Na+ stoichiometry consistent with the antiport being overall exergonic.
Hereinafter, the electrical potential of the cytosol (N phase) relative to the outside medium (P phase) is represented as Ψ CO.
Organisms with ion-pumping rhodopsins
Ion-pumping rhodopsins are light energy transducers that bring about active ion transport as the sole product of the photochemical reaction usable in cell metabolism (Oesterhelt & Stoeckenius, Reference Oesterhelt and Stoeckenius1973). The photoreaction and ion transport occurs using a single retinol-binding opsin protein, and brings about positive charge (H+ or Na+) flux from the N to the P side of the membrane, or negative charge (Cl−) flux from the P to the N side of the membrane, where ‘N’ and ‘P’ are as defined by Mitchell (Reference Mitchell1979). For the plasmalemma, the cytosol is the N side and the outside medium is the P side (see equation (1)).
Some marine bacteria have rhodopsins that pump H+ or Na+ out of the cells, or Cl− into the cells; some marine Archaea have been shown to have H+ efflux or Cl− influx rhodopsins, but there are no reports of Na+ efflux rhodopsins in Archaea (Oesterhelt & Stoeckenius, Reference Oesterhelt and Stoeckenius1973; Bejá & Lanyi, Reference Bejá and Lanyi2014; Yoshizawa et al., Reference Yoshizawa, Kumuga, Kim, Ogawa, Hayashi, Iwasaki, DeLong and Kogure2014; Larkum et al., Reference Larkum, Ritchie and Raven2018). Unexpectedly, there is an inwardly directed H+ pump driven by xenorhodopsin in Nanosalina (Shevchenko et al., Reference Shevchenko, Mager, Kovalev, Polovinkin, Alekseev, Juettner, Chizhov, Bamann, Vavourakis, Ghai, Gushchin, Borshchevskiy, Rogachev, Melnikov, Popov, Balandin, Rodriguez-Valera, Manstein, Bueldt, Bamberg and Gordelly2017); the significance of this energized, but energetically downhill, flux is not clear. Some marine Archaea and bacteria have autotrophic CO2 assimilation pathways, but none of these are energized entirely by ion-pumping rhodopsins despite the possibility of such energization (Raven & Smith, Reference Raven and Smith1981; Raven, Reference Raven2009a, Reference Raven2009b; Berg et al., Reference Berg, Kockelhorn, Ramos-Vera, Say, Zarzycki, Hügler, Alber and Fuchs2010; Bejá & Lanyi, Reference Bejá and Lanyi2014; Larkum et al., Reference Larkum, Ritchie and Raven2018). A marine aerobic anoxygenic photoheterotrophic bacterium, with bacteriochlorophylls, also has an H+-pumping rhodopsin, presumably in the plasmalemma (Larkum et al., Reference Larkum, Ritchie and Raven2018). There seem to be no reports of ion-pumping rhodopsins in marine aerobic or anaerobic anoxygenic photosynthetic bacteria, or in marine photosynthetic cyanobacteria, although a freshwater/terrestrial cyanobacterium (Gloeobacter) has a H+ efflux rhodopsin, with respiratory and photosynthetic H+ pumps, in the plasmalemma (Larkum et al., Reference Larkum, Ritchie and Raven2018). In an analysis of the energetics of phototrophic marine picoplankton, ion-pumping rhodopsins are major energy-transducing pigments in the ocean in organisms that are small enough to pass through a 2 μm filter (Gómez-Consarnau et al., Reference Gómez-Consarnau, Raven, Levin, Cutter, Wang, Seegers, Arístegui, Fuhrman J, Gasel and Sañudo-Wilhelmy2019; see also Kirchman & Hanson, Reference Kirchman and Hanson2013).
Ion-pumping rhodopsins also occur in eukaryotes. Among marine photosynthetic eukaryotes, the best-characterized case of ion-pumping rhodopsin in the plasmalemma is in Acetabularia, which contains an H+-pumping rhodopsin. Paradoxically, this rhodopsin moves H+into the cytosol, rather than the expected direction of acting as a H+ efflux pump (Raven, Reference Raven2009a; Wada et al., Reference Wada, Shimono, Kikukawa, Hato, Shinya, Ki, Kimura-Somega, Shirouza, Taogami, Miyachi, Juing, Kamo and Yokoyama2011; Bejá & Lanyi, Reference Bejá and Lanyi2014; Tamogami et al., Reference Tamogami, Kikuyawa, Wada, Demura, Kimura-Someya, Shirouzo, Yokayama, Miauchi, Simono and Kamo2017; Larkum et al., Reference Larkum, Ritchie and Raven2018), i.e. in the same direction as the inwardly directed H+ pump driven by xenorhodopsin in the bacterium Nanosalina (Shevchenko et al., Reference Shevchenko, Mager, Kovalev, Polovinkin, Alekseev, Juettner, Chizhov, Bamann, Vavourakis, Ghai, Gushchin, Borshchevskiy, Rogachev, Melnikov, Popov, Balandin, Rodriguez-Valera, Manstein, Bueldt, Bamberg and Gordelly2017). The Cl− ATPase in the plasmalemma of Acetabularia generates a Ψ CO of −180 mV, inside negative, in the light that, with a probable cytosol pH between 7 and 8, means an inwardly directed H+ electrochemical difference. This means that the H+-transporting rhodopsin ‘pumps’ H+ downhill. Other cases, where it is thought that the H+-pumping rhodopsin is in the plasmalemma and functions in H+ efflux, are found in some diatoms where it is thought to be an Fe-sparing way of energizing the plasmalemma (Raven, Reference Raven2009a; Slamovits et al., Reference Slamovits, Okamoto, Burri, James and Keeling2011; Marchetti et al., Reference Marchetti, Corlett, Hoplinson, Ellis and Cassar2015; Cohen et al., Reference Cohen, Ellis, Lampe, McNair, Twining, Maldonado, Brzezinski, Kizminov, Thamatrakoln, Till, Bruland, Sunda, Bangu and Marchetti2017; Larkum et al., Reference Larkum, Ritchie and Raven2018). In at least one case (the dinoflagellate Noctiluca which lacks oxygenic photosynthesis, except by symbiosis) the H+-pumping rhodopsin is found in a digestive vacuole; H+-pumping rhodopsins also occur in dinoflagellates with oxygenic photosynthesis (Slamovits et al., Reference Slamovits, Okamoto, Burri, James and Keeling2011; Vader et al., Reference Vader, Laughinghouse IV, Griffiths, Jakobsen and Gabrielsen2018).
It is clear that there is a significant variation in the phylogenetic analysis, and the richness of relevant data available among taxa. This is also the case for data considered below for organisms with bacteriochlorophylls and chlorophylls.
Organisms with bacteriochlorophylls
The anoxygenic photosynthetic bacteria function as photolithotrophs with reductants other than water in anaerobic environments or photoheterotrophically by assimilating CO2 with an organic reductant more oxidizing than H2 in anaerobic environments. In both of these cases autotrophic CO2 assimilation pathways are used (Larkum et al., Reference Larkum, Ritchie and Raven2018). These photolithotrophic organisms occupy geographically limited benthic marine anoxic illuminated habitats. The other marine bacteria with bacteriochlorophyll are the planktonic aerobic anoxygenic bacteria (Kolber et al., Reference Kolber, Gerald, Lang, Beatty, Blankenship, VanDover, Vetriani, Ratheber and Falkowski2001) that lack autotrophic CO2 assimilation pathways, and so are photoheterotrophs (Larkum et al., Reference Larkum, Ritchie and Raven2018; Gómez-Consarnau et al., Reference Gómez-Consarnau, Raven, Levin, Cutter, Wang, Seegers, Arístegui, Fuhrman J, Gasel and Sañudo-Wilhelmy2019). Bacteriochlorophylls are less significant in terms of photon absorption than ion-pumping rhodopsins or than chlorophylls as energy-transducing pigments in the ocean in organisms capable of passing through a 2 μm filter (Gómez-Consarnau et al., Reference Gómez-Consarnau, Raven, Levin, Cutter, Wang, Seegers, Arístegui, Fuhrman J, Gasel and Sañudo-Wilhelmy2019; see also Kirchman & Hanson, Reference Kirchman and Hanson2013).
Bacteriochlorophylls, like chlorophylls but unlike ion-pumping rhodopsins, are light energy transducers that indirectly bring about active transport of H+. The primary photochemistry moves an electron from a high potential electron donor on the P side of the membrane (sensu Mitchell, Reference Mitchell1979) to a low potential acceptor on the N side of the membrane (sensu Mitchell, Reference Mitchell1979) (equation (1)). H+ active transport from the P to the N side of the membrane is a result of secondary, thermodynamically downhill, redox reactions.
The freshwater and marine Chlorobi and Chloroflexi and, in inland hot springs, Acidobacteria, are photolithotrophic bacteria with light-harvesting bacteriochlorophyll c in chlorosomes. These organisms have no intracellular membranes, and have either Type 1 (Chlorobi, Acidobacteria) or Type 2 (Chloroflexi) reaction centres and associated redox catalysts, and the (photo-)redox H+ pumps and H+ electrochemical difference-driven FOF1 ATP synthases, are in their plasmalemma (Adams et al., Reference Adams, Cadby, Robinson, Tsukatani, Tank, Wen, Blankenship, Bryant and Hunter2013; Larkum et al., Reference Larkum, Ritchie and Raven2018). This plasmalemma location of photochemistry is also the case for the firmicute Heliobacterium without chlorosomes and with Type 1 reaction centres. H+ efflux across the plasmalemma is driven by light energy in the photoperiod, and by non-photochemical redox reactions in the scotophase. The H+ free energy difference across the plasmalemma in the light is large enough to synthesize ATP, granted the H+:ATP ratio of the bacterial FOF1 ATP synthase (Larkum et al., Reference Larkum, Ritchie and Raven2018).
The marine photosynthetic Proteobacteria with Type 2 reaction centres are the anaerobic photolithotrophic purple sulphur bacteria, and the anaerobic or aerobic photoheterotrophic purple non-sulphur bacteria. These organisms have their Type 2 reaction centres in plasmalemma invaginations and/or intracellular vesicles or flattened thylakoids (Larkum et al., Reference Larkum, Ritchie and Raven2018). These structural features mean that most, or all, of the photochemistry and associated H+ pumping is in membranes other than the plasmalemma that directly exchanges solutes with the bulk medium. It is, however, unclear what primary ion pumps occur in those parts of the plasmalemma that are not invaginated, and so are involved in nutrient uptake.
The marine anaerobic anoxygenic photosynthetic bacteria of the Chlorobi, Chloroflexi and Proteobacteria make a very small contribution (less than 0.1%) to global marine primary productivity (Johnson et al., Reference Johnson, Wolfe-Simon, Pearson and Knoll2009; Raven, Reference Raven2009b). Photons absorbed by marine aerobic anoxygenic photoheterotrophic bacteria probably make a larger contribution (0.5–5%) to global marine primary productivity than the anaerobic anoxygenic photolithotrophic bacteria (Kolber et al., Reference Kolber, VanDover, Niederman and Falkowski2000, Reference Kolber, Gerald, Lang, Beatty, Blankenship, VanDover, Vetriani, Ratheber and Falkowski2001; Goericke, Reference Goericke2002; Johnson et al., Reference Johnson, Wolfe-Simon, Pearson and Knoll2009; Raven, Reference Raven2009b; Kirchman & Hanson, Reference Kirchman and Hanson2013; Gómez-Consarnau et al., Reference Gómez-Consarnau, Raven, Levin, Cutter, Wang, Seegers, Arístegui, Fuhrman J, Gasel and Sañudo-Wilhelmy2019).
Organisms with chlorophylls
Cyanobacteria
Some phylogenetic evidence is consistent with a freshwater/terrestrial origin of cyanobacteria (Blank & Sánchez-Baracaldo, Reference Blank and Sánchez-Baracaldo2010; Blank, Reference Blank2013b; Sánchez-Baracaldo et al., Reference Sánchez-Baracaldo, Ridgwell and Raven2014). Data from freshwater cyanobacteria show that respiratory and photosynthetic redox and proton pumping reactions, and the associated CFOCF1 ATP synthase, occur in thylakoids. The exception is in Gloeobacter, where there are no thylakoids and photosynthesis and respiration, and ion-pumping rhodopsin, as well as nutrient transporters, occur in the plasmalemma (Mullineaux, Reference Mullineaux2014; Lea-Smith et al., Reference Lea-Smith, Bombelli, Vasudevan and Howe2016). The plasmalemma of thylakoid-containing freshwater Cyanobacteria has vanadate-sensitive (presumably ATP-driven) H+ efflux (Scherer & Böger, Reference Scherer and Böger1984; Kaplan et al., Reference Kaplan, Scherer and Lerner1989; Schultze et al., Reference Schultze, Forberich, Rexroth, Dyczmons, Roegner and Appel2009), and can oxidize NAD(P)H and reduce O2; and contains plastoquinone (PQ) and ATP synthase (Mullineaux, Reference Mullineaux2014; Lea-Smith et al., Reference Lea-Smith, Bombelli, Vasudevan and Howe2016), and moves protons out of the cell (Scherer et al., Reference Scherer, Stürzl and Böger1984). While this is consistent with primary active H+ efflux at the plasmalemma in freshwater cyanobacteria, the work of Ritchie (Reference Ritchie1992) shows that there is an electrogenic Na+ efflux pump at the plasmalemma of Synechococcus R2, and that the H+ efflux involves a Na+:H+ antiporter. This Na+ active efflux occurs over a wide range of external pH (5–10), K+ (0.1–300 mol m−3) and Na+ (0.1–300 mol m−3). The halophilic, alkalophilic cyanobacterium Aphanothece halophytica has a Na+-dependent FOF1 ATP synthase which, working as an ATPase in the plasmalemma, could act in active Na+ efflux (Wiangno et al., Reference Wiangno, Raksajit and Incharoensakdi2007; Soontharapirakkul & Incharoensakdi, Reference Soontharapirakkul and Incharoensakdi2010; Soontharapirakkul et al., Reference Soontharapirakkul, Promden, Yamada, Kageyama, Incharoensakdi, Iwamoto-Kihara and Takabe2011; see prediction by Mitchell, Reference Mitchell1979). Gabbay-Azaria et al. (2000) demonstrated cytochrome oxidase activity in the plasmalemma of the marine cyanobacterium Spirulina subsalsa (now Arthrospira subsalsa) that could be involved in active H+ efflux (Gabbay-Azaria et al., Reference Gabbay-Azaria, Schonfeld, Tel-Or, Messinger and Tel-Or1992). Bergman et al. (Reference Bergman, Siddiqui, Carpenter and Pescher1993) found cytochrome oxidase in the plasmalemma as well as the thylakoid membranes of the marine diazotrophic cyanobacterium Trichodesmium thiebaultii. A P-type Ca2+ ATPase has been found in a marine cyanobacterium that can excavate solid CaCO3 (Garcia-Pichel et al., Reference Garcia-Pichel, Ramirez-Reinat and Guo2010).
Na+ symport of HCO3− is one of the means of concentrating inorganic C in freshwater cyanobacterial cells, although these organisms also have other HCO3− transporters, one of which is an ABC transporter (Omata et al., Reference Omata, Price, Badger, Okamura, Gohta and Ogawa1999; Badger & Price, Reference Badger and Price2003). Freshwater cyanobacteria also use ABC transporters for NO3− (Maeda & Omata, Reference Maeda and Omata1997). However, the HCO3− and NO3− transporters in marine cyanobacteria are not ABC transporters such as occur in their freshwater counterparts (Wang et al., Reference Wang, Li and Post2000; Badger & Price, Reference Badger and Price2003; Maeda et al., Reference Maeda, Murakami, Ito, Tanaka and Omata2015).
Eukaryotic algae: relation to the supergroups in the New Tree of Life
Almost all eukaryotic photolithotrophs have a photosynthetic apparatus most of whose core genes were derived from endosymbiosis of a freshwater β-cyanobacterium with a non-photosynthetic unicellular eukaryote to comprise the Archaeplastida (Burki et al., Reference Burki, Roger, Brown and Simpson2020; Palmgren et al., Reference Palmgren, Sorenson, Hallström, Säll and Broberg2020). ‘Green’ Archaeplastida with chlorophyll b produced, by endosymbiosis in an Excavate cell, the secondarily photosynthetic Euglenophyta and, by endosymbiosis in a cell of the Rhizaria in the TSAR (Telonemia, Stramenopila, Alveolata, Rhizaria) supergroup, the secondarily photosynthetic Chlorarachniophyta. In a further set of secondary endosymbioses, ‘Red’ Archaeplastida with phycobilins produced, in cells of the Cryptista, the phycobilin-containing Cryptophyta and, with the loss of light-harvesting phyobilins, in cells of the Haptista to produce the Haptophyta, and in cells of the TSAR supergroup to produce the chromerids and dinoflagellates in the Alveolata, and the Ochrista in the Stramenopila. A very small minority of photolithotrophic eukaryotes (the genus Paulinella) arose by endosymbiosis of an α-cyanobacterium in a rhizarian (TSAR supergroup) (Nowack, Reference Nowack2014).
Eukaryotic algae: Archaeplastida (Glaucophyta, Rhodophyta, Chlorophyta, algal members of the Streptophyta)
The primary endosymbiosis of a β-cyanobacterium in an aerobic eukaryote that gave rise to chloroplasts of the Archaeplastida involved a cyanobacterium whose closest living relative is the freshwater Gloeomargarita lithophora (Brasier, Reference Brasier2013; Blank, Reference Blank2013a; Lewis, Reference Lewis2017; Ponce-Toledo et al., Reference Ponce-Toledo, Deschamps, Lôpez-García, Zivanovic, Benzenara and Moreira2017; Sánchez-Baracaldo et al., Reference Sánchez-Baracaldo, Raven, Pisani and Knoll2017). The basal extant Archaeplastida are the freshwater Glaucophyta; the basal Rhodophyta, the Cyanidiophyceae, are non-marine, but their habitat of acid hot springs cannot be described as freshwater (Dittami et al., Reference Dittami, Heesch, Olsen and Collén2017). There have been numerous freshwater–marine transitions in the Rhodophyta and, especially, in the Chlorophyta, but less so in the predominantly freshwater algal Streptophyta (Dittami et al., Reference Dittami, Heesch, Olsen and Collén2017). These habitat variations make it difficult to predict which primary active ion transporters occur in the plasmalemma of these organisms.
In the Rhodophyta, the non-marine (acid hot spring) Cyanidium caldarium, Cyanidioschyzon merolae and Galdieria sulphuraria (Cyanidiophyceae) have P-type H+-ATPases but not Na+-ATPases (Ohta et al., Reference Ohta, Shirakawa, Uchida, Yoshida, Matuo and Ehami1997; Lee et al., Reference Lee, Ghosh and Saier2017). Two Na+-ATPases (PyKPA1 and PyKPA2) have been characterized in the marine bangiophycean Porphyra yezoensis (now Pyropia yezoensis) (Barrero-Gil et al., Reference Barrero-Gil, Garciadeblás and Benito2005; Uji et al., Reference Uji, Hirata, Mikami, Mizuto and Saga2012a, Reference Uji, Monma, Mizuta and Saga2012b; see also Chan et al., Reference Chan, Zäuner, Wheeler, Grossman, Prochnik, Blouin, Zhuang, Benning, Berg, Yarish, Ekiksen, Klein, Lin, Levine, Brawley and Bhattachaya2012; Kishimoto et al., Reference Kishimoto, Shimajiri, Oshima, Hase, Mikami and Akama2013). This organism also has two plasmalemma Na+/H+ antiporters (Uji et al., Reference Uji, Monma, Mizuta and Saga2012b). An Na+:H+ antiporter has also been described in Pyropia haitanensis by Chen et al. (Reference Chen, Wang, Xu, Xu, Ji, Chen and Xie2019), who assume, on the basis of vanadate inhibition, that there is a H+-ATPase at the plasmalemma of this alga. However, vanadate inhibition is the case for all P and F ATPases (Müller et al., Reference Müller, Jensen and Taiz1999; Araki & González, Reference Araki and González1998; Kuhlbrandt, Reference Kuhlbrandt2004; Hong & Pedersen, Reference Hong and Pedersen2008; Pedersen et al., Reference Pedersen, Axelsen, Harper and Palmgren2012), but to a much smaller extent for V ATPases (Müller et al., Reference Müller, Irkens-Kiesecker, Rubinstein and Taiz1996; Araki & González, Reference Araki and González1998). Accordingly, vanadate is a general inhibitor of H+, Na+ and Ca2+ P-ATPases, and is not specific for H+ ATPases. Reed et al. (Reference Reed, Collins and Russell1981) and Reed & Collins (Reference Reed and Collins1981) examined the energetics of ion transport in Porphyra purpurea over a wide range of hypo- and hyper-saline (relative to seawater) media. Under all salinities (1/16 seawater to 3× seawater) there is active Na+ efflux (Reed et al., Reference Reed, Collins and Russell1981). It should be noted that the measurement of Ψ CO in the essentially non-vacuolate Porphyra purpurea cells by Reed and co-workers was determined using the distribution of the lipophilic cation TPMP+; there are reservations about the use of this method of measuring transplasmalemma electrical potential differences in eukaryotes (Ritchie, Reference Ritchie1982, Reference Ritchie1984). The floridiophycean Chondrus crispus has a P-type Na+-ATPase but no H+-ATPase (Lee et al., Reference Lee, Ghosh and Saier2017). There seem to be no relevant data for freshwater red algae.
In the Chlorophyta, the microalgal Prasinophyceae occur in marine and also freshwater habitats (Tragin & Vaulot, Reference Tragin and Vaulot2018; Del Cortona et al., Reference Del Cortona, Jackson, Bucchini, Van Bel, D'hondt, Škaloud, Delwiche, Knoll, Raven, Verbruggen, Vandepoel, De Clerck and Leliaert2020). There is functional evidence for the presence in the plasmalemma of the marine Tetraselmis (=Platymonas) viridis of an ATP-driven Na+ pump (Balnokin & Popova, Reference Balnokin and Popova1994; Balnokin et al., Reference Balnokin, Popova and Gimmler1997, Reference Balnokin, Popova and Andreev1999, Reference Balnokin, Popova, Pagis and Andreev2004; Gimmler, Reference Gimmler2000; Pagis et al., Reference Pagis, Popova, Andreev and Balnokin2001, Reference Pagis, Popova, Andreev and Balnokin2003; Popova & Balnokin, Reference Popova and Balnokin2013) and an ATP-driven H+ pump (Popova & Balnokin, Reference Popova and Balnokin1992; Gimmler, Reference Gimmler2000; Pagis et al., Reference Pagis, Popova, Andreev and Balnokin2003). There is genomic evidence of a P-type Na+-ATPase in the marine Ostreococcus tauri (Rodríguez-Navarro & Benito, Reference Rodríguez-Navarro and Benito2010). Gimmler (Reference Gimmler2000) provides an energetic background to infer the presence of active H+ and Na+ efflux in Tetraselmis viridis, although there are no data from that organism on the electrical potential difference across the plasmalemma, or Na+ and H+ concentrations in the cytoplasm.
The Chlorophyta: Chlorophyceae are mainly freshwater but with some marine representatives (Tragin & Vaulot, Reference Tragin and Vaulot2018; Del Cortona et al., Reference Del Cortona, Jackson, Bucchini, Van Bel, D'hondt, Škaloud, Delwiche, Knoll, Raven, Verbruggen, Vandepoel, De Clerck and Leliaert2020), the P-type ATPases of the genus Dunaliella has been the subject of considerable investigation (Wolf et al., Reference Wolf, Slayman and Gradmann1995; Weiss & Pick, Reference Weiss and Pick1996; Popova et al., Reference Popova, Belyaev, Shuvalov, Yurchenko, Matalin, Khramov, Orlova and Balnokin2018). Sequences encoding P-type H+-ATPases have been found in the genomes of the halophilic Dunaliella bioculata (Smahel et al., Reference Smahel, Hamann and Gradmann1990; Wolf et al., Reference Wolf, Slayman and Gradmann1995), Dunaliella salina (Weiss & Pick, Reference Weiss and Pick1996; Katz et al., Reference Katz, Waridel, Shevchenko and Pick2007), Dunaliella bioculata (Bertucci et al., Reference Bertucci, Tambutté, Tambutté, Allemand and Zoccola2010) and Dunaliella tertiolecta (Popova et al., Reference Popova, Belyaev, Shuvalov, Yurchenko, Matalin, Khramov, Orlova and Balnokin2018), as well as the acidophilic Dunaliella acidophila (Weiss & Pick, Reference Weiss and Pick1996; Bertucci et al., Reference Bertucci, Tambutté, Tambutté, Allemand and Zoccola2010). While Popova et al. (Reference Popova, Belyaev, Shuvalov, Yurchenko, Matalin, Khramov, Orlova and Balnokin2018) could not find a P-type Na+-ATPase in the genome of Dunaliella tertiolecta, Popova et al. (Reference Popova, Shumkova, Andreev and Balnokin2005; see also Shumkova et al., Reference Shumkova, Popova and Balnokin2000) functionally identified an electrogenic Na+-translocating ATPase in Dunaliella maritima using inside-out plasmalemma vesicles (see also Popova & Balnokin, Reference Popova and Balnokin2013). The possibility of a primary H+ pump with H+:Na+ antiport was ruled out by the observation of stimulation, rather than inhibition, of Na+ transport by the uncoupler CCCP (m-chlorophenyl carbonyl cyanide phenylhydrazone). Popova et al. (Reference Popova, Shumkova, Andreev and Balnokin2005) did not attempt to identify whether the catalyst of Na+ transport was a P-type ATPase. Further investigation is needed into the possibility of direct redox energization of Na+ efflux from Dunaliella salina (Katz & Pick, Reference Katz and Pick2001). The freshwater Chlamydomonas reinhardtii has a P-type Na+-ATPase (Barrero-Gil et al., Reference Barrero-Gil, Garciadeblás and Benito2005; Rodríguez-Navarro & Benito, Reference Rodríguez-Navarro and Benito2010), as well as a P-type H+-ATPase (Campbell et al., Reference Campbell, Coble, Cohen, Ch'ng, Russo, Long and Armbrust2001; Barrero-Gil et al., Reference Barrero-Gil, Garciadeblás and Benito2005; Bertucci et al., Reference Bertucci, Tambutté, Tambutté, Allemand and Zoccola2010).
An important aspect of the functioning of primary active transport of H+ and Na+ in driving symport, antiport and uniport of other solutes by Dunaliella is the free energy difference across the plasmalemma. There are several reports of intracellular Na+ concentrations (Katz & Avron, Reference Katz and Avron1985; Bental et al., Reference Bental, Degani and Avron1986; Pick et al., Reference Pick, Kanni and Avron1986; Wegmann, Reference Wegmann1986; Gimmler, Reference Gimmler2000), cytoplasmic pH (Burns & Beardall, Reference Burns and Beardall1987; Gimmler et al., Reference Gimmler, Kugel, Leibfritz and Mayer1988; Ginzburg et al., Reference Ginzburg, Ratcliffe and Southon1988; Gimmler, Reference Gimmler2000) and Ψ CO (Gimmler & Greenway, Reference Gimmler and Greenway1983; Oren-Shamir et al., Reference Oren-Shamir, Pick and Avron1990; see the critique by Ritchie, Reference Ritchie1982, Reference Ritchie1984) in a variety of halophilic Dunaliella species. Ψ CO of Dunaliella acidophila has been measured using the preferable microelectrodes method (Remis et al., Reference Remis, Simonis and Gimmler1992), but not so far for halophilic Dunaliella species. The thermodynamic analysis by Gimmler (Reference Gimmler2000) dealt with Dunaliella salina and suggested active efflux of both H+ and Na+, and pre-dates the discovery of the electrogenic Na+ ATPase in Dunaliella maritima (Popova et al., Reference Popova, Shumkova, Andreev and Balnokin2005; see also Shumkova et al., Reference Shumkova, Popova and Balnokin2000). Khramov et al. (Reference Khramov, Matalin, Karpichev, Balnokin and Popova2019) showed increased transcript abundance of a putative H+ P-ATPase under hypersaline conditions in Dunaliella maritima.
As with the Chlorophyceae, the Chlorophyta: Trebouxiophyceae are mainly freshwater but with some marine members (Tragin & Vaulot, Reference Tragin and Vaulot2018; Del Cortona et al., Reference Del Cortona, Jackson, Bucchini, Van Bel, D'hondt, Škaloud, Delwiche, Knoll, Raven, Verbruggen, Vandepoel, De Clerck and Leliaert2020). There is genomic evidence of both Na+-ATPase and H+-ATPases in the halotolerant Picochlorum sp. (Foflonker et al., Reference Foflonker, Price, Qiu, Palenik, Wang and Bhattacharya2014). The only contribution to a thermodynamic analysis of the need for active H+ and/or Na+ efflux is the work of Bock et al. (Reference Bock, Jacob, Kirst, Leibfritz and Mayer1996) who, using 31P NMR, found a cytoplasmic pH of 7.8 in the high intertidal–supralittoral macroalga Prasiola crispa. For a freshwater Chlorella sp. there is genomic evidence of a Na+ -ATPase (Uji et al., Reference Uji, Hirata, Mikami, Mizuto and Saga2012a), and functional evidence consistent with a H+-ATPase (Komor et al., Reference Komor, Cho, Schricker and Schobert1989).
The earliest Chlorophyta: Ulvophyceae fossils are from marine sediments, and most extant ulvophyceans are marine, exceptions being the freshwater Dichotomosiphon and some species of Cladophora and the terrestrial Trentepohliales (Del Cortona et al., Reference Del Cortona, Jackson, Bucchini, Van Bel, D'hondt, Škaloud, Delwiche, Knoll, Raven, Verbruggen, Vandepoel, De Clerck and Leliaert2020). Blount & Levedahl (Reference Blount and Levedahl1960) found active electrogenic Na+ efflux and Cl− influx in the marine gametophyte Halicystis ovalis phase of the marine ulvophycean Derbesia marina. There is genomic evidence for a P-type Na+ ATPase of the marine ulvophycean Flabellia petiolata (formerly Udotea petiolata) (Rodríguez-Navarro & Benito, Reference Rodríguez-Navarro and Benito2010), and for a number of P-type ATPases in Ulva (Zhang et al., Reference Zhang, Ye, Liang, Mou, Fan, Xu, Xu and Zhuang2012; De Clerck et al., Reference De Clerck2018).
Blinks (Reference Blinks1940, Reference Blinks1949) showed that Halicystis ovalis (the giant-celled coenocytic gametophyte phase of Derbesia marina) had a potential difference between the vacuole and seawater medium (Ψ VO) of −75 to −80 mV; for Halicystis osterhoutii (the gametophyte phase of Derbesia osterhoutii) the value is −65 to −70 mV. Replacing external Cl− with NO3− (or SO42−, or a number of organic anions) caused the potential difference to become negligible, or positive. This is consistent with the short-circuit current experiments of Blount & Levedahl (Reference Blount and Levedahl1960) showing the occurrence of an electrogenic Cl− influx pump in Halicystis ovalis. Graves & Gutknecht (Reference Graves and Gutknecht1976, Reference Graves and Gutknecht1977a, Reference Graves and Gutknecht1977b) examined ion concentrations and fluxes, and the effects of decreased external Cl− and low temperatures and of clamped Ψ VO, in the gametophyte Halicystis parvula phase of Derbesia tenuissima, and also concluded that there is an electrogenic Cl− influx pump. Graves & Gutknecht (Reference Graves and Gutknecht1976, Reference Graves and Gutknecht1977a, Reference Graves and Gutknecht1977b) infer that the Cl− pump is at the plasmalemma, as is the case for Acetabularia, discussed below.
Saddler (Reference Saddler1970a, Reference Saddler1970b) measured ion (K+, Na+, Cl−) fluxes between uninucleate giant-celled marine Acetabularia and the seawater medium, and electrical properties of the cell, concluding that there was an electrogenic Cl− influx pump. The occurrence of active electrogenic Cl− influx was confirmed by Mummert & Gradmann (Reference Mummert and Gradmann1976) and by Gradmann et al. (Reference Gradmann, Tittor and Goldfarb1982). The Ψ CO in the light is −170 mV (Saddler, Reference Saddler1970a) to −180 mV (Amtmann & Gradmann, Reference Amtmann and Gradmann1994). Saddler (Reference Saddler1970a, Reference Saddler1970b) suggested that cytoplasmic Cl− in Acetabularia is the same (500 mol m−3) as that in seawater, although this is not consistent with observed inhibitory effects of Cl− on metabolism (Raven, Reference Raven2017). Using the value of 500 mol m−3 for cytoplasmic Cl−, the electrochemical potential gradient for Cl− is 17–18 kJ mol−1, driving Cl− from the cytoplasm to the medium (Saddler, Reference Saddler1970a, Reference Saddler1970b). Mummert & Gradmann (Reference Mummert and Gradmann1991a) showed the role of Cl− in the action potential of Acetabularia, and also (Mummert & Gradmann, Reference Mummert and Gradmann1991a, Reference Mummert and Gradmann1991b) discovered the role of vesicular transport of ions across the cytosol to the vacuole. The Cl− influx is driven by a Cl−-ATPase (Gradmann et al., Reference Gradmann, Tittor and Goldfarb1982; Goldfarb & Gradmann, Reference Goldfarb and Gradmann1983; Ohhashi et al., Reference Ohhashi, Katsu and Ikeda1992) inhibited by vanadate (Smahel et al., Reference Smahel, Hamann and Gradmann1992; see discussion of vanadate inhibition of primary active ion-pumping ATPase under ‘Rhodophyta’ above). Goldfarb et al. (Reference Goldfarb, Sanders and Gradmann1984) reported that the Cl− pump can be reversed, coupled to net ATP synthesis (as in the prediction of Mitchell, Reference Mitchell1979). Ikeda et al. (Reference Ikeda, Schmid and Oesterhelt1990a, Reference Ikeda, Schmid and Oesterhelt1990b) showed that the Cl−-ATPase was not identical with the (C)F ATPase of the FOF1/CFOCF1 ATP synthase, but Ikeda et al. (Reference Ikeda, Kadowaki, Ikeda, Moritani and Kanazawa1997) demonstrated the interchangeability of the b subunit of the Acetabularia Cl−-ATPase and the β-subunit of the Escherichia coli F-ATPase. Finally, Moritani et al. (Reference Moritani, Ohnashi, Kadowaki, Tagaya, Lottspeich, Oesterhelt and Ikeda1997) determined the primary structure of the b subunit of the Acetabularia Cl−-ATPase. There seems to have been no further work on this Cl−-ATPase other than studies on action potentials (Raven, Reference Raven2017).
For the major ions commonly subject to primary active transport, Na+ and H+, the cytoplasmic Na+ concentration in Acetabularia is 60 mol m−3 (Amtmann & Gradmann, Reference Amtmann and Gradmann1994), and the cytoplasmic pH is estimated at 8.0–8.4 using pH indicators, and pH 7.6–7.7 from the pH at which isolated intact chloroplasts exhibit their highest rate of photosynthesis (Dodd & Bidwell, Reference Dodd and Bidwell1971). If external Na+ is 450 mol m−3, the internal:external Na+ concentration difference is 0.133, the electrochemical potential difference for Na+ is 22 kJ mol−1 and will drive Na+ into the cell. For H+, the indicator dye-measured cytoplasmic pH of 8.0–8.2 (Dodd & Bidwell, Reference Dodd and Bidwell1971) is essentially identical to that of seawater, so the electrochemical potential difference for H+ is 17 kJ mol−1, driving H+ into the cell. No evidence is available for the occurrence of primary active transport processes at the plasmalemma of Acetabularia other than the inward Cl− pump and the inward H+ pump. If secondary active efflux of Na+ is driven by Cl− symport, granted the electrochemical potentials for the two ions calculated above, the Cl−:Na+ ratio must be not less than 2. Active H+ efflux cannot be driven by the inwardly directed H+-pumping rhodopsin. Since the calculated electrochemical potential differences for H+ and Cl− are equal and opposite, net H+ efflux driven by Cl− efflux requires a ratio of Cl−:H+ > 1.0.
Other large-celled ulvophyceans include the Siphonocladales and Cladophorales, mainly marine and comprising one or more coenocytic cells. In the cases examined, the vacuole-positive electrical potential difference across the tonoplast is much greater than in other vacuolated organisms, in some cases making the inside-positive tonoplast–medium electrical potential difference greater than the inside-negative cytosol–medium electrical potential difference (Hope & Walker, Reference Hope and Walker1975). There are data for the giant-celled marine Chaetomorpha darwinii (now Chaetomorpha coliforme) on the Ψ CO of −72 mV, cytoplasmic Na+ concentration (25 mol m−3) and cytoplasmic pH (pH 8.0–8.3 in the light; pH 7.5−7.8 in the dark), with external Na+ 500 mol m−3 and external pH 8.0. The free energy difference across the plasmalemma, cytosol relative to medium, for H+ is −7.6 kJ mol−1, driving H+ into the cell, and −12.6 kJ mol−1 for Na+, also driving Na+ into the cell (Dodd et al., Reference Dodd, Pitman and West1966; Findlay et al., Reference Findlay, Hope, Pitman, Smith and Walker1971; Raven and Smith, Reference Raven and Smith1980), in the light. No data are available for electrical potentials, or intracellular ion concentrations, in the dark. Less information is available for the closely related large-celled alga, i.e. species of Valonia, Valoniopsis and Ventricaria (Bisson et al., Reference Bisson, Beilby and Shepherd2006), but the available information is consistent with a situation similar to that in Chaetomorpha darwinii.
Finally among the Ulvophyceae, there are important data on Ulva spp., now incorporating the genera Ulva and Enteromorpha (Hayden et al., Reference Hayden, Blomster, Maggs, Silva, Stanhope and Walland2003). Ulva is multicellular, with small cells of which half or less of the volume is taken up by a vacuole. Ritchie (Reference Ritchie1985) examined the energetics of ion transport in Enteromorpha (=Ulva) intestinalis. The cytoplasmic pH was not measured in that study so Ritchie (Reference Ritchie1985) used a value of pH 7.3 from a wide range of studies on (mostly non-marine) cyanobacteria, eukaryotic algae and plants, with an external pH of 8.0. With the measured Ψ CO of −54 ± 5 mV in seawater in the light, and −30 ± 5 mV in the dark, the proton electrochemical potential difference across the plasmalemma is not significantly different from zero (Ritchie, Reference Ritchie1985). For Na+, the ion most likely to be subject to primary active efflux in Ulva, the electrochemical potential difference (cytosol relative to medium) across the plasmalemma is −15.5 kJ mol−1 in the light and −13.1 kJ mol−1 in the dark (Ritchie, Reference Ritchie1985).
There are also data for the closely related Ulva lactuca. Reed & Collins (Reference Reed and Collins1981) used the distribution of the lipid-soluble cation TPMP+ to measure Ψ CO for Ulva lactuca cells in seawater; they found values of −54 ± 1.8 mV in the light and −44 ± 3.5 mV in the dark. Ritchie (Reference Ritchie1988), using microelectrodes, showed that Ψ CO of U. lactuca in seawater was −39 ± 1.1 mV in the light and −25 ± 1.9 mV in the dark. In view of the comments by Ritchie (Reference Ritchie1982, Reference Ritchie1984) on problems with the use of lipid-soluble cations to estimate the electrochemical potential difference between cells and the medium in eukaryotes, the following calculations use the electrical potential difference values of Ritchie (Reference Ritchie1988). Thus, using intracellular and extracellular Na+ concentrations, we calculate the electrochemical potential difference for Na+ (cytosol relative to medium) across the plasmalemma to be −13.8 kJ mol−1 in the light and −12.2 kJ mol−1 in the dark. Ritchie (Reference Ritchie1988) does not give values for the electrochemical potential differences for H+ across the plasmalemma, but with the assumption made by Ritchie (Reference Ritchie1985) for cytoplasmic pH (7.3), the H+ electrochemical potential difference (cytosol relative to medium) across the plasmalemma is +0.30 kJ mol−1 in the light and +1.65 kJ mol−1 in the dark. The choice of using the cytoplasmic pH value of Ritchie (Reference Ritchie1988) is supported by Lundberg et al. (Reference Lundberg, Welch, Jensén and Vogel1989) who, using 31P NMR, found a cytoplasmic pH of 7.2 in U. lactuca.
The algal members of the Streptophyta are the Charophyceae sensu lato (Del Cortona et al., Reference Del Cortona, Jackson, Bucchini, Van Bel, D'hondt, Škaloud, Delwiche, Knoll, Raven, Verbruggen, Vandepoel, De Clerck and Leliaert2020). The most morphologically complex of the Charophyceae are the Charales with most of the thallus volume occupied by giant cells (Beilby, Reference Beilby2015; Nishiyama et al., Reference Nishiyama2018). Most of the Charales are freshwater, although some species occur in brackish waters (e.g. in the Baltic) and Lamprothamnium spp. grows in coastal lagoons with very large changes in salinity, with the highest values twice that of seawater (Beilby, Reference Beilby2015). Energization of transport at the plasmalemma of Lamprothamnium, like that of freshwater Charales, involves a P-type H+ efflux ATPase, with active Na+ efflux driven by H+ antiport and active Cl− influx driven by H+ symport (Beilby, Reference Beilby2015). The electrochemical potential difference across the plasmalemma for H+, with external pH of 8 and cytoplasmic pH of 7.7 and a ΨCO of −160 mV (Kirst & Bisson, Reference Kirst and Bisson1982) at 25°C, is −13.7 kJ mol−1. For the major ions the electrochemical potential differences, cytosol relative to medium, are −24.2 kJ mol−1 (Na+), −3.14 kJ mol−1 (K+) and +9.27 kJ mol−1 (Cl−) (Kirst & Bisson, Reference Kirst and Bisson1982). The primary active efflux of H+ energizes, directly or indirectly, the active efflux of Na+ and K+ and active influx of Cl−.
Eukaryotic algae: organisms with secondary and tertiary chloroplast endosymbiosis
The earliest fossils of the diatoms (Bacillariophyceae sensu lato; Ochrophyta) are from marine habitats, with later invasion of fresh waters (Falkowski et al., Reference Falkowski, Katz, Knoll, Quigg, Raven, Schofield and Taylor2004; Siver et al., Reference Siver, Velez, Clivetti and Binda2018). There is functional evidence of a plasmalemma Na+-ATPase in the colourless marine diatom Nitzschia alba (Bhattacharya & Volcani, Reference Bhattacharya and Volcani1980). The work of Flynn et al. (Reference Flynn, Öpik and Syrett1987) on plasmalemma vesicles of Phaeodactylum tricornutum is also consistent with the occurrence of an Na+-ATPase. The available evidence on cytoplasmic pH is for pH 7.6 in a non-vacuolate marine pennate diatom Phaeodactylum tricornutum (Burns & Beardall, Reference Burns and Beardall1987), and pH 7.3 in the centric marine diatom Thalassiosira weissflogii at an external pH of 8 (Hervé et al., Reference Hervé, Derr, Douady, Quinet, Moisan and Lopez2002). The Ψ CO is −60 to −90 mV for the marine centric diatom Coscinodiscus wailesii (Gradmann & Boyd, Reference Gradmann and Boyd1995, Reference Gradmann and Boyd1999a, Reference Gradmann and Boyd1999b) and −84 mV for the marine centric diatom Odontella sinensis (Taylor et al., Reference Taylor, Brownlee and Wheeler2017). These values for a range of marine diatoms are consistent with the occurrence of active H+ efflux. For Na+ the only intracellular concentration values are for whole cells of the marine Coscinodiscus granii (46 mol m−3) and Coscinodiscus wailesii (125 mol m−3) (Kesseler, Reference Kesseler1974). Boyd & Gradmann (Reference Boyd and Gradmann1999) assume that these mean intracellular concentrations, dominated by the concentration in the largest cell compartment, the vacuole, also apply to the cytosol. With this assumption, and Ψ CO of −75 mV across the plasmalemma in the marine C. wailesii (Gradmann & Boyd, Reference Gradmann and Boyd1999a, Reference Gradmann and Boyd1999b), the Na+ free energy difference across the plasmalemma is 12 kJ mol−1, inside negative. Jones & Morel (Reference Jones and Morel1988) suggested that redox reactions in the plasmalemma of Thalassiosira weissflogii can act as an H+ efflux pump. Bertucci et al. (Reference Bertucci, Tambutté, Tambutté, Allemand and Zoccola2010) cite genomic evidence for a P-type H+-ATPase in the diatom Phaeodactylum tricornutum. Diatoms also have vacuolar H+ pyrophosphatases and V-type H+-ATPases (Bussard & Lopez, Reference Bussard and Lopez2014). While there is evidence from metazoans for the expression of V-type H+-ATPases in the plasmalemma (Beyenbach & Wieczorek, Reference Beyenbach and Wieczorek2006), there is no evidence as to whether this occurs in diatoms (Wieczorek et al., Reference Wieczorek, Brown, Grinstein, Ehrenfeld and Harvey1999; Bussard & Lopez, Reference Bussard and Lopez2014).
Almost all of the extant Ochrophyta: Phaeophyceae are marine. For the marine phaeophycean Ectocarpus siliculosus there is genomic evidence for a P Na+-ATPase (Uji et al., Reference Uji, Hirata, Mikami, Mizuto and Saga2012a, Reference Uji, Monma, Mizuta and Saga2012b). The only estimates of the electrochemical potential differences across the plasmalemma of the Phaeophyceae are for the development of the just-fertilized eggs of the fucoid Pelvetia fastigiata (Allen et al., Reference Allen, Jacobsen, Joaquen and Jaffe1972; Gibbon & Kropf, Reference Gibbon and Kropf1993) and the unfertilized eggs of the fucoid Fucus serratus (Taylor & Brownlee, Reference Taylor and Brownlee1993). Gibbon & Kropf (Reference Gibbon and Kropf1993) used microelectrodes to measure the Ψ CO of −60 mv, inside negative), and the pH difference (0.5 units, inside low) between the cytosol and seawater medium. Gibbon & Kropf (Reference Gibbon and Kropf1993) calculated the proton motive force across the plasmalemma, equivalent to an electrochemical potential difference, cytosol relative to medium for H+ of −2.5 kJ mol−1, i.e. close to electrochemical equilibrium. The data for ion content of Allen et al. (Reference Allen, Jacobsen, Joaquen and Jaffe1972) are for the whole zygote, rather than the cytosol, so calculations of electrochemical potential difference across the plasmalemma for K+, Na+ and Cl− are less accurate than the value for H+ (Gibbon & Kropf, Reference Gibbon and Kropf1993). The calculations of Gibbon & Kropf (Reference Gibbon and Kropf1993) show that while K+ and Cl− are near electrochemical equilibrium, the electrochemical potential difference, cytosol relative to medium, for Na+ is −14 kJ mol−1. Gibbon & Kropf (Reference Gibbon and Kropf1993) therefore suggest that the primary active transport at the plasmalemma is for Na+, with Na+:H+ antiport generating the much smaller H+ electrochemical potential difference. Taylor & Brownlee (Reference Taylor and Brownlee1993) examined the electrical properties of the plasmalemma, and the ion content, of unfertilized eggs of Fucus serratus in seawater. The Ψ CO using microelectrodes is −40 to −65 mV, with a mean of −50 mV. With the same cautions as for Pelvetia fastigiata zygotes, the driving force for H+ is 1 kJ mol−1 directed inwards, for Na+ 12 kJ mol−1, directed inwards, and for Cl−, zero kJ mol−1, i.e. equilibrium. These values are consistent with the occurrence of a Na+ efflux, with very weak evidence of a H+ efflux pump, and for Cl− at equilibrium. However, replacement of external Cl− by isethionate− makes the plasmalemma electrical potential difference 20 mV less negative, consistent with active electrogenic Cl− influx, and a lower Cl− concentration in the cytosol than in some other intracellular compartments. Further experiments are needed to examine this possibility.
Klenell et al. (Reference Klenell, Snoeijs and Pedersén2002, Reference Klenell, Snoeijs and Pedersén2004) suggested that Laminaria digitata and Laminaria saccharina (now Saccharina latissima) have a P-type H+-ATPase that is involved in acidifying part of the thallus surface; however, the evidence (inhibition by vanadate) does not distinguish a P-type H+ ATPase from a P-type Na+-ATPase in parallel with a H+:Na+ antiporter (see discussion above for Rhodophyta). Klenell et al. (Reference Klenell, Snoeijs and Pedersén2004) also used erythrosin B as an inhibitor of the plasmalemma P-type H+-ATPase; however, erythrosin B also inhibits a range of other processes (Gimmler, Reference Gimmler1988).
In the Ochrophyta: Raphidophyceae, inverted plasmalemma vesicles of the marine Heterosigma akashiwo show ATP-dependent Na+ accumulation. The Na+ accumulation was inhibited by vanadate, and was shown not to be an accumulation down an inside-negative electrical potential, or a result of Na+:H+ exchange following H+ accumulation by an H+-ATPase (Shono et al., Reference Shono, Wada and Fujii1995, Reference Shono, Hara, Wada and Fujii1996). These findings are supported by genomic evidence of a Na+-ATPase (Shono et al., Reference Shono, Wada, Hara and Fuji2001; Jo et al., Reference Jo, Shono, Wada, Ito, Nomoto and Hara2010; Uji et al., Reference Uji, Hirata, Mikami, Mizuto and Saga2012a).
For the Haptophyta the earliest known fossils of the calcified coccoliths of coccolithophores in the Class Prymnesiophyceae are from marine strata, and there is no evidence that the coccolithophores ever invaded fresh waters (Falkowski et al., Reference Falkowski, Katz, Knoll, Quigg, Raven, Schofield and Taylor2004). The coccolithophore Emiliania huxleyi (Haptophyta: Prymnesiophyceae) has a putative P-type H+-ATPase in the plasmalemma (Lohbeck et al., Reference Lohbeck, Riebesell and Reusch2014). The Ψ CO of a calcifying strain of Emiliania huxleyi (Sikes & Wilbur, Reference Sikes and Wilbur1982) is −81 mV using K+-valinomycin and −145 mV using a fluorescent dye (cf. Ritchie, Reference Ritchie1982, Reference Ritchie1984). However, using the preferable microelectrode technique, Taylor et al. (Reference Taylor, Chrachri, Wheeler, Goddard and Brownlee2011) found a Ψ CO of −46 mV. There are conflicting reports on the intracellular pH of Emiliania huxleyi (Dixon et al., Reference Dixon, Brownlee and Merrett1989; Nimer et al., Reference Nimer, Brownlee and Merrett1994; see also Suffrian et al., Reference Suffrian, Schulz, Gurowska, Riebesell and Bleich2011). Using the fluorescent dye method the internal pH was determined as 7.28 (Dixon et al., Reference Dixon, Brownlee and Merrett1989), 7.03 (Nimer et al., Reference Nimer, Brownlee and Merrett1994) and 7.0 (Gibbin et al., Reference Gibbin, Putnam, Davy and Gates2014), at an external pH of 8 in the presence of 2 mol m−3 inorganic carbon. Lower values for internal pH are found with the DMO method and/or in the absence of inorganic C (Dixon et al., Reference Dixon, Brownlee and Merrett1989; Nimer et al., Reference Nimer, Brownlee and Merrett1994). Another coccolithophore, Pleurochrysis sp., has a P-type Ca2+-ATPase (Araki & González, Reference Araki and González1998).
The earliest known fossil Dinophyta (Alveolata) are from marine sediments with subsequent invasion of fresh waters (Lenz et al., Reference Lenz, Wilde and Riegel2002; Falkowski et al., Reference Falkowski, Katz, Knoll, Quigg, Raven, Schofield and Taylor2004). Symbiotic dinoflagellates of the Symbiodiniaceae may have a Na+-ATPase in the plasmalemma (Goiran et al., Reference Goiran, Allemand and Galgani1997), although a H+-ATPase and H+-Na+ antiport has not been ruled out as the mechanism of Na+ efflux. Bertucci et al. (Reference Bertucci, Tambutté, Tambutté, Allemand and Zoccola2010) and Mies et al. (Reference Mies, Van Sluys, Metcalfe and Sumida2017a, Reference Mies, Voolstra, Castro, Pires, Calderon and Sumida2017b) showed that the gene for a plasmalemma-located P-type H+-ATPase in the Symbiodiniaceae shows increased expression during symbiosis with corals, possibly related to acidification of the perisymbiotic space (Bertucci et al., Reference Bertucci, Tambutté, Tambutté, Allemand and Zoccola2010). It is not clear which ion is pumped by the P-ATPase in the calcifying dinoflagellate Thoracosphaera helmii, but it is probably Ca2+ (Van de Waal et al., Reference Van de Waal, John, Ziveri, Hoins, Sluija and Röst2013).
Submerged marine flowering plants: seagrasses
Fernández et al. (Reference Fernández, Garcia-Sánchez and Felle1999), Garcia-Sánchez et al. (Reference Garcia-Sánchez, Jaime, Ramos, Sanders and Fernández2000) and Rubio et al. (Reference Rubio, Belver, Venene, Garcia-Sánchez and Fernández2011) provided electrophysiological evidence of a H+-ATPase of the leaf and root cell plasmalemmas of Zostera marina. The Ψ CO is −150 to −160 mV, inside negative, and is hyperpolarized by fusicoccin, a compound known to stimulate non-halophytic flowering plant plasmalemma H+-ATPases; the cytosolic pH is 7.3 (Fernández et al., Reference Fernández, Garcia-Sánchez and Felle1999; Garcia-Sánchez et al., Reference Garcia-Sánchez, Jaime, Ramos, Sanders and Fernández2000). With an external seawater pH of 8.0 and the cytosol −160 mV negative relative to the seawater, the H+ electrochemical potential difference, cytosol relative to medium, across the plasmalemma is thus −13.7 kJ mol−1 kJ per mol, favouring H+ entry. Rubio et al. (Reference Rubio, Linares-Rueda, Garcia-Sánchez and Fernández2005) showed that the cytosol Na+ concentration (measured with Na+-selective microelectrodes) of 10.7 ± 3.3 mol m−3 in cells of Zostera marina in seawater has a Ψ CO of −150 mV, so the electrochemical potential difference across the plasmalemma is 25 kJ mol−3, driving Na+ into the cytosol. Following Pak et al. (Reference Pak, Fukuhara and Nitta1995), Fukuhara et al. (Reference Fukuhara, Pak, Ohwaki, Tsujimura and Nitta1996) and Muramoto et al. (Reference Muramoto, Harada, Ohkaki, Takagi and Fukuhara2002) characterized a salt-tolerant P-type H+-ATPase in the plasmalemma of Zostera marina. Generation of the −25 kJ mol−1 gradient (cytosol relative to medium) for Na+ using a Na+:H+ antiporter and a −13.7 kJ mol−1 gradient for H+ requires a Na+:H+ ratio of 0.5 or lower. Rubio et al. (Reference Rubio, Garcia, Garcia-Sánchez, Niell, Felle and Fernández2017, Reference Rubio, Garcia-Pérez, Garcia-Sánchez and Fernández2018) showed that the cytosol pH and the electrical potential difference across the plasmalemma of the seagrass Posidonia oceanica are very similar to those of Zostera marina.
Emergent marine flowering plants: tidal (=salt) marsh plants (herbaceous) and mangroves (trees)
Brügemann & Janiesch (Reference Brügemann and Janiesch1989) compared the plasma membrane ATPase from control specimens and those grown with added NaCl of the tidal marsh plant Plantago maritima. Wu & Seliskar (Reference Wu and Seliskar1998) investigated salinity adaptations in the H+-ATPase of the tidal marsh plant Spartina patens. The H+-ATPase also energizes NaCl secretion by salt glands in recretohalophytic tidal marsh plants and mangroves (Yuan et al., Reference Yuan, Leng and Wang2016; Dassanayake & Larkin, Reference Dassanayake and Larkin2017). The suggestion that the Limonium salt gland has primary active transport involving a Cl−-ATPase has not been substantiated (Raven, Reference Raven2017).
Evolutionary aspects of primary active ion pumps in marine photosynthetic organisms
The occurrence of primary active H+, Na+ and Cl− at the plasmalemma of marine photosynthetic organisms is summarized in Table 3, encapsulating the outcomes of the analysis in the previous section. Among the Archaeplastida, the brackish Characeae and brackish and marine flowering plants only have P(II)-type H+-ATPases. However, the Streptophyta are not basal among the Archaeplastida, so it cannot be concluded that P(II)-type H+-ATPase is the ancestral energizer of the plasmalemma. No data seem to be available for Glaucophyta; among Rhodophyta, marine representatives have P(II)-type Na+-ATPases and acidophilic ‘freshwater’ Cyanidiophyceae have P(II)-type H+-ATPases. Marine Chlorophyta have both P(II)-type H+-ATPases and P(II)-type Na+-ATPases. In these two phyla of Archaeplastida it is likely that horizontal gene transfer has been involved, e.g. via virus-encoded P-ATPases: an example is a Ca2+-ATPase in a freshwater Chlorella virus (Bonza et al., Reference Bonza, Martin, Kang, Lewis, Greiner, Giacometti, Van Etten, De Michelis, Thiel and Moroni2010). A range of transporters are encoded by other viruses (Greiner et al., Reference Greiner, Moroni, Van Etten and Thiel2018) and horizontal gene transfer has been shown for ATPases in prokaryotes (Hilario & Gogarten, Reference Hilario and Gogarten1993) and other transporters in eukaryotic algae (Chan et al., Reference Chan, Reyes-Prieto and Bhattacharya2011, Reference Chan, Zäuner, Wheeler, Grossman, Prochnik, Blouin, Zhuang, Benning, Berg, Yarish, Ekiksen, Klein, Lin, Levine, Brawley and Bhattachaya2012). Similar horizontal gene transfer is required to account for the distribution of P(II)-type H+-ATPases and P(II)-type Na+-ATPases among marine algae with plastids derived from secondary or tertiary endosymbiosis (Chan et al., Reference Chan, Reyes-Prieto and Bhattacharya2011, Reference Chan, Zäuner, Wheeler, Grossman, Prochnik, Blouin, Zhuang, Benning, Berg, Yarish, Ekiksen, Klein, Lin, Levine, Brawley and Bhattachaya2012; Burki et al., Reference Burki, Roger, Brown and Simpson2020). The origin of the F-ATPase that pumps Cl− in some ulvophycean Chlorophyta (Table 3) is unclear; metazoan Cl−-ATPases seem to be P-ATPases (Gerencser & Zhang, Reference Gerencser and Zhang2003).
Table 3. Phylogenetic distribution of plasmalemma ion transport ATPases in marine photosynthetic eukaryotes

For references see text.
Conclusions
In Archaea there are ion-pumping rhodopsins that actively transport H+ out of, and Cl− into, the cells; in one archaean there is an inward H+-pumping rhodopsin. In Bacteria all three of the ions H+, Na+ and Cl− are moved (H+ and Na+ out, Cl− in) by ion-pumping rhodopsins. There is an H+influx rhodopsin in Acetabularia, and (probably) H+ efflux rhodopsins in diatoms. Photochemistry based on bacteriochlorophyll exports H+ from the cytosol in some marine anoxygenic photosynthetic bacteria, but chlorophyll-based redox reactions do not export H+ from cells of marine cyanobacteria. Exergonic redox reactions export H+ and Na+ in photosynthetic bacteria, H+ in cyanobacteria and possibly H+ in eukaryotic algae. H+-and/or Na+-ATPases occur in the plasmalemma of all photosynthetic marine organisms tested. P-type H+ efflux ATPases occur in the marine Streptophyta, i.e. marine charophycean algae and seagrasses and emergent marine flowering plants. P-type Na+-ATPases are the main primary active ion pumps in the plasmalemma of other marine green algae and non-green algae. However, there may be P-type H+-ATPases in some cases, and a F-type Cl−-ATPase occurs in the ulvophycean Acetabularia. Some assignments of P-type ATPases as H+ or as Na+ pumps using genomics are not conclusive. Despite the insights that genomics can provide, it seems that there are still large gaps in the availability of electrophysiological data from many of the ecologically (and economically) important marine (and freshwater) phototrophs. There is thus a need for more such data to improve our understanding of the functioning of primary active transport in these organisms.
Acknowledgements
The University of Dundee is a registered Scottish charity, No. 015096. Discussions over the years with Jim Barber, Mary Beilby, Mary Bisson, Jack Dainty, Geoff Findlay, Mario Giordano, Alex Hope, Tony Larkum, Enid MacRobbie, Ray Ritchie; Hugh Saddler, Dale Sanders, Vladimir Skulachev, F Andrew Smith, Roger Spanswick, N Alan Walker and Philip White have been very helpful.





