INTRODUCTION
‘I have met in a morning's walk successively a lawyer, a plumber, and a college student each going about his respective business, and each with a skin as yellow as an orange … But the cases have occurred principally among the young, and in that class the disease has been epidemic.’
So remarked Sweet, a physician in Geneva, New York, of a visitation of jaundice in his hometown in 1887 [Reference Sweet1]. He was intrigued not only that the outbreak was widespread but also that children were particularly affected. Specifically, ‘Children from 6 to 12 years of age have for the most part been the subjects of the malady [Reference Sweet1].’ Earlier, regarding the 1881 jaundice outbreak in Washington, D.C., Garnett commented, ‘it has not hitherto occurred to me to meet with this disease in the form of an epidemic confining its attacks exclusively to young children [Reference Garnett2].’ Davis, from Chicago, averred, ‘Epidemic jaundice among children is of very exceptional occurrence [Reference Davis3].’ Across Maine in the decade before and continuing to the next century – exemplified by the reports of outbreaks in Cumberland County (1869) [Reference Foster4], multiple locations in the state including Minot (1886–1890) [Reference Walker5] and Andover with its neighbouring logging camps (1909) [Reference Leslie6] – the emergence of epidemic jaundice striking children primarily was also becoming notable.
What Sweet, Garnett and their contemporaries were witnessing was the harbingering of a new wave of jaundice epidemics in the USA, beginning in the 1870s. The documentations of Neill [Reference Neill7] and Blumer [Reference Blumer8] confirmed that epidemics started becoming widespread during the closing decades of the 19th century. Blumer enumerated eight of 59 jaundice outbreaks (14%) between 1812 and 1886 (confined mostly to the south of the Mason–Dixon line), but 51 (86%) during the short period from 1886 to 1920 (dispersed across the country) [Reference Blumer8].
Thereafter, the number of outbreaks rose even more markedly. From 1920 through to 1922, >200 outbreaks struck New York State alone [Reference Williams9]. By then, demographic features considered atypical by the 19th-century observers [Reference Sweet1–Reference Leslie6] had become hallmarks of the epidemic: highest clinical attack rates among children <15 years compared with older age-groups; no significant difference in attack rates between males and females; and higher morbidity and fatality rates among young children (<5 years) compared with older children (5–14 years). These characteristics define community-wide hepatitis A in the pre-vaccine era [Reference Sherman and Eichenwald10–Reference Capps, Bennett, Mills, Ettinger, Drake and Stokes15]. Through the greater duration of the 20th century, epidemics continued cyclically. They led to large-scale administration of pooled gamma globulin for prophylaxis of exposed contacts (Fig. 1). The epidemics were held in abeyance only in the 1990s with the introduction of routine vaccination against hepatitis A in children [Reference Wasley, Fiore and Bell16].
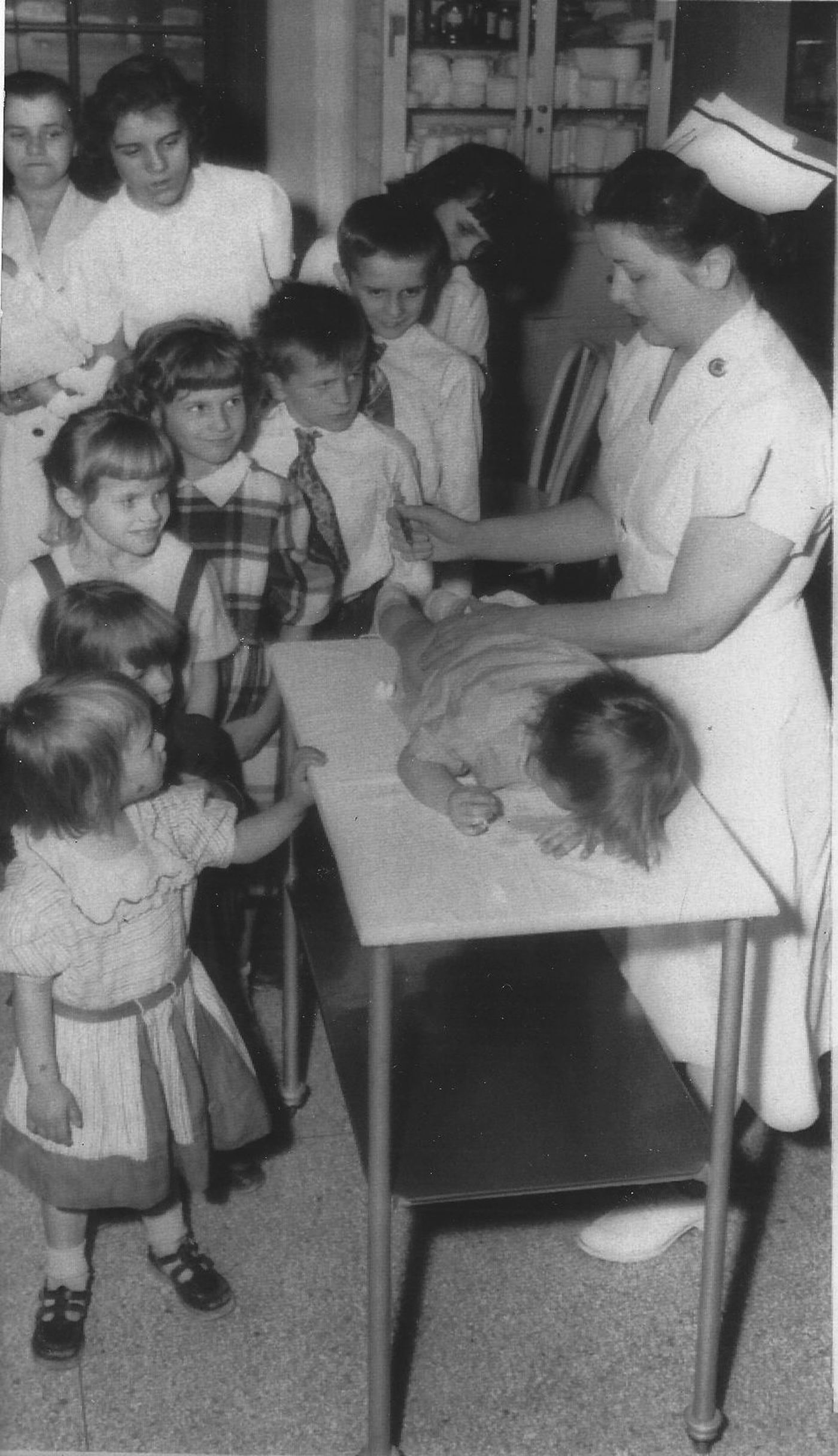
Fig. 1. Ten children of the Heckmann family lining up for gamma globulin shots at the Children's Hospital, Pittsburgh, during an outbreak of jaundice (1954). Nurse administering an injection to a boy atop a table, with mother at the end of the line carrying an infant (partially out of view). (Credit: United Press.).
THE ENQUIRY
Some of the outbreaks documented by Neill [Reference Neill7] and Blumer [Reference Blumer8] did not show a predilection for children and early adolescents (hereafter, ‘juveniles’), which would suggest that they were not hepatitis A. If so, what other diseases might underlie those non-juvenile-predominant outbreaks? That question is addressed in the first part of the enquiry. The enquiry is then extended to examine if hepatitis A could be associated with the jaundice epidemic of the American Civil War, which broke out before the 1870s, the period when epidemic hepatitis A had not yet emerged.
PRELIMINARY CONSIDERATIONS
Varieties of epidemic jaundice
Up to the end of the 19th century, the causes of epidemic jaundice were unknown. The discovery of what is now called Leptospira hemorraghica during World War I [Reference Neill7] led to the distinction of ‘spirochetal’ jaundice (subsequently termed leptospirosis) and Weil's disease from the more benign forms of epidemic jaundice. One variant, essentially the ‘filth disease’ form, was associated with outbreaks in military and civilian populations, and was called variously ‘epidemic catarrhal jaundice’, ‘infective hepatitis’, ‘infectious hepatitis’, ‘epidemic hepatitis’, etc. [Reference Ackerknecht, Stevenson and Multhauf17]. Another variant, transmitted from syringes, was referred to as serum or homologous serum hepatitis. Following definitive human transmission experiments during World War II, these two forms came to be known as hepatitis A and hepatitis B [Reference Zuckerman, Deinhardt and Deinhardt18], the prototypes of faecal–orally transmitted and blood-borne hepatitis, respectively. Subsequently, faecal–orally transmitted hepatitis resolved to hepatitis A and E, and blood-borne hepatitis to hepatitis B, C and D. The pathogens causing these hepatitides – all viruses – bear alphabetical assignments corresponding to the diseases, i.e. hepatitis viruses A to E.
Toxic jaundice
Epidemic jaundice need not always ensue from microbial infection. Nonetheless, all outbreaks considered in the current enquiry have not been reported to be associated with exposure to hepatotoxins [Reference Zimmerman19]. They are therefore presumed to be infectious in origin.
Sporadic jaundice
Past descriptions of painless jaundice – the principal clinical manifestation of a viral hepatitide – potentially provide nosologic clues. (Distinction is made between painless and painful jaundice. The latter points to a disparate class of disease, one associated with biliary obstruction, of which cholelithiasis is prominent [Reference Gordon-Taylor20].) Records of painless jaundice that ran a benign course and occurring sporadically in the American Continent date back to the 17th and 18th centuries, and are exemplified by the correspondence in 1682 between the sons of the Rev. Richard Mather of Massachusetts [21], and the reminiscences of Dr Jacob Roebeck whose practice in the 1790s was based in Grand Isle, Vermont [Reference Reynolds and Hemenway22]. However, the information therein and in such like documents does not permit inferences of which disease underlay the occasions of jaundice. With regard to sporadic, fatal jaundice in pregnancy, the earliest documentation is in the form of a case report of ‘leucinosis’ published in 1867 [Reference Wood23]. Autopsy revealed a shrunken liver, with microscopy showing replacement of hepatocytes by fatty globules, pointing to the acute fatty liver of pregnancy rather than a viral hepatitide. Prospects of identifying the cause(s) of sporadic, painless jaundice from historical records are dim.
Community vs. military and institutional epidemics
Community outbreaks tend to originate from persons from a wide age range and both sexes. If the disease in question exhibits a predilection for a particular population group, community-wide outbreaks can permit assessments of disease causation. Mortality and morbidity data pertaining to military outbreaks concern males in their adolescence through to middle-aged adulthood, so are too circumscribed demographically for inferring what the causative disease might be. Similarly, data from outbreaks in civilian institutional populations, e.g., schools, colleges, hostels and workplaces, also tend to be associated with specific age-groups and sex.
THE FIRST ENQUIRY
The initial enquiry examines what non-hepatitis-A-like diseases might underlie the jaundice outbreaks of the 19th century and early 20th century. These were outbreaks for which the cases were non-juvenile predominant.
Reports of jaundice outbreaks were examined, guided by the documentations of Neill [Reference Neill7] and Blumer [Reference Blumer8]. Other publications outside their purview were also looked into. Military and civilian institutional outbreaks were excluded from this phase of the enquiry.
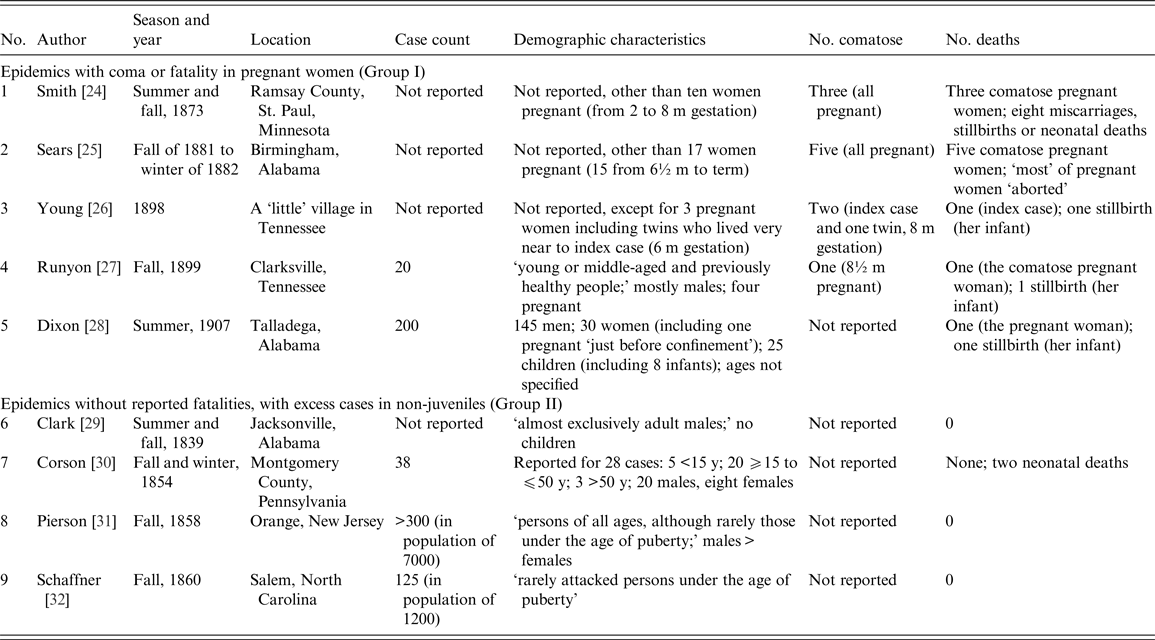
Altogether, nine community-wide, non-juvenile-predominant outbreaks were considered [Reference Smith24–Reference Shaffner32]. Observational data reported from them are summarised in Table 1. The outbreaks can be classified into two groups: Group I (n = 5), notable for coma or fatality in pregnant women, with or without data indicating excess cases of jaundice among adults; and Group II (n = 4), not notable for coma or fatality in pregnant women but with excess cases of jaundice among non-juveniles. The Group I outbreaks happened later (1873–1907) than those in Group II (1839–1860) (Fig. 2).
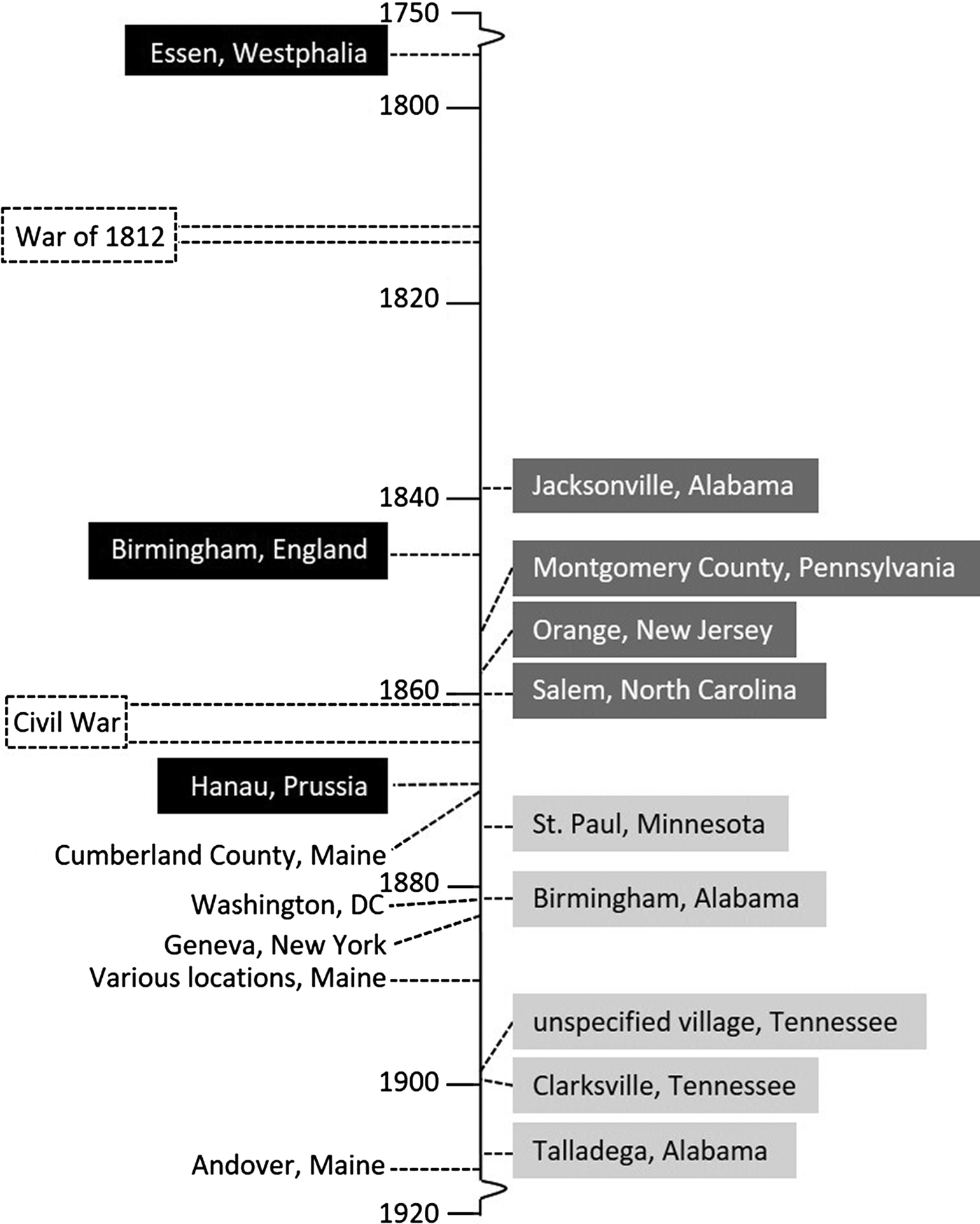
Fig. 2. Timeline of jaundice outbreaks specified in the current study. Light grey box: Group I outbreaks (non-juvenile-dominant); dark grey box: Group II outbreaks (non-juvenile-dominant); black box: juvenile-dominant outbreaks in Europe that preceded those in the United States; and unboxed: early juvenile-dominant outbreaks in the United States.
Table 1. Non-juvenile-dominant, community-wide jaundice outbreaks in the USA, 19th century to early 20th century
Hepatitis E-like epidemics
In the Group I outbreaks, almost all the pregnant women developed coma (n = 12) or died comatose (n = 11), mostly during the 3rd trimester. Another feature was the high number of intrauterine and neonatal deaths (>11) among the affected women (Table 1).
Reports of outbreaks from Group I display interesting features. In Smith's report [Reference Smith24], jaundice in St. Paul, Minnesota, ‘so generally spread that it might be said to have been an epidemic,’ resolved ‘in almost all instances.’ But outcomes for ten cases – all pregnant women – were less salutary: three perished in a coma and miscarried; and five of the seven survivors also miscarried. The epidemic in Birmingham, Alabama, was reported by Sears [Reference Sears25] to have ‘exhibited some peculiar features,’ viz., five of 17 pregnant women among the cases died. All fatalities resulted ‘not from the effects of fever, but from severe nervous symptoms, resulting in delirium, convulsions and coma.’ Young's account of the jaundice outbreak in a Tennessean village [Reference Young26] focused on three cases, all pregnant. Its space-time clustering is distinct: ‘the victims lived in the same little village not more than 100 yards apart’ and jaundice ‘developed within a few days of each other.’ Vividly described is the natural history of fulminant hepatitis in the index case. She was a 35-year-old grand multigravida, who, when first attended to, was in ‘remarkably good spirits, laughing and jesting,’ though deeply jaundiced. When visited again ten days later, coma had set in; by the next afternoon she had expired. In the outbreak in Clarksville, Tennessee, reported by Runyon [Reference Runyon27], four pregnant women were among the cases; one died in a coma but the rest survived. The last outbreak in Group I, in Talladega, Alabama, would have been unremarkable if not for the mention of fatality in a pregnant woman and her infant [Reference Dixon28].
In all Group I outbreaks, no deaths were reported other than among pregnant women. These outbreaks are redolent of classic (faecal–orally transmitted or waterborne) hepatitis E. Community-wide epidemic hepatitis E exhibits distinct demographic characteristics: highest clinical attack rates are observed among persons in the 15–44-year age group, with males predominating. Mortality is particularly heavy among women in gestation: whereas the case-fatality rate for the general population is seldom >1%, for pregnant women it can reach up to 50%. Invariably, death is preceded by coma. Hepatitis A, in particular, is unlikely because of the exceeding rarity of pregnancy deaths associated with it. No epidemic disease other than hepatitis E, whether infective or toxic, shows such a peculiar, grave predilection for gestational women (to a lesser extent, parturient and postpartum women). This signature characteristic is the outcome of the propensity for hepatitis E virus (HEV) belonging to genotype 1 (more rarely, genotype 2) to trigger massive liver necrosis in gestational women, thereby leading to hepatic failure and encephalopathy. Intrauterine and neonatal deaths that frequently arise are due to vertical HEV transmission [Reference Teo33].
The Group I outbreaks happened over an approximate three-decade period (from the late 1880s to the first decade of the 20th century). It overlaps the period of emergence of hepatitis A (1–6) (Fig. 2). Their coevality suggests that hepatitis E either had previously established endemicity before that period, or was – like hepatitis A – also in emergence. Thereafter, given the absence of Group-1-like outbreaks after the 1907 Talladega epidemic [Reference Dixon28] (Fig. 2), classic hepatitis E subsided – unlike hepatitis A, which continued to spread.
Non-hepatitis-E-like outbreaks
The four outbreaks in Group II (between 1839 and 1860) are noted for their preponderance in adults [Reference Sears25, Reference Young26] or rarity in persons ‘under the age of puberty’ [Reference Pierson31, Reference Shaffner32], and in males [Reference Clark29–Reference Pierson31]. In none of the outbreaks were deaths reported (Table 1). Clark's account of the outbreak in Jacksonville, Alabama [Reference Clark29], is notable as a case series, among the earliest of its kind reported in the USA. Compared with that, the reports of Corson [Reference Corson30], Pierson [Reference Pierson31] and Shaffner [Reference Shaffner32] are prosaic, with the possible exception of Pierson's description which noted that among those jaundiced were three of the five physicians practicing in Orange, New Jersey [Reference Pierson31]. Population data were provided for the outbreak there, and the one in Salem, North Carolina [Reference Shaffner32], permitting calculation of clinical attack rates as being 1 and 4·5%, respectively.
The Group II epidemics are unlikely to be due to hepatitis E, since no deaths were observed, let alone among pregnant women. Nor could they be hepatitis A, as they were not juvenile-predominant. Moreover, hepatitis A outbreaks with excess attack rates in non-juveniles are consequences of lowered herd immunity that follow improvements in sanitation, hygiene and poverty: they are phenomena encountered in the modern era [Reference Jacobsen and Wiersma34], not before World War I. Other icterogenic infectious diseases (e.g., leptospirosis, typhus, louse-borne relapsing fever, etc.) [Reference MacArthur35] are possibilities. These tend to be associated with extra-icteric features and high morbidity and mortality rates, whereas all the Group II outbreaks ran a benign course. Accordingly, non-A, non-E viral hepatitis may not be discounted. The features of these epidemics do not provide sufficient information to determine if they are from a single aetiology.
THE SECOND ENQUIRY
The enquiry into community-wide jaundice outbreaks has provided a chronological perspective of the outbreak occurrences and an insight into what the causative agents may be. Those now permit an examination of what might underlie the military outbreaks in the 19th century, in particular, the great jaundice epidemic of the American Civil War.
Preliminary consideration: contemporaneous epidemics in Europe
In Europe, occurrences in the 19th century of the military, institutional and community-wide jaundice epidemics have amply been documented [Reference Hirsch and Creighton36, Reference Hinssen37] as have hepatitis E outbreaks in particular [Reference Teo33]. Like in the USA, outbreaks in Europe that were predominantly non-juvenile (i.e. similar to the Group II outbreaks), antedate those that were principally juvenile. These include military outbreaks. Notably, juvenile-predominant outbreaks in Europe preceded those in the USA [Reference Sweet1–Reference Neill7], e.g. 1772 in Essen, Westphalia [Reference Brüning38], 1852 in Birmingham, England [Reference Barker39] and 1868 in Hanau, Prussia [Reference Rehn40] (Fig. 2). Descriptions of juvenile-dominant outbreaks in Europe started to burgeon from the late 19th century, flourishing as the next century arrived and progressed [Reference Anderson41–Reference Bachmann and Rodenwaldt43].
Military epidemics in the USA
In the USA, regimental outbreaks of jaundice have been reported, two during times of war and several more in between [44]. The first wartime outbreak happened sometime between 1812 and 1814, according to a report by Faulkner [Reference Faulkner45]; he alluded to a militia man who encountered jaundice in ‘almost every man’ in his company which was then mobilising in Virginia against the British [Reference Butler46]. A few decades later loomed the American Civil War (1861–1865). Jaundice was rife among the combatants, whether encamped, imprisoned or otherwise quartered. The medical corps of the Union Army recorded 87 236 cases of jaundice [44]. Case counts for the Confederate Army were not as comprehensively compiled [Reference Sharp47] but correspondences of field physicians provide insights into how widespread jaundice was [Reference Roberts, Whayne and Gatewood48, Reference Baird49].
What caused these outbreaks? With regard to the War of 1812, no mention of extra-icteric features or deaths among the Virginian militiamen was made but the ailment seemed innocuous [Reference Faulkner45]. An enterically transmitted viral hepatitide could have been responsible. The epidemic in the Civil War was considered ‘a troublesome and tedious, but not a dangerous affection [Reference Woodward50],’ although it elicited a 5% case-fatality rate [44]. Coinfection with leptospirosis, malaria, yellow fever, typhoid and typhus [Reference Neill7, 44, Reference Steiner51] possibly contributed to the fatalities, as could scurvy [Reference Woodward50]. Nonetheless, the most likely candidate jaundice-engendering disease remains a viral hepatitide.
Which one? Hepatitis E seems improbable. Particularly in regard to the Civil War, during which women mingled closely and multifariously with the soldiers in and out of the field [Reference Harper52], no reports of fatal jaundice among pregnant women are apparent. How about hepatitis A? Given that hepatitis A outbreaks in the USA would not have emerged until the last three decades of the 19th century (Fig. 2), and therefore hepatitis A virus (HAV) was not circulating until that period, hepatitis A may not be the candidate, contrary to the received view [Reference Zuckerman, Deinhardt and Deinhardt18]. However, as juvenile-predominant (hepatitis-A-like) jaundice epidemics had already broken out in Europe [Reference Brüning38, Reference Barker39] prior to the Civil War (Fig. 2), the possibility that HAV was introduced from Europe to the American Continent before or at onset of the war cannot be discounted (Woodward reported that within the first year of war there were already >10 000 cases of jaundice in the Union Army [Reference Woodward50]). If so, the Civil War – that ‘last great armed conflict in the world fought without knowledge of the germ theory of disease [Reference Sartin53]’ – could have facilitated the establishment of HAV endemicity in the USA. Given its upheaval and disruption, that war may have set the stage for the hepatitis A epidemics that would soon break out across the country [Reference Sweet1–Reference Wasley, Fiore and Bell16]. A caveat against ascribing a European introduction of HAV to participants of the Civil War is that, unlike the American War of Revolution and the War of 1812, it was not fought against Europeans or involved European military intervention [Reference Doyle54]. European military personnel might not have imported hepatitis A to the USA.
LIMITATIONS
The enquiry into community-wide outbreaks was not based on formal notification data, whether regional or national. All case counts in the Group I and II outbreaks, even if reported with population counts, allow only crude attack rates to be determined. Sex- or age-adjusted rates cannot be calculated as data on the sex distribution of the cases and base populations were seldom reported, if at all. These limitations blunt assessments of disease predilections for specific sex and age groups. Furthermore, the dearth of juveniles or pregnant women at the outbreak locations could lead to the non-assignment of Group II outbreaks to hepatitis A or E, respectively. Lastly, the outbreaks in both groups related only to clinically overt cases, with asymptomatic cases unaccounted for.
The chronological assessment showing the Civil War as having occurred before the emergence of epidemic hepatitis A and the Group I outbreaks (Fig. 2) rests on a small number of outbreak reports, and is therefore tenuously based. Examination of other historical documents, if extant and available, may confer more precision to chronological assessments. PCR and sequencing studies when successfully carried out in tissues archived from jaundiced patients [Reference Ganova-Raeva, Punkova, Campo, Dimitrova, Skums and Vu55] from the 19th century may also help to identify the icterogenic agents.
CONCLUSIONS
Excess clinical attack rates among juveniles feature in community-wide outbreaks of jaundice caused by hepatitis A, as do excess mortality rates among pregnant women during outbreaks of hepatitis E. The unique and disparate demographic predilections of these two viral hepatitides permit inferences of their emergence and endemicity. In the USA, epidemic hepatitis A emerged over the last three decades of the 19th century. During this period, hepatitis-E-like outbreaks were also in occurrence, suggesting either co-emergence with hepatitis A or prior establishment of hepatitis E endemicity. The hepatitis-E-like outbreaks then ceased, contrasting with hepatitis A outbreaks whose number grew. Another class of outbreaks, those that were non-juvenile-predominant but not bearing hepatitis E features, antedated the 1870s; the disease(s) underlying those outbreaks remain(s) unknown. The Civil War broke out before that period. If hepatitis A had not yet established endemicity, it would not have caused jaundice that was widespread during the war. However, hepatitis A could have been introduced just prior to the war or during its early phases. If so, the war may be viewed to have fostered the spread of hepatitis A through to the 20th century.
ACKNOWLEDGEMENTS
The author records his gratitude to MA Purdy and TV Murphy for their critical reading of the manuscript.






