Herpes simplex virus (HSV) 1 and 2 are rare causes of transverse myelitis,Reference Nardone, Versace and Brigo 1 , Reference Steiner, Kennedy and Pachner 2 but there are no reports in the literature of HSV transverse myelitis causing hypoglycorrhachia and extremely high CSF protein. We report a case of HSV 2 transverse myelitis associated with hypoglycorrhachia and hyperproteinemia.
A 56-year-old healthy Caucasian man from Ontario presented to another hospital with headache, vomiting, general malaise and progressive nuchal rigidity. Basic blood work within a week of symptom onset and non-contrast CT head were normal, and a lumbar puncture done at day 10 showed CSF glucose 2.1 mmol/L (2.2–3.9 mmol/L), protein 1.32 g/L (0.15–0.45 g/L) and total nucleated cell count (TNC) 253×106/L (100% lymphocytes). Viral studies for HSV 1 and 2 and Varicella-zoster virus were negative. He was discharged home with a presumptive diagnosis of viral meningitis. A day after returning home, he noted bilateral upper extremity (UE) numbness and weakness that progressed over the next 3 weeks to his lower extremities (LE) as well as his perineum. He had difficulty fully emptying his bladder and fully ejaculating, and by the day of his presentation to our hospital, he was unable to walk without assistance. He was afebrile during the entire duration of his symptoms. There was no associated weight loss, new rashes or genital lesions. He had not recently traveled and did not have high-risk sexual behaviors. Within 2 days of admission, he progressed to 0/5 power of his right LE and worsening power in his proximal left LE (3/5), along with bilateral extensor plantar response. He had a right T8 and left L1 sensory level with decreased sensation in the UE to the elbow.
MRI spine showed a relatively long segment of T2 hyperintense signal in the cervical cord from C4-C7 with circumferential leptomeningeal/dural enhancement, more marked posteriorly (Figure 1A–1F) suggesting focal inflammatory change with cord edema. MRI brain was normal with no leptomeningeal enhancement. Repeat CSF studies showed CSF glucose 0.8 mmol/L, protein 7.14 g/L, TNC 1603×106/L (98% lymphocytes). Flow cytometry did not show clonal B or T cell populations and pathology revealed atypical lymphocytes consistent with inflammation. Herpes simplex virus-2 DNA was positive by polymerase chain reaction in CSF and other viral, bacterial and fungal studies including testing for mycobacteria and HIV were negative. Paraneoplastic markers, Venereal Disease Research Laboratory, ANA panel and aquaporin-4 antibodies were negative. Angiotensin-converting enzyme, thyroid stimulating hormone and vitamin B12 levels were within the normal limits. CT chest, abdomen and pelvis was normal.
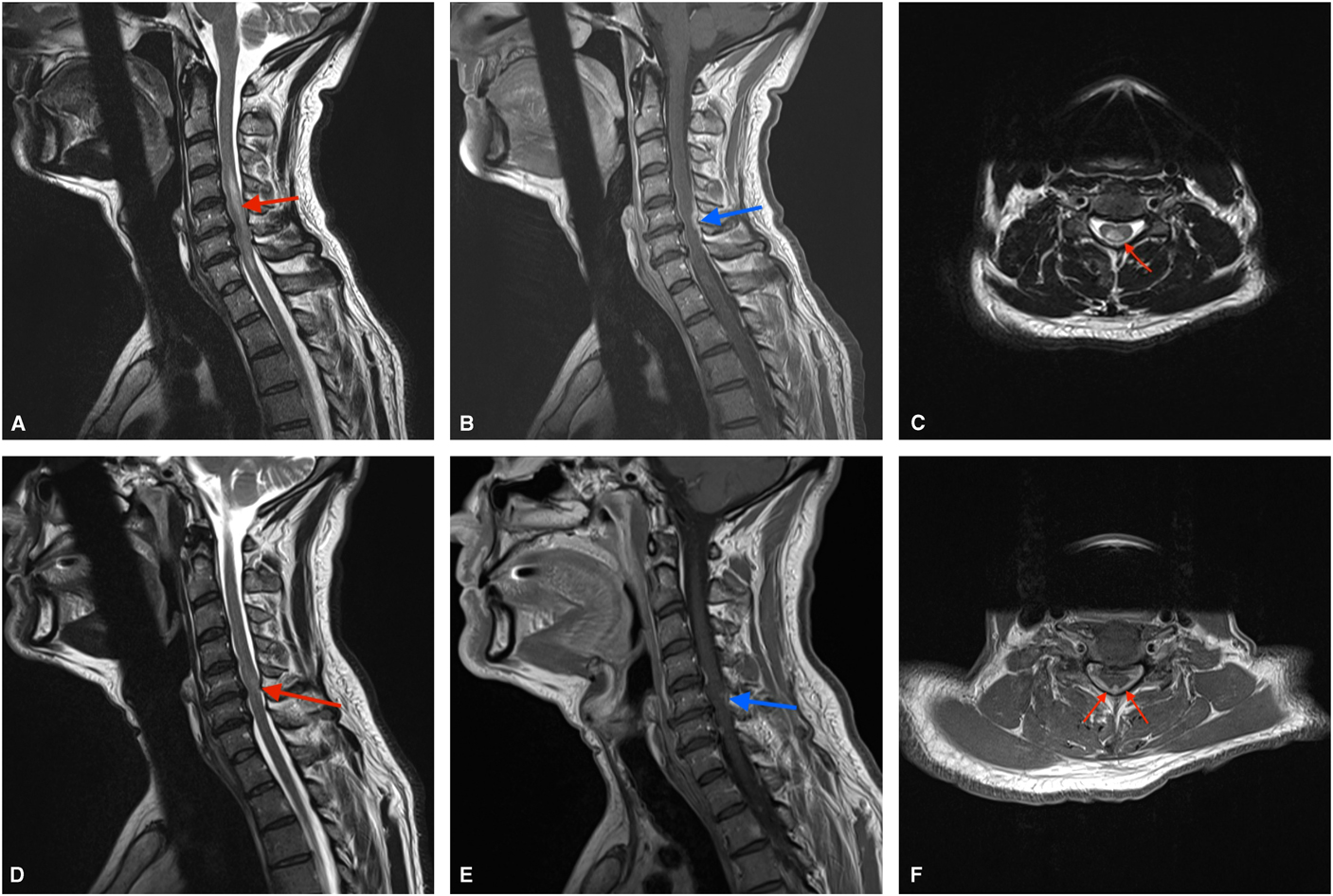
Figure 1 Sagittal MRI at presentation showing T2 hyperintensity within the cervical cord from C4-C7 (A) as well as meningeal enhancement posteriorly (B). Axial sections from the initial MRI further detail the T2 signal changes within the cord (C) and posterior enhancement (F). Follow-up sagittal images show marked improvement in cord signal change (D) as well as resolution of a large portion of the meningeal enhancement (E) after treatment.
He was treated with 5 days of intravenous (IV) methylprednisolone as well as 14 days of IV acyclovir. MRI spine after his steroid treatment showed significant improvement in cord edema but some persistent dural enhancement (Figure 1D, 1E). One month later, his CSF had normalized with unremarkable cytometry and pathology. Clinically, he regained almost full motor control and was ambulating well with the use of a cane.
Herpes simplex virus-2 is a neurotropic virus known to be primarily associated with genital herpes. It may rarely cause a spectrum of neurologic complications, including aseptic meningitis and myelitis.Reference Nardone, Versace and Brigo 1 , Reference Steiner, Kennedy and Pachner 2 Although HSV-2 myelitis is typically attributed to reactivation of the virus, lying dormant in dorsal root ganglia, recent work has shown that the virus may reach the spinal cord independent of dorsal root ganglia, perhaps via autonomic neurons.Reference Ohashi, Bertke, Patel and Krause 3 It is possible that in our case, the initial HSV-2 infection caused sequential aseptic meningitis and myelitis with detectable levels of HSV-2 occurring after the first CSF sample was taken.
Herpes simplex virus-2 myelitis has been reported to occur in patients with an underlying malignancy or immune deficiency and a recent literature review found only 11 case reports in immunocompetent individuals.Reference Nardone, Versace and Brigo 1 Similar to our patient, many presented without herpetic skin lesions or fever. The progression of symptoms and recovery was extremely variable, with the majority having persistent neurologic deficits. Our patient differed from this population in that he had striking hypoglycorrhachia and very high CSF protein. This is most often associated with bacterial or mycobacterial infection but has been reported in association with HSV-2 meningitis.Reference Brenton 4 In review of the literature, there were no reports of HSV-2 myelitis associated with hypoglycorrhachia and hyperproteinemia, however, given the absence of evidence to suggest co-existing malignancy, sarcoidosis or bacterial, mycobacterial or fungal infection, one may extrapolate that this finding was a direct effect of HSV-2 infection, particularly given the normalization of CSF glucose values on follow-up.
This case emphasizes the consideration of herpetic infections with hypoglycorrhachia and high CSF protein and reinforces the combination of acyclovir and methylprednisolone as an appropriate treatment regimen for this rarely encountered condition.
Disclosures
CT, CF, and KP have nothing to disclose.
Statement of Authorship
CT: Manuscript writing and review; CF: image acquisition and manuscript editing; KP: manuscript editing and supervision.



