Dietary proteins are frequently claimed to reduce appetite and then food intake during subsequent meals beyond what can be accounted for by their energy content in humans( Reference Anderson, Tecimer and Shah 1 – Reference Westerterp-Plantenga, Nieuwenhuizen and Tome 4 ). The mechanisms underlying these protein-induced satiety effects, involving different and complex pathways that include both indirect vagus-mediated signals and the direct sensing of blood amino acids (AA), nutrients and hormones by specific brain areas, are not fully understood( Reference Fromentin, Darcel and Chaumontet 5 ).
The current thinking is that proteins are more efficient than fats( Reference Porrini, Santangelo and Crovetti 6 ) or carbohydrates (CHO)( Reference Bertenshaw, Lluch and Yeomans 7 , Reference Poppitt, McCormack and Buffenstein 8 ) in inducing satiety. However, discrepancies have been observed between studies regarding the satiating capacity of proteins( Reference Raben, Agerholm-Larsen and Flint 9 ), although these could be attributed to methodological conditions that hamper the interpretation of the results, such as the macronutrient content and other food parameters, texture( Reference Wanders, Mars and Borgonjen-van den Berg 10 ), structure, energy level or the time period elapsing between the preload and the test meal( Reference Blundell, de Graaf and Hulshof 11 ). This period is frequently fixed, and only a few studies have observed the satiety response in human subjects who were free to request the next meal at any time( Reference Chapelot and Payen 12 – Reference Douglas, Ortinau and Hoertel 14 ). Subjects may also differ in their ability to sense nutrient and energy intakes and adapt their overall food intake accordingly, which may particularly be the case in overweight subjects or obese as opposed to lean subjects( Reference Brennan, Luscombe-Marsh and Seimon 15 , Reference Ho, Kennedy and Dimitropoulos 16 ). Lastly, different protein sources have also been suspected of differently influencing the induction of satiety, with contrasting results( Reference Anderson, Tecimer and Shah 1 , Reference Bowen, Noakes and Clifton 2 , Reference Lang, Bellisle and Oppert 17 , Reference Lam, Moughan and Awati 18 ). Particularly, the satiating effect of whey protein and casein in relation to their digestive kinetics and subsequent postprandial changes in plasma AA and hormones has been extensively addressed but remains controversial( Reference Hall, Millward and Long 3 , Reference Lorenzen, Frederiksen and Hoppe 19 , Reference Juvonen, Karhunen and Vuori 20 ). The direct link between satiety responses to milk protein fractions and their digestion rate can, however, be hardly challenged in a unique protocol due to the problems inherent in obtaining access to the gastrointestinal tract.
The present study was designed to assess whether the type of milk protein fractions, particularly differing in terms of their digestion kinetics, could modulate the satiating effect of a protein load in overweight subjects. For this purpose, the satiating effect of three different protein snacks (casein, whey protein or a 50:50 mixture of the two) was assessed in each subject against a basal CHO load, especially on the basis of the duration of satiety. Digestive kinetics and subsequent postprandial changes in plasma AA induced by different milk proteins were determined using a 15N-labelled meal test in a subgroup of subjects equipped with a jejunal tube.
Materials and methods
Participants and study design
All participants were certified to be in good health after a thorough physical examination performed by medical staff in the Human Nutrition Research Centre (HNRC) at Avicenne Hospital (Bobigny, France), as well as routine biochemical tests. The inclusion criteria were 25 < BMI < 30 kg/m2 and 18 < age < 40 years. The exclusion criteria were positive serology for HIV, hepatitis B and C virus, any pathology, allergy to dairy proteins, pregnancy or an absence of contraception in women. The purpose and potential risks of the study were fully explained to the subjects. The study was conducted accorded to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the Ethics Committee of Saint-Germain-en-Laye Hospital. Written informed consent was obtained from all participants. The study was registered at www.ClinicalTrials.gov (NCT00862329, SURPROL). It was performed in the HNRC at Avicenne Hospital under single-blind conditions according to a three-arm parallel design.
Subjects were recruited between March 2008 and June 2010, and recruitment was halted when the groups contained at least twenty-five subjects, including eight subjects for a digestive and metabolic investigation. Finally, eighty-two healthy overweight subjects took part in the study: thirty-eight females and forty-four males, with a mean BMI of 28 (sd 1·8) kg/m2 and a mean age of 29 (sd 7) years. The subjects were allocated by the data manager to one of three groups (n 26–28 per group): casein (CAS group); whey protein (WP group); a 50:50 mix of the two (MIX group). During the final third of the study, BMI, age and sex were used to homogenise the groups. Sex ratio, weight, BMI and BMR values did not differ between the groups (Table 1).
Table 1 Characteristics of the study subjects (Mean values and standard deviations)
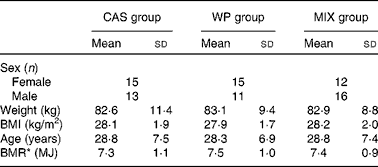
CAS, casein; WP, whey protein; MIX, equal mix of casein and whey protein.
* BMR was calculated from the equations of Harris and Benedict.
Protocol
For satiety, each subject completed a control (snack without protein) and treatment (snack with protein) test. The subjects were thus their own controls (Fig. 1). A subgroup of twenty-four subjects underwent a digestive and metabolic test after ingesting the protein snack (n 8 per group).

Fig. 1 Experimental design. Eighty-two subjects, divided into three groups (casein (CAS), whey protein (WP) and an equal mix of the two (MIX)), followed two satiety tests after ingestion of a control snack (without protein) on day 1 (D1) and a protein snack on day 7 (D7). A subgroup of twenty-four subjects underwent a digestive and metabolic exploration after ingesting the protein snack on day 10 (D10).
Satiety assessment
On day 1, the subjects underwent a control satiety test. They visited the HNRC in the morning after an overnight fast. The subjects were placed in a room with no time cues (closed curtains and no television, watch or personal computer). They were allowed to read and listen to pre-recorded music. They were given a standardised breakfast (1170 kJ) at 08.00 hours, including 120 ml skimmed milk, 30 g cornflakes, 100 ml orange juice, 20 g sugar, and tea or coffee, which they had to ingest in 30 min. At 11.00 hours, they had to ingest in 15 min a liquid control snack (1003 kJ) composed of 60 g maltodextrin (Roquette). The snack was dissolved in water to reach a final volume of 500 ml and flavoured with orange. After the control snack, the subjects were asked to request for lunch when they felt hungry. The meal proposed contained an excess quantity of food, including pasta, tomato sauce, cottage cheese, fruit salad and water. The time period elapsing between the snack and the spontaneous meal request was recorded, as was the energy intake at lunch. Regularly throughout the satiety test, the subjects completed visual analogue scales to evaluate their appetite feelings.
After the control test, volunteers returned home and were asked to consume every morning one liquid protein snack for 5 d. The protein snacks were isoenergetic with the control snack and composed of 30 g maltodextrin, and 30 g casein, whey protein or mix (1003 kJ). Proteins were purchased from Ingredia. The subjects were given five shakes containing the protein load powder and orange flavour for self-administration after its dissolution in 500 ml water. The nature of the protein load was not revealed to the subjects in respect of the single-blind design.
On day 7, the subjects came back to the HNRC for the same satiety test as on day 1, but with the protein snack. The satiating power of the protein snack was assessed in each subject as against the control snack. Satiety parameters were thus the main outcome of the study.
Digestive and metabolic measurements
On day 8, a subgroup of twenty-four subjects (n 8 per group) was used to investigate digestive, hormonal and metabolic parameters that might be associated with satiety. They were equipped with a double-lumen nasogastric tube that migrated to the proximal jejunum, as described previously( Reference Bos, Airinei and Mariotti 21 , Reference Bos, Juillet and Fouillet 22 ). The location of the sampling site was controlled by radiography. On day 9, after the subjects had fasted overnight, a solution of polyethylene glycol (PEG)-4000, used as a non-absorbable marker, was infused through the intestinal tube. After the baseline sampling of jejunal effluents and plasma, the subjects ingested their liquid protein snack in the same way as during the control satiety test, but in this case the proteins were intrinsically labelled with 15N, as described previously( Reference Lacroix, Bos and Leonil 23 ). Intestinal effluents were collected continuously on ice for 6 h after the snack, and blood samples were collected every 30 min for 3 h and hourly thereafter. The subjects were given 80 ml water every hour. At the end of this test period, the gastrointestinal tube was removed and the subjects returned home after eating a complete meal.
Analyses
Effluents were pooled into 30 min periods and freeze-dried until analysis. The concentration of PEG-4000 in digesta samples was measured using a turbidimetric method, as described previously( Reference Hyden 24 ), to determine the liquid flow rate. Enrichment of total N and 15N was determined by the elemental analysis-isotope ratio MS method, as described previously( Reference Boutrou, Gaudichon and Dupont 25 ).
Plasma AA were analysed by ion-exchange chromatography after protein precipitation, with the addition of norleucine as an internal standard (Biotech Instrument). The 15N enrichment of AA was determined by isotope ratio MS (IsoPrime, GV Instrument) coupled to an elemental analyser (Euro Elemental Analyser 3000; EuroVector), after purification on Dowex AG-50 X8 resin, as described previously( Reference Lacroix, Bos and Leonil 23 , Reference Airinei, Gaudichon and Bos 26 ). The dietary N level in plasma AA was calculated as follows:
where Nplasma AA was calculated from the sum of N in individual plasma AA (on the basis of concentrations) and the estimated volume of plasma as 5 % of body weight( Reference Gannong 27 ). APEsample and APEmeal are the 15N enrichment in the digesta and meal, respectively, where APE stands for atom percentage excess.
Concentrations of plasma insulin, glucagon, glucose-dependent insulinotropic peptide (GIP), glucagon-like peptide 1 (GLP-1), peptide YY and ghrelin (active form) were analysed using a human endocrine panel (Milliplex; Millipore) on a Bioplex 200 system (Bio-Rad Laboratories, Inc.). The intra-assay CV ranged from 5·5 % for GLP-1, insulin and peptide YY to 10 % for glucagon. The inter-assay CV ranged from 6·5 % for GIP to 23 % for GLP-1.
Statistical analyses
Data are presented as means with their standard errors. The number of subjects necessary to recruit was calculated from a power test based on the time period elapsing between the snack and request for lunch, using the software GPower 3.1. Only a few data were available in the literature to draw hypothesis on the differences between a CHO and protein snack, depending on the type of protein. In the study of Marmonier et al. ( Reference Marmonier, Chapelot and Fantino 28 ), the time period elapsing between the snack and meal request was 471 min after a CHO snack v. 429 min after a high-protein snack, associated with a within standard deviation of 30 min. We targeted differential effects of 10 min between two groups with a standard deviation of 25, resulting in an effect size of 0·35. It was then calculated that at least eighty subjects needed to be enrolled to ensure a statistical power of 80 % and thus to detect differences between the three groups using a one-way ANOVA, with an α level of 5 %.
The effect of qualitative (group and sex) and quantitative (BMI, weight, BMR and values of the control snack) variables on satiety parameters was also tested within ANOVA or ANCOVA models, using the generalised linear model procedure of SAS.
Changes in visual analogue scales after the snack were compared between the control and protein snacks using a mixed model with two repeated factors, time and test (PROC MIXED, SAS 9.1). The effects of the protein snack on digestive kinetics and hormones were compared between groups using a mixed model, with time as the repeated factor and BMR as a covariate, to take into account the fact that the amount of the snack was similar among subjects. P≤ 0·05 was taken as the criterion for statistical significance.
Results
Digestion kinetics
Flow rates of dietary N in the jejunum (Fig. 2(a)) differed significantly as a function of group, with a substantial delivery during the first 3 h after ingesting whey protein or mix while casein was delivered progressively in two phases during the first 3 h and between 4 and 5 h. The rate of appearance of dietary AA in plasma (Fig. 2(b)) reflected the rapid and slow gastric emptying of whey protein and casein, respectively, with an intermediate value for the mix of the two. Indeed, the casein snack did not trigger any peak for dietary AA but a progressive rise throughout the 6 h, whereas whey protein resulted in a massive appearance within 3 h. After the mix snack, a maximal appearance of dietary AA was observed as soon as 1 h and was followed by a plateau.

Fig. 2 Flow rates of dietary nitrogen in the jejunum (a) and dietary amino acid appearance in the plasma (b) in a subgroup of twenty-four subjects, after ingestion of a protein snack containing 15N-labelled milk proteins (casein (![]() ), whey protein (
), whey protein (![]() ) and an equal mix of the two (
) and an equal mix of the two (![]() )). Values are means (n 8 per group), with their standard errors represented by vertical bars. * Time point at which a group effect was observed (P< 0·05). The effects of group, time and interaction were tested in a mixed model with time as a repeated factor. There were significant effects observed for time (P< 0·0001, for both (a) and (b)) and the time × group interaction ((a) P= 0·001 and (b) P= 0·006).
)). Values are means (n 8 per group), with their standard errors represented by vertical bars. * Time point at which a group effect was observed (P< 0·05). The effects of group, time and interaction were tested in a mixed model with time as a repeated factor. There were significant effects observed for time (P< 0·0001, for both (a) and (b)) and the time × group interaction ((a) P= 0·001 and (b) P= 0·006).
Levels of gastrointestinal and pancreatic hormones were also determined (see Fig. S1, available online). The secretion of insulin and GIP in response to the ingestion of the protein snack was similar in the three groups, with a peak at 0·5 h to reach approximately 300 pmol/l and a return to the baseline at 3 h. Peptide YY, glucagon and GLP1 did not vary significantly over time while ghrelin levels gradually rose during the investigation. A trend for a group effect (P= 0·06) was obtained with GLP-1, with a globally lower secretion in the CAS group than in the other two groups.
Influence of snacks on satiety
Post-snack feelings of hunger (Fig. 3(a)) and fullness (Fig. 3(b)) did not differ between the groups (mixed model with time and snack as repeated factors). There was also no significant difference between the control and protein snacks.

Fig. 3 Feelings of hunger (a) and fullness (b) after ingestion of the control (n 82) or protein (n 26–28 per group) snack. The snack was given at t= 0 min. Values are means, with their standard errors represented by vertical bars. There was no significant effect observed for snack (protein v. control (![]() )) or the type of protein snack (casein (
)) or the type of protein snack (casein (![]() ), whey protein (
), whey protein (![]() ) and an equal mix of the two (
) and an equal mix of the two (![]() ); mixed model with protein as a factor and time as a repeated factor).
); mixed model with protein as a factor and time as a repeated factor).
The time period elapsing between the control CHO snack and request for lunch was 133 (sem 7) min (median 126 min, range 15–385 min). The time period elapsing after the protein snack, whatever the type of protein, was 150 (sem 7·5) min (median 150 min, range 15–345 min). As a result, the protein snack significantly increased this period by 17 (sem 7) min compared with the control snack (P= 0·02). There was no significant effect of group (CAS, MIX or WP) on either the time period elapsing after the protein snack or the difference from the values obtained after the control snack, as shown in Fig. 4(a). Besides, the time period elapsing after the protein snack was significantly dependent on the time period elapsing after the control snack (ANCOVA, P< 0·0001), indicating that the duration of satiety was strongly subject-dependent. In contrast, there was no effect of subject-related variables such as sex, body weight, BMI or BMR.

Fig. 4 Effects of the protein snack v. the control carbohydrate snack on the time period elapsing before the request for lunch in all subjects (n 82) (a) and in early eaters (n 41) (b). The satiating effect of the protein snack is represented whatever the type of protein snack as well as in each protein group (intention-to-treat, n 26–28; early eaters, n 10–16). Data are expressed as the difference between the values obtained after ingestion of the protein snack and the control snack, respectively. Early and late eaters were split on the basis of the time period elapsing after ingesting the control snack (cut-off point: median 126 min). Values are means, with their standard errors represented by vertical bars. Mean value was significantly different from 0 min: * P≤ 0·05, ** P≤ 0·01, *** P≤ 0·001. There was no effect observed for the protein group (ANOVA with group as a factor). CAS, casein; MIX, equal mix of casein and whey protein; WP, whey protein.
Owing to the significant relationship between the control test and the satiety response to the protein snack, a split analysis was performed in subjects displaying an initially short time period (early eaters) or long time period (late eaters) for meal request after the control snack (cut-off point: median 126 min). In early eaters, the time period for lunch request after the protein snack was increased by 32·4 (sem 7·5) min (P= 0·0001), whereas in late eaters, the protein snack did not have any effect. Although the ANOVA did not reveal any significant effect of the type of protein snack on the delay in this subgroup of subjects (Fig. 4(b)), the extension of the duration of satiety compared with the control snack was only significant in the WP and MIX groups, but not in the CAS group.
Energy intake at lunch was 4·2 (sem 0·1) MJ after the control snack (median 4·1 MJ, range 1·8–6·5 MJ). After the protein snack, energy intake at lunch was 4·2 (sem 1·3) MJ and did not differ compared with the control snack. Energy intake after the protein snack was not influenced by the type of protein but by the energy intake at the control test (ANCOVA, P< 0·0001). In contrast to the time period elapsing between the snack and request for lunch, subject-related variables influenced energy intake at lunch, such as sex (P= 0·0005) and BMR (P= 0·0007). Baseline energy intake was also positively linked to weight (R 0·027, P= 0·01) but not to BMI. Energy intake at lunch was not influenced by the length of time after the control snack.
Discussion
The present study addressed the sensitivity of overweight subjects to the satiating effect of different milk protein snacks, differing in terms of protein type and consequently digestive kinetics. Milk proteins, whatever their type, moderately but significantly extended the appetite-suppressant effect of a liquid CHO snack. A post hoc analysis revealed that the satiating effect of proteins were only efficient in subjects displaying a short duration of satiety. The absence of any effect of milk protein fractions despite the marked difference in their digestion kinetics indicates that in our experimental conditions, the modulation of the kinetics of dietary AA delivery did not influence the duration of satiety.
Although kinetic profiles were modulated between the three different protein snacks, displaying marked differences in the rate of appearance of dietary AA in plasma, there was no difference between the protein sources in relation to the effect on the duration of satiety. This modulation was characterised in a subgroup of subjects, but not at the same time as the satiety test, because the presence of the intestinal tube was not compatible with assessing satiety. At the jejunal level, the half delivery time of dietary N was 50 min with whey protein and 2 h with casein, but with an estimated complete emptying time (based on cumulated recovery) of 2·5 h with whey protein and 6 h with casein. The mix snack displayed similar kinetics to that for whey protein (half delivery time of 1 h) but with an estimated complete digestion time of 4 h. The appearance of 15N in plasma AA provided a good discrimination of kinetics, with a peak time at 90 min for whey protein and the absence of a peak for casein, while the mix of the two displayed an early maximum (1 h) followed by a plateau. These digestive and plasma AA kinetic profiles are in agreement with those observed previously( Reference Lacroix, Bos and Leonil 23 , Reference Boirie, Dangin and Gachon 29 , Reference Mahé, Benamouzig and Gaudichon 30 ), with the peculiarity that due to clotted aggregates in the stomach, casein did not trigger a plasma AA peak. We were thus able to verify that mixing casein and whey protein in equal proportion produced an intermediate kinetic.
Despite these differences, there were no effects on appetite rating. Several studies have compared the satiating effects of different milk proteins, of which the most widely studied are whey protein and casein or total milk proteins. However, the results of the studies reported to date have been contradictory. Some authors have found a stronger effect of whey protein( Reference Hall, Millward and Long 3 ), casein( Reference Abou-Samra, Keersmaekers and Brienza 31 ) or total milk proteins( Reference Lorenzen, Frederiksen and Hoppe 19 ), or no difference( Reference Bowen, Noakes and Clifton 2 ). This latter study was performed in overweight women in order to compare liquid preloads (milk shake, 1·1 MJ) containing 50 g whey protein, soya, gluten or glucose. Energy intake during a subsequent buffet meal was lower by 10 % 3 h after ingesting all the protein preloads when compared with the glucose treatment, but without there being any differences between the protein sources. It should be noted that unlike the present experiment, all these studies implemented a fixed period between the load and the meal. Interestingly, in young men, a modification to food texture by increasing the viscosity of a liquid casein snack using transglutaminase increased fullness compared with a liquid casein or whey protein snack, but decreased the secretion of GLP-1 and cholecystokinin( Reference Juvonen, Karhunen and Vuori 20 ). In the present study, no differences between casein and whey protein were observed, although the total gastric emptying time after ingestion of the casein snack was delayed by about 4 h. This could suggest that an excessive slowing of digestive kinetics, together with a high-protein dose (50 g), is necessary to obtain an effect on the duration of satiety. Furthermore, it shows that higher peaks of plasma GLP-1 and cholecystokinin observed during the early postprandial phase are not necessarily linked to a stronger satiating effect.
We also did not find any marked effects of the type of protein snack on hormonal profiles. A high variability was observed, especially for gastrointestinal hormones, which may have been partly due to the presence of the intestinal tube and to the fact that the energy load of the snack was unique whatever the corpulence of the subjects, resulting in the contribution of 10 to 17 % to the BMR, although without any differences between the protein groups. Insulin and GIP were principally triggered by the presence of maltodextrin (30 g) in the snack, buffering the insulinotropic capacity of whey protein( Reference Calbet and MacLean 32 ). The only effect on GLP-1 was global, without affecting the kinetics, with a lower level in the CAS group. The absence of difference between casein and whey protein is in line with most previous findings( Reference Bowen, Noakes and Clifton 2 , Reference Juvonen, Karhunen and Vuori 20 , Reference Calbet and Holst 33 ), but not all( Reference Hall, Millward and Long 3 ). However, all these studies were performed with 50 g protein without CHO, whereas the present study used a dose more compatible with a supplementation strategy, meaning 30 g. At a lower dose (15 g), higher levels of GLP-1 and cholecystokinin secretion have been reported after ingestion of total milk proteins than that of whey protein( Reference Diepvens, Haberer and Westerterp-Plantenga 34 ).
The present study also addressed the effect of the protein snack against the CHO snack, and particularly in relation to the duration of satiety, a parameter that has been scarcely assessed. In one study, an increase in time period elapsing after a high-protein load compared with a high-CHO load( Reference Marmonier, Chapelot and Fantino 28 ) has been reported; however, most of the investigations were performed with a fixed time interval between the load and the meal. We found that the duration of the satiating effect was increased by approximately 17 min when the snack contained proteins, in comparison to an isovolumic and isoenergetic (1 MJ) snack containing maltodextrin. The result of the present study is consistent with that of Douglas et al. ( Reference Douglas, Ortinau and Hoertel 14 ) who found an increase in the duration of satiety from 150 min with a low-protein yogurt to 180 min with a high-protein one. In contrast, there was no effect on energy intake at lunch. This illustrates the fact that because the period was not fixed and subjects received their lunch when they were hungry, this criterion was no longer discriminating. Chungchunlam et al. ( Reference Chungchunlam, Moughan and Henare 35 ) did not find a significant effect of the timing of the preload varying from 30 to 120 min on energy intake; however, this does not preclude the fact that after 120 min, the effect of the preload altered the result. Moreover, by contrast, with the time period elapsing after the snack, we found that energy intake was influenced by sex, as shown previously( Reference Lam, Moughan and Awati 18 ), and also by BMI and BMR, thus increasing the factors influencing this criterion. Therefore, under the present experimental conditions, energy intake at meal was not a sensitive criterion, while the time period elapsing was the most appropriate one. As stated by Blundell et al. ( Reference Blundell, de Graaf and Hulshof 11 ), the period during which a preload maintains a state of satiety can be considered as a good indicator to assess its satiety power. Interestingly, we found that the periods obtained after the protein snack varied considerably (15–385 min) and were strongly dependent on the periods observed following the control snack. We thus reanalysed the results after splitting the subjects as early and late eaters, and found that the duration of the satiating effect of proteins was extended by 32 min in early eaters but not in late eaters. This shows that the efficacy of proteins in extending the duration of satiety depends on their sensitivity to an energy load. In subjects in whom a non-protein snack triggers a sufficient duration of satiety, proteins do not exert any additional benefit. Interestingly, it has recently been shown that the usual mealtime interval of subjects influences the extent to which they could lose weight during dietary intervention( Reference Garaulet, Gomez-Abellan and Alburquerque-Bejar 36 ).
This split was performed with two subgroups in order to retain sufficient statistical power to further test the effect of proteins. However, an analysis with three subgroups produced similar results, with a significant effect of proteins in early and medium eaters, and no effect in late eaters. The present study thus provides new insights that should be investigated specifically in further studies. Lastly, the split analysis suggests that the presence of whey protein could extend the duration of satiety in early eaters. Indeed, compared with the CHO snack, the satiating effect of proteins was only significant in the WP and MIX groups, but we were unable to find a group effect, probably due to a loss of statistical power subsequent to splitting. A specific study on subjects recruited for their ability to respond to an energy load would therefore be necessary to ascertain a higher satiating power of whey protein in subjects displaying a short duration of satiety.
The present study was performed using an original approach that could investigate in the same subjects both the jejunal flux of dietary protein and the satiety response to a snack. A second original approach was that the design of the present study first included a control satiety test, allowing the classification of subjects in the absence of any protein in the snack, and then a second test to determine the additional effect of proteins, after habituation to the snack. Because of this original approach, the present study had some limitations. In particular, a cross-over design could not be implemented, leading to a loss of statistical power for between-group analysis. Furthermore, we could not verify the effect of snack order. Lastly, although the subjects were habituated to the snack – a design that has been rarely employed in other studies – the habituation period remained short. It would be interesting to evaluate the effect of a longer adaptation period and its subsequent impact on food consumption and weight. To our knowledge, very few studies have assessed this long-term effect. A sustainable effect on hunger feelings has been reported in subjects regularly consuming a satiating snack for 18 weeks after weight loss, together with better weight maintenance( Reference Diepvens, Soenen and Steijns 37 ); however, this result cannot be generalised. The present study focused on the duration of satiety and did not allow for any speculation regarding its possible effects on food intake.
In conclusion, the kinetics of dietary AA delivery do not play a major role in satiety responses. The present results also show that the effect of proteins in inducing satiety needs to be interpreted in terms of target subjects, particularly relative to their sensitivity to satiety. The variability in the duration of satiety was extremely high among individuals, as has already been shown for daily energy intake between individuals of the same sex, age, body composition and activity levels( Reference George, Tremblay and Despres 38 ), and we showed that proteins were efficient when compared with CHO alone in early eaters. To our knowledge, this is the first study to demonstrate that sensitivity to satiety (short or long satiety period) influences the effect of specific macronutrients on satiety responses. Such new criteria could be taken into account when using loads enriched with macronutrients for the control of energy intake.
Supplementary material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S0007114514001470
Acknowledgements
The authors thank Marie Claude Amar from the Volunteer Research Center at Avicenne Hospital for recruiting the subjects and collecting the samples, as well as Laure Chevalier and Cécile Bos for their active participation in the study.
The authors are indebted to the Corrine Marmonier and Jean-Pierre Guyonnet, from the CNIEL, for constructive scientific discussions.
The present study was supported by a grant from the CNIEL and the French Agency for Research and Technology (Program PNRA 2006; SURPROL). The authors are participants of the EU-funded COST action INFOGEST (COST FA 1005).
The authors' contributions are as follows: A. M.-B. and C. G. designed the research; A. M.-B., C. G., G. A., C. P., J. P., R. B. and D. R. conducted the research; J. L. supplied the labelled milk proteins; C. G., A. M.-B. and C. P. analysed the data; A. M.-B., C. G., G. F. and D. T. wrote the paper; A. M.-B. and C. G. had primary responsibility for the final content. All authors read and approved the final version of the paper.
The authors declare that there are no conflicts of interest.







