Iodine is an essential micronutrient for the human body and an integral component of thyroid hormones. Iodine plays a physiological role in thyroid hormones, promotes the metabolism of substances and energy and promotes growth and development(Reference Ma, Skeaff and Pearce1,Reference Eastman and Zimmermann2) . Iodine intake must be within a certain range; if too low or too high, iodine will have adverse effects on the body(Reference Yadav and Pandav3,Reference Leung and Braverman4) .
Over the past two decades, the universal salt iodisation (USI) strategy has been recognised as a safe, economic and sustainable policy to eliminate iodine deficiency diseases (IDD). China is one of the most seriously affected areas of IDD in the world. In 1995, China began to implement USI, which has played a major role in eliminating IDD(Reference Shen5,Reference Liu, Wang and Liu6) .
In recent years, there have been a number of reports of iodine overdoses leading to autoimmune thyroiditis(Reference Luo, Kawashima and Ishido7–Reference Armando, Lidia and Norman9). At the same time, it has been shown in domestic epidemiological investigations that the nationwide fortification of iodine can also increase the prevalence of thyroid diseases, especially hyperthyroidism(10–Reference Wassie, Yelland and Smithers12). Some studies have reported that long-term fortification of iodine after iodine deficiency will have an impact on thyroid autoimmune status(Reference Lind, Kumnig and Heinisch13,Reference Pedersen, Knudsen and Jorgensen14) ; however, similar studies are generally small in scale and quantity, and there is a lack of large sample epidemiological studies in China. So, what is the status of thyroid disease in China’s iodine-deficient (ID) areas after 25 years of USI? What is the thyroid immune status in this region?
In the current study, we conducted an epidemiological investigation of abnormal thyroid antibodies in three regions of China with different water iodine contents. The iodine nutritional status of people in the different water iodine areas was determined, and the relationship between serum iodine, urine iodine and thyroid function in patients with abnormal thyroid antibody was ascertained to provide theoretical bases for the prevention of autoimmune thyroid disease (AITD).
Materials and methods
Survey areas and subjects
In this survey, three geographic areas with different iodine levels were selected, including ID areas with a median water iodine (MWI) level <10 μg/l, iodine-adequate (IA) areas with a MWI between 40 and 100 μg/l and the iodine-excess (IE) areas with a MWI > 300 μg/l(Reference Jia, Zhang and Shen15). A MWI ≤ 10 μg/l is defined as an ID area according to the Chinese national standard(Reference Shen, Su and Ge16). Iodised salt was provided in the ID areas included in this survey, and the coverage of household use of qualified iodised salt was >90 % according to the Chinese national standard(Reference Zhang, Chen and Ge17). Iodised salt was not provided in the IA and IE areas.
A multi-stage stratified random sampling method was used in this study. Based on the available data from the most recent national IDD surveillance and water iodine surveillance in China(18), Shandong Province was selected as the survey area. In the first stage, one district/county was randomly selected from the three different water iodine regions in Shandong Province. In the second stage, one town was randomly selected in each district/county. In the third stage, according to the expected sample size, 1–2 villages were randomly selected from each town. In the final stage, individuals meeting the inclusion criteria in the selected villages were randomly selected as the subjects of the study.
Based on the sampling method, Dongtan and Qianlv villages in Jiaxiang County located in Jining City were selected as the ID areas. Liuxiangzhuang and Dongding villages in Weishan County of Jining City were selected as IA areas, and Jieyuanji village in Mudan District of Heze City was selected as the IE area. These villages are located in the southwestern part of Shandong Province in China (Fig. 1).

Fig. 1. Geographical distribution of survey areas in Shandong Province of China. ![]() , Median water iodine (MWI) ≤ 10 μg/l (iodised salt fortification);
, Median water iodine (MWI) ≤ 10 μg/l (iodised salt fortification); ![]() , 40 < MWI < 100 μg/l;
, 40 < MWI < 100 μg/l; ![]() , MWI > 300 μg/l;
, MWI > 300 μg/l; ![]() , river;
, river; ![]() , city boundary.
, city boundary.
More than 20 years has elapsed since the USI was implemented in China; the prevalence of AITD varies widely across areas with different water iodine levels(Reference Jin, Guan and Shen19–Reference Hou, Liu and Wang21). At the same time, the prevalence of AITD in the survey areas has not been previously reported. This study was cross-sectional in design, and the sample size was computed using the following formula: N = Z 2α/2 P (1 − P)/d 2 (α = 0·05, P = 0·5, d = 0·05)(Reference Aday and Cornelius22). Based on the calculation, the expected sample for each area was at least 384 adults.
Finally, a total of 1225 adults were recruited in this study. Of the 1225 adults enrolled in this study, 409, 392 and 424 were from the ID, IA and IE areas, respectively. The recruitment criteria included residing in the survey areas for more than 5 years and 18–60 years of age. To control for confounding factors, the following were excluded: patients with congenital thyroid disease and taking anti-thyroid drugs or thyroxine in the past year; patients with autoimmune, endocrine, heart, chronic and family genetic diseases; and pregnant women. The three areas mentioned above were villages with similar living standards, dietary habits and environmental conditions.
Survey methods and indicators
A standard questionnaire was used to collect information on the demographic characteristics, including sex, name, age, BMI (weight/height2 (kg/m2)), education level, family income, MWI, current smoking status, alcohol consumption, radiation history (including whether or not radiation examinations have been performed in the past 10 years, whether or not there is a source of radiation near the residence, and the frequency of using computers and mobile phones) and a personal or family history of thyroid disease (including type of thyroid disease). The questionnaire was administered by trained research assistants in face-to-face interviews. The BMI was classified according to the Chinese Public Health Standards as follows: underweight, BMI < 18·5 kg/m2; normal weight, 18·5 ≤ BMI < 24·0 kg/m2; overweight, 24 ≤ BMI < 28·0 kg/m2; and obese, BMI ≥ 28·0 kg/m2(Reference Chen, Zhao and Yang23). According to the WHO, an individual is a smoker if he/she smokes at least one cigarette per day for >1 month, and if a person drinks at least once a week for >1 year, the individual is considered an alcohol consumer.
Based on the recommendation from the WHO, urinary iodine concentration (UIC) and thyroid-stimulating hormone (TSH) levels were used to evaluate the iodine nutrition status of the population(24). In view of the limitations of these indicators, free triiodothyronine (FT3), free thyroxine (FT4), thyroglobulin antibody (TGAb) and thyroid peroxidase antibody (TPOAb) levels were also measured. In addition, given the sensitivity of serum iodine in evaluating individual iodine nutrition status, the stability of population iodine nutrition status and the significance of screening in people at high risk for thyroid disease, serum iodine was also included for testing(Reference Jin, Jiang and Liu25). Thyroglobulin (Tg) is a biomarker of iodine deficiency and iodine nutrition status(Reference Ma and Skeaff26,Reference Du, Gao and Feng27) . However, a wide range of methods are used to analyse Tg, making it difficult to compare studies(Reference Ma and Skeaff26). Therefore, Tg was not determined in the present study. Moreover, we collected salt samples from all participants for the determination of salt iodine content.
This project was approved by the Ethical Review Board of Harbin Medical University (no. hrbmuecdc20200320). Written informed consent was obtained from all participants before the survey was conducted.
Water sample collection and iodine determination
Before the survey was conducted, water iodine levels were determined to verify whether or not the three areas met the criteria for inclusion in the study. The samples of water were collected according to the Chinese national standard(28). Samples of the daily drinking water of participants were collected in clean plastic tubes. For ID areas (Dongtan and Qianlv villages) with a dispersed water supply, five samples each were collected from the east, west, south, north and central locations in each village. In total, ten samples were collected from the ID areas. For IA (Liuxiangzhuang and Dongding villages) and IE (Jieyuanji village) areas with a centralised water supply, two parallel samples (the average value was calculated) were collected from each village, giving a total of six samples from the IA and IE areas. Each water sample was at least 15 ml. The water samples were kept at room temperature, and the determination was completed within 24 h. The iodine concentration of drinking water was determined using the method of As3+–Ce4+ catalytic spectrophotometry recommended by the Chinese National Reference Laboratory for Iodine Deficiency Disorders, Chinese Centre for Disease Control and Prevention(Reference Wang, Liu and Li29).
Salt sample collection and iodine determination
Salt samples were collected from all of the participants in the three areas. Each participant provided at least 50 g of table salt in a clean, labelled ziploc bag. The iodine content in the salt samples was determined using the general test method of the salt industry(Reference Tong and Huo30). The standard salt iodine content in the ID areas of Shandong Province was 25 mg/kg, and non-iodised salt was supplied in IA and IE areas. The salt type was classified into the following categories: non-iodised salt (salt iodine content < 5 mg/kg), qualified iodised salt (18 mg/kg ≤ salt iodine content ≤ 33 mg/kg) and unqualified iodised salt (5 mg/kg < salt iodine content <18 or >33 mg/kg).
Urine sample collection and iodine determination
From each participant, the fasting single-spot urine sample was collected in the morning (08.00–11.00 hours) in clean, well-labelled plastic tubes and stored at 4°C. The test was completed within 2 weeks. UIC was measured according to the China Health Standard Method of Determination of Iodine in Urine by As3+–Ce4+ catalytic spectrophotometry(Reference Zhang, Yan and Liu31).
Blood sample collection and determination
Five millilitres of venous blood (no anticoagulant) was collected from the study subjects and centrifuged at 3000 g after standing at room temperature for 2 h. Serum was separated and stored in a low-temperature refrigerator at –80°C.
Serum iodine concentration (SIC) was measured using an inductively coupled plasma-MS system (PerkinElmer NexION 350). The levels of FT3, FT4, TSH, TPOAb and TGAb were determined using chemiluminescent immunoassay (Siemens Healthcare Diagnostics Inc.).
The reference values were 52–109 µg/l SIC (Quest Diagnostics(Reference Han, Wu and Yu32)), 3·1–6·8 pmol/l FT3, 11·5–22·7 pmol/l FT4, 0·27–4·2 µIU/ml TSH, 0–60 U/ml TPOAb and 0–60 U/ml TgAb. In addition, experienced radiologists used portable ultrasound instruments equipped with 7·5 MHz transducers to evaluate thyroid glands. TPOAb and TGAb are of great significance in the diagnosis of autoimmune thyroiditis. Weetman(Reference Weetman33) considered that pure TGAb, TPOAb-positive and double antibody-positive indicated a diagnosis of AITD. Thus, the diagnostic criteria for thyroid disease were as follows: hypothyroxinaemia: FT4 < 11·5 pmol/l and TSH within the normal range; overt hypothyroidism: TSH > 4·20 µIU/ml and FT4 < 11·5 pmol/l; subclinical hypothyroidism: TSH > 4·20 µIU/ml and FT4 within the normal range; overt hyperthyroidism: TSH < 0·27 µIU/ml, FT4 > 22·7 pmol/l and FT3 > 6·8 pmol/l; subclinical hyperthyroidism: TSH < 0·27 µIU/ml, and FT3 and FT4 within the normal range; AITD: TPOAb (+)/TGAb (+) (TPOAb- and/or TGAb-positive) and both test values >60 U/ml were positive; and goitre: thyroid volume >25 ml (male) and >18 ml (female). All of the above thyroid diseases, except AITD, include individuals with positive thyroid antibody titers.
Statistical analysis
Statistical analyses were performed using Excel 2016 and SPSS software (version 17.0) for Windows. Non-normally distributed data are expressed as medians and interquartile ranges. The three groups were compared using one-way ANOVA on normally distributed variables. The variables with a skewed distribution were assessed with the Kruskal–Wallis test. If a difference was found between multiple groups, a post hoc examination was performed accordingly. The χ 2 test was adopted for comparing the rates between groups with categorical outcomes. A binary logistic regression model was applied to calculate the OR and 95 % CI for AITD from the three areas. The model for AITD was adjusted for age, sex and BMI. A P < 0·05 was considered statistically significant.
Results
Demographic characteristics of the adults included in this study
The demographic characteristics of the adults in the three areas are presented in Table 1. In this study, the MWI concentration in the ID, IA and IE areas was 2·6, 71·4 and 325·0 μg/l, respectively. The coverage of household use of qualified iodised salt in the ID areas was 93·9 %, which is in agreement with the evaluation content and criteria for the elimination of IDD in China (coverage of household use of qualified iodised salt > 90·0 %)(Reference Shen, Su and Ge16). Except for five iodised salt samples detected in IA areas (participants who provided iodised salt will be excluded in later data analyses), other salt samples in the IA and IE areas were in agreement with Chinese national standards (non-iodised salt should be provided to IA and IE areas).
Table 1. Demographic characteristics, urinary iodine, serum iodine and thyroid function in three areas
(Normally distributed mean values and standard deviations; non-normally distributed medians and 25th and 75th percentiles (P25–P75), and percentages)*

ID, iodine-deficient areas; IA, iodine-adequate areas; IE, iodine-excess area; MWI, median water iodine; UIC, urinary iodine concentration; SIC, serum iodine concentration; FT3, free triiodothyronine; FT4, free thyroxine; TSH, thyroid-stimulating hormone; TPOAb, thyroid peroxidase antibody; TGAb, thyroglobulin antibody; TPOAb (+), thyroid peroxidase antibody-positive; TGAb (+), thyroglobulin antibody-positive; TPOAb (+) & TGAb (+), TPOAb- and TGAb-positive.
* The Kruskal–Wallis test was adopted for UIC, SIC, FT3, FT4, TSH; the χ 2 test was used for TPOAb (+), TGAb (+), and TPOAb (+) & TGAb (+). P < 0·05 was considered significant.
† ID and IE groups compared with the IA group, respectively. ID group compared with the IA group: SIC (Z = −2·403, P = 0·016), TSH (Z = −3·399, P = 0·001), TGAb (+), n (%) (χ 2 = 11·735, P = 0·001) and IE group compared with the IA group: UIC (Z = −9·606, P < 0·001), SIC (Z = −5·664, P < 0·001), FT4 (Z = −6·751, P < 0·001).
‡ IE group compared with the ID group. UIC (Z = −11·920, P < 0·001), SIC (Z = −3·194, P = 0·001), FT3 (Z = 13·271, P < 0·001), FT4 (Z = 74·320, P < 0·001), TSH (Z = 14·809, P < 0·001), TGAb (+), n (%) (χ 2 = 9·740, P = 0·002).
Iodine nutrition status and thyroid function in adults
The UIC and thyroid function status of adults in the three areas are shown in Table 1. The UIC of the adults in the ID, IA and IE areas was 228·4, 243·9 and 390·4 μg/l, respectively. According to the recommendations of the WHO/UNICEF/ICCIDD(24), the iodine status of ID and IA areas was sufficient, while the iodine status in the IE area was excessive.
The median urinary iodine and SIC, FT3, FT4, TSH levels, and TGAb-positive rate of adults in the ID, IA and IE areas differed significantly (H = 160·425, P < 0·001; H = 32·354, P < 0·001; H = 13·630, P = 0·001; H = 82·974, P < 0·001; H = 17·768, P < 0·001 and χ 2 = 15·551, P < 0·001, respectively).
The urine iodine and serum iodine levels in the IE area were significantly higher than the IA areas (Z = −9·606, P < 0·001 and Z = −5·664, P < 0·001, respectively), and the serum iodine level in ID areas was significantly higher than the IA areas (Z = −2·403, P = 0·016). Compared with ID areas, the IE area had higher urinary iodine and serum iodine levels (Z = −11·920, P < 0·001 and Z = −3·194, P = 0·001, respectively).
Compared with the ID areas, the FT3 level in the IE area was significantly lower (Z = 13·271, P < 0·001). Compared with the levels in the IA and ID areas, the FT4 level in the IE area was significantly higher (Z = −6·751, P < 0·001 and Z = 74·320, P < 0·001, respectively). Compared with the IA areas, the TSH level in the ID areas was significantly lower (Z = −3·399, P = 0·001), while the TSH level in the IE area was significantly higher than the ID areas (Z = 14·809, P < 0·001). No statistical difference was detected in the TPOAb-positive rate and double antibody-positive rate. Compared with the IA areas, the TGAb-positive rate in the ID areas was significantly higher (χ 2 = 11·735, P = 0·001), while the TGAb-positive rate in the IE area was significantly lower than the ID areas (χ 2 = 9·740, P = 0·002).
Thyroid disease in the three areas
In the ID, IA and IE areas, the prevalence of subclinical hypothyroidism and AITD differed significantly among adults (χ 2 = 15·847, P < 0·001 and χ 2 = 14·885, P = 0·001, respectively). Compared with the IA areas, the prevalence of subclinical hypothyroidism in the ID areas was significantly lower (χ 2 = 4·991, P = 0·025), but significantly higher in the IE area than the ID areas (χ 2 = 15·853, P < 0·001). The prevalence of AITD was significantly higher in the ID areas than the IA areas (χ 2 = 12·783, P < 0·001). Compared with the ID areas, the prevalence of AITD in the IE area was lower (χ 2 = 7·666, P = 0·006). However, we observed no significant difference in the prevalence of other thyroid diseases among the three areas (Table 2).
Table 2. Prevalence of thyroid disease among adults in three areas
(Numbers and percentages)*
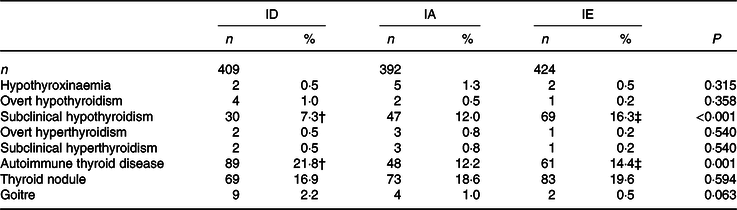
ID, iodine-deficient areas; IA, iodine-adequate areas; IE, iodine-excess area.
* The χ 2 test was used for hypothyroxinaemia, overt hypothyroidism, subclinical hypothyroxinaemia, overt hyperthyroidism, subclinical hyperthyroidism, autoimmune thyroid disease, thyroid nodule and goitre. P < 0·05 was considered significant.
† ID, IE groups compared with the IA group, respectively and P < 0·05. ID group compared with the IA group, P < 0·05. Subclinical hypothyroidism (χ 2 = 4·991, P = 0·025) and autoimmune thyroid disease (χ 2 = 12·783, P < 0·001).
‡ IE group compared with the ID group, P < 0·05. Subclinical hypothyroidism (χ 2 = 15·853, P < 0·001), autoimmune thyroid disease (χ 2 = 7·666, P = 0·006).
Thyroid function, and urinary and serum iodine levels in autoimmune thyroid disease and thyroid peroxidase antibody (−) & thyroglobulin antibody (−) adults in the three areas
Thyroid function, and urinary and serum iodine levels in AITD and TPOAb (−) & TGAb (−) (TPOAb- and TGAb-negative) adults from the three areas are shown in Table 3. Significant sex differences were observed between the AITD group in the ID, IA and IE areas when compared with the TPOAb (−) & TGAb (−) group (χ 2 = 21·013, 5·458 and 6·259; P ≤ 0·001, 0·019 and 0·012, respectively). The proportion of AITD in the females in each area was higher than males (ID, 27·6 v. 6·9 %; IA, 14·7 v. 6·2 %; and IE, 17·3 v. 8·1 %). There was no significant difference in age between the three areas. However, the FT3 level in the AITD group in the ID and IE areas was significantly lower than the TPOAb (−) & TGAb (−) group (Z = −2·467 and −2·040; P = 0·014 and 0·041, respectively). Furthermore, the FT4 level in the AITD group was significantly lower in the IA areas (Z = −2·028, P = 0·043). Comparatively, the TSH level in the AITD group in each of the three areas was higher than the level in the TPOAb (−) & TGAb (−) group, although these differences were only significant in the ID and IE areas (Z = −3·621 and −3·580; and all P < 0·001, respectively). The BMI, SIC and UIC showed no statistical differences.
Table 3. Thyroid function, urinary iodine and serum iodine in adults of autoimmune thyroid disease (AITD) and thyroid peroxidase antibody (TPOAb) (−) & thyroglobulin antibody (TGAb) (−) in three areas
(Normally distributed mean values and standard deviations; non-normally distributed medians and 25th and 75th percentiles (P25–P75), and percentages)*
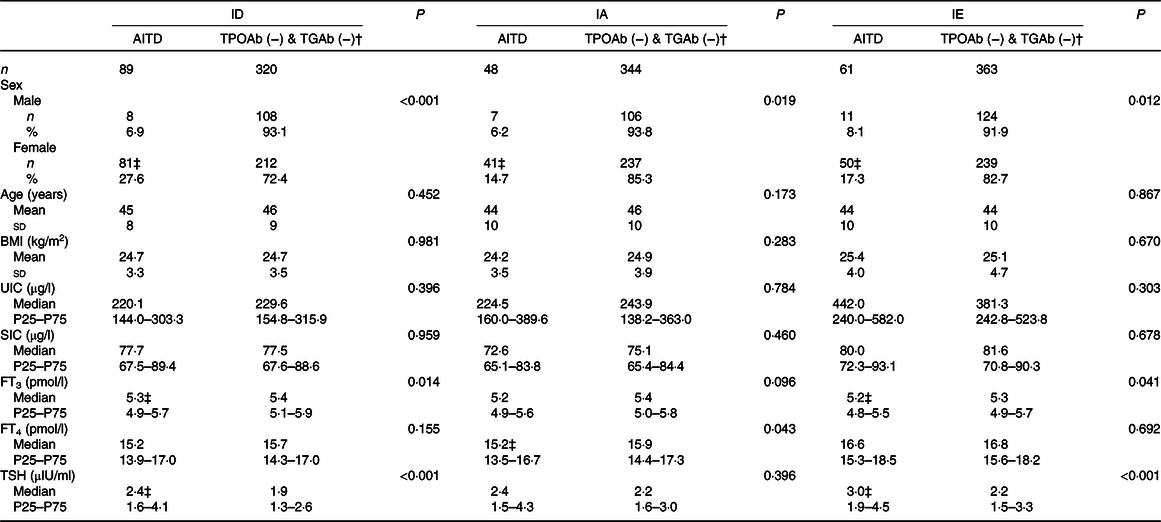
ID, iodine-deficient areas; IA, iodine-adequate areas; IE, iodine-excess area; TPOAb (−) & TGAb (−), TPOAb- and TGAb-negative; UIC, urinary iodine concentration; SIC, serum iodine concentration; FT3, free triiodothyronine; FT4, free thyroxine; TSH, thyroid-stimulating hormone.
* The χ 2 test was used for sex; the independent-samples t test was used for age, BMI; the Mann–Whitney U test was adopted for UIC, SIC, FT3, FT4 and TSH. P < 0·05 was considered significant.
† TPOAb (−) & TGAb (−) group as the control in each area.
‡ AITD group compared with TPOAb (−) & TGAb (−) group in each area, P < 0·05. ID: sex (χ 2 = 21·013, P < 0·001), FT3 (Z = −2·467, P = 0·014), TSH (Z = −3·621, P < 0·001; IA: sex (χ 2 = 5·458, P = 0·019), FT4 (Z = −2·028, P = 0·043); IE: sex (χ 2 = 6·259, P = 0·012), FT3 (Z = −2·040, P = 0·041), TSH (Z = −3·580, P < 0·001).
Thyroid disease distribution of autoimmune thyroid disease and thyroid peroxidase antibody (−) & thyroglobulin antibody (−) in adults in the three areas
The thyroid disease distribution among the AITD and TPOAb (−) & TGAb (−) adults in the three areas is shown in Table 4. Comparatively, the prevalence of any form of thyroid disease and the total prevalence of thyroid disease among the AITD groups in the three areas did not differ significantly. However, among the TPOAb (−) & TGAb (−) group, the total prevalence of thyroid disease (16·8 %, 95 % CI 12·9 %, 20·7 %) in the IE area was significantly higher than the prevalence in the ID areas (8·1 %, 95 % CI 5·1 %, 11·1 %; χ 2 = 11·526, P = 0·001), especially with respect to subclinical hypothyroidism (χ 2 = 23·253, P < 0·001), but the prevalence of goitre in the ID areas (1·9 %) was significantly higher than the IA (0·3 %) and IE areas (0·0 %).
Table 4. Thyroid disease distribution of autoimmune thyroid disease (AITD) and thyroid peroxidase antibody (TPOAb) (−) & thyroglobulin antibody (TGAb) (−) in adults of three areas
(Numbers and percentages)*

ID, iodine-deficient areas; IA, iodine-adequate areas; IE, iodine-excessive area; TPOAb (−) & TGAb (−), TPOAb- and TGAb-negative.
* The χ 2 test was used for hypothyroxinaemia, overt hypothyroidism, subclinical hypothyroxinaemia, overt hyperthyroidism, subclinical hyperthyroidism, thyroid nodule, goitre and total thyroid disease. P < 0·05 was considered significant.
† TPOAb (−) & TGAb (−) group in the ID and IE areas compared with the TPOAb (−) & TGAb (−) group in the IA areas, P < 0·05. ID areas compared with the IA areas: subclinical hypothyroidism, goitre and total thyroid disease (χ 2 = 10·403, P = 0·001; χ 2 = 3·989, P = 0·046; χ 2 = 6·196, P = 0·013).
‡ TPOAb (−) & TGAb (−) group in the IE area compared with the TPOAb (−) & TGAb (−) group in the ID areas, P < 0·05. Subclinical hypothyroidism, goitre and total thyroid disease (χ 2 = 23·253, P < 0·001; χ 2 = 10·249, P = 0·001; χ 2 = 11·526, P = 0·001). The AITD groups of three areas were compared; the prevalence of any form of thyroid disease and the total prevalence of thyroid disease did not differ significantly.
§ AITD group compared with TPOAb (−) & TGAb (−), P < 0·05. Total thyroid disease had differed significantly in each area (ID: χ 2 = 25·430, P < 0·001; IA: χ 2 = 16·076, P < 0·001; IE: χ 2 = 7·018, P = 0·008). The TPOAb (−) & TGAb (−) groups of three areas were compared, subclinical hypothyroidism, goitre and total thyroid disease had differ significantly (χ 2 = 22·895, P < 0·001; χ 2 = 10·000, P = 0·007; χ 2 = 11·603, P = 0·003).
The total prevalence of thyroid disease in the AITD group in the ID, IA and IE areas was higher than the TPOAb (−) & TGAb (−) group (ID, χ 2 = 25·430, P < 0·001; IA, χ 2 = 16·076, P < 0·001; and IE, χ 2 = 7·018, P = 0·008). The distribution of thyroid disease in the adults from the three areas in the AITD and TPOAb (−) & TGAb (−) groups is shown in Fig. 2. Thyroid disease in the TPOAb (−) & TGAb (−) group was mainly subclinical hypothyroidism and thyroid nodules; however, more thyroid diseases were observed in the AITD group than the TPOAb (−) & TGAb (−) group.
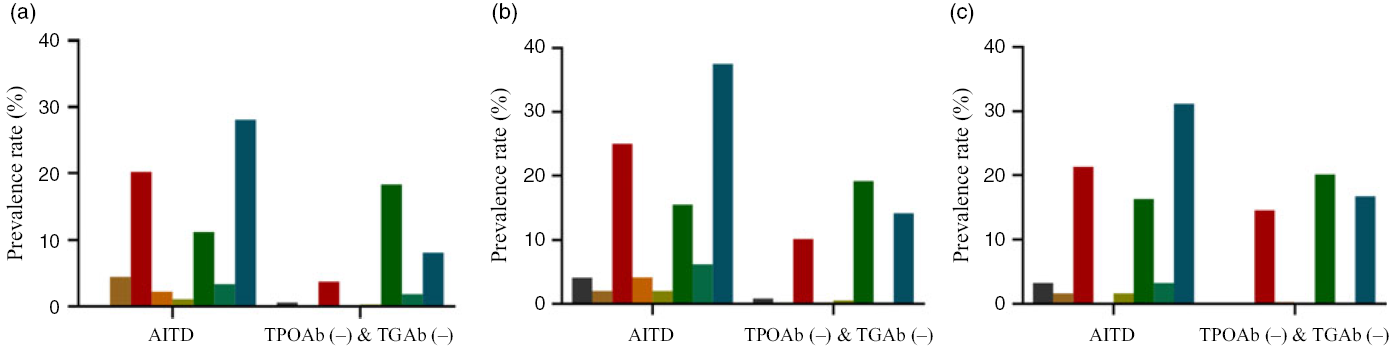
Fig. 2. Thyroid disease distribution of autoimmune thyroid disease (AITD) and thyroid peroxidase antibody (TPOAb (−)) & thyroglobulin antibody (TGAb (−)) in adults of three areas. (a) Iodine-deficient areas; (b) iodine-adequate areas; (c) iodine-excessive area. ![]() , Hypothyroxinaemia;
, Hypothyroxinaemia; ![]() , overt hypothyroidism;
, overt hypothyroidism; ![]() , subclinical hypothyroidism;
, subclinical hypothyroidism; ![]() , overt hyperthyroidism;
, overt hyperthyroidism; ![]() , subclinical hyperthyroidism;
, subclinical hyperthyroidism; ![]() , thyroid nodule;
, thyroid nodule; ![]() , goitre;
, goitre; ![]() , total thyroid disease. TPOAb (−) & TGAb (−), TPOAb- and TGAb-negative.
, total thyroid disease. TPOAb (−) & TGAb (−), TPOAb- and TGAb-negative.
Relationship between serum and urinary iodine and thyroid function in adults with autoimmune thyroid disease
All subjects with AITD were divided into five groups based on serum iodine levels, as follows: <50, 50–69·9, 70–89·9, 90–109·9 and ≥110 μg/l. Non-linear correlations between serum and urinary iodine, as well as FT3, FT4 and TSH, were demonstrated. This relationship has been described using scatter plots, as shown in Fig. 3. The levels of urinary iodine and FT4 increased with increasing median serum iodine values (Fig. 3(a) and (c), whereas the levels of FT3 increased, then decreased with increasing levels of the median serum iodine (Fig. 3(b)). The levels of TSH tended to decrease, then increased with increasing levels of the median serum iodine (Fig. 3(d)).
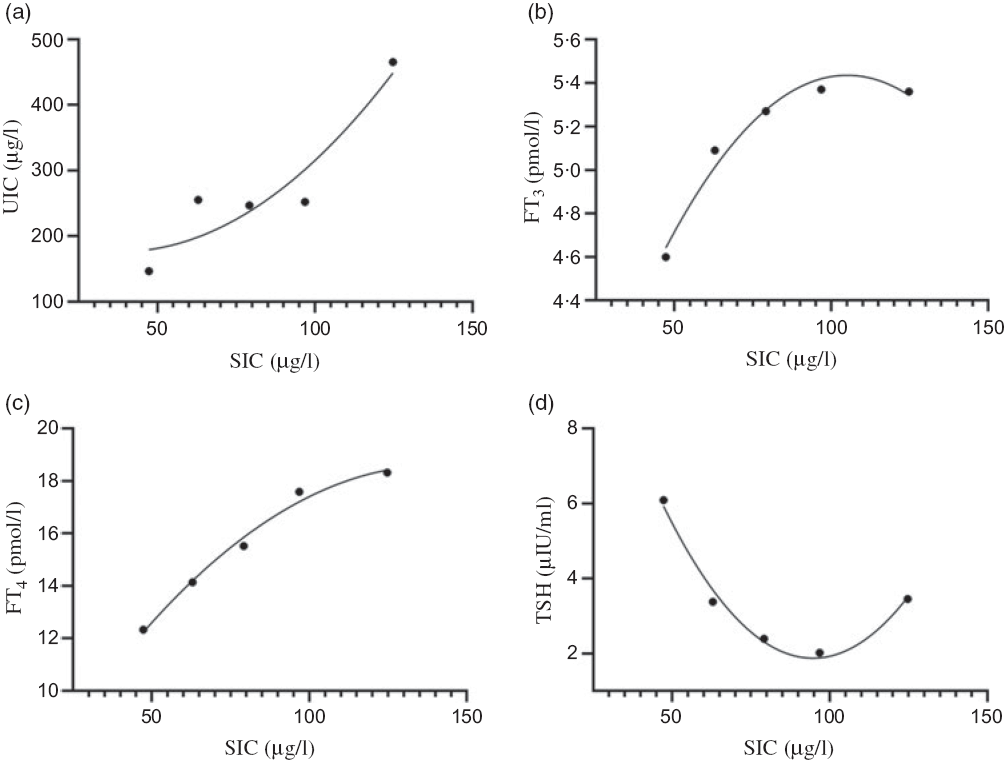
Fig. 3. Relationships between serum iodine and urinary iodine and thyroid function in adults of autoimmune thyroid disease. (a) Serum iodine concentration (SIC) and urinary iodine concentration (UIC); (b) SIC and free triiodothyronine (FT3); (c) SIC and free thyroxine (FT4); (d) SIC and thyroid-stimulating hormone (TSH). (a), R 2 = 0·8700; (b), R 2 = 0·9748; (c), R 2 = 0·9886; (d), R 2 = 0·9849.
Analysis of the risk factors for autoimmune thyroid disease in adults
The association between the independent variables (sex, age, BMI, education level, family income, MWI, current smoking status, alcohol consumption, UIC, SIC, TSH, goitre, nodules and radiation history) was explored using a univariate logistic regression model. The results showed that sex, education level, economic income, MWI, cigarette smoking, alcohol consumption, TSH, SIC and frequency of using computers and mobile phones were associated with AITD. Multivariable logistic regression analysis showed that AITD was more likely in females than males (OR = 2·868; 95 % CI 1·411, 5·831; P = 0·004). In addition, a MWI ≤ 10 μg/l (iodised salt fortification) (OR = 3·012; 95 % CI 1·920, 4·726; P < 0·001), TSH < 0·27 µIU/ml (OR = 5·428; 95 % CI 1·723, 17·096; P = 0·004), TSH > 4·2 µIU/ml (OR = 3·176; 95 % CI 2·010, 5·019; P < 0·001), SIC > 110 μg/l (OR = 2·228; 95 % CI 1·110, 4·471; P = 0·024) and goitre (OR = 6·878; 95 % CI 1·898, 24·930; P = 0·003) were independently associated with AITD (Table 5).
Table 5. Analysis of the risk factors for autoimmune thyroid disease (AITD) in adults
(Odds ratios and 95 % confidence intervals)*
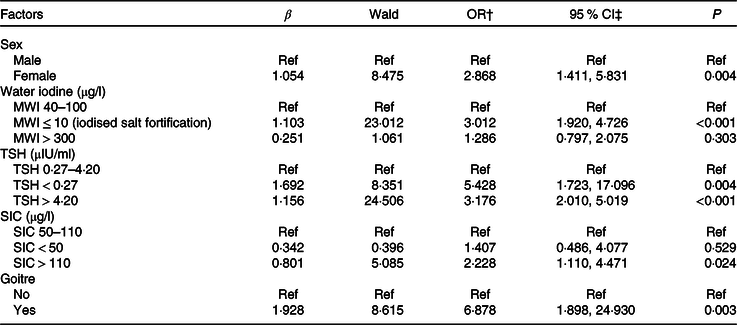
Ref, reference; MWI, median water iodine; TSH, thyroid-stimulating hormone; SIC, serum iodine concentration.
* TSH > 4·20 µIU/ml including cases of hypothyroidism, subclinical hypothyroidism; TSH < 0·27 µIU/ml including cases of hyperthyroidism and subclinical hyperthyroidism; goitre, thyroid volume >25 ml (male) and >18 ml (female).
† Model for AITD was adjusted for age, sex and BMI.
‡ Binary logistic regression analysis was used.
Discussion
Iodine is an essential microelement that participates in the synthesis of thyroxine(Reference Ma, Skeaff and Pearce1,Reference Eastman and Zimmermann2) . When ingestion is insufficient or in excess of the body’s needs, abnormalities can develop. According to the recommendations of WHO/UNICEF/ICCIDD, the median urinary iodine is a good indicator reflecting recent iodine intake and the iodine nutritional status of the population(24). In this study, the UIC of adults in the ID, IA and IE areas was 228·4, 243·9 and 390·4 μg/l, respectively. Thus, the iodine status in the ID and IA areas was sufficient, and the iodine status in the IE area was in excess. The results of this study suggested that the USI policy implemented in China with an iodine concentration <10 μg/l significantly improved the iodine nutritional status of adults. However, the serum iodine levels in the three areas were slightly different. The serum iodine levels in the ID and IE areas were higher than the IA areas, and the serum iodine levels in the IE area were the highest. Some studies have shown that people living in areas with different concentrations of water iodine have significantly different urine iodine levels(Reference Henjum, Barikmo and Gjerlaug34,Reference Teng, Shan and Teng35) . Compared with the urine iodine, serum iodine is an important biomarker of iodine metabolism. It is relatively stable and can truly reflect the body’s recent iodine nutrition status; excess iodine is excreted through renal metabolism. The serum iodine level is representative of serum precipitable iodine and is related to thyroid functional status(Reference Keating and Albert36,Reference McConahey, Keating and Power37) . Therefore, the iodine nutrition status of the study population reflected by the serum iodine level may be more stable than urine iodine(Reference Jin, Jiang and Liu25). If serum iodine was used to evaluate the iodine nutritional status of the population of this study, the iodine nutritional status in the ID areas was lower than the IE area, but higher than the IA areas.
Animal experiments and demographic epidemiological surveys have shown that excessive iodine intake can lead to abnormal thyroid function, thus, increasing the incidence of thyroid diseases(Reference Teng, Shan and Teng35,Reference Calil-Silveira, Serrano-Nascimento and Laconca38) . In the present study, the TSH levels of adults in the ID and IE areas were diverse compared with the IA areas. Previous studies have reported that the adult TSH levels increased with an increase in iodine intake(Reference Laurberg, Pedersen and Hreidarsson39,Reference Wang, Jin and Teng40) , which is consistent with the previous data. At the same time, the level of FT3 in the IE area was lower than the other two areas, while the level of FT4 was higher, suggesting that high iodine might cause changes in serum FT3 and FT4 levels. In this study, an interesting finding was that although iodine status was sufficient for the ID population, the positive rate of TGAb in ID areas was significantly higher than the other two areas. Although the positive rate of TGAb in the IE area was higher than the IA areas, the positive rate of TGAb was relatively lower than the ID areas, which warrants further study. When analysing the distribution of thyroid diseases in the three areas, it was found that the prevalence of AITD in the ID and IE areas was higher than the IA areas and the prevalence in the ID areas reached 21·8 %. Many studies(Reference Lind, Kumnig and Heinisch13,Reference Pedersen, Knudsen and Jorgensen14) have shown that in ID areas, thyroid-specific autoantibodies (TGAb, TPOAb and TRAb) among residents were higher after iodised salt fortification compared with the levels before iodised salt fortification. Similarly, some non-specific IgA and IgG were also higher than the levels before iodised salt fortification. Nevertheless, there is another issue to consider in this study. Because this study was cross-sectional in design and data on thyroid antibody levels in the ID areas were missing before iodised salt consumption, we cannot fully confirm that the higher thyroid antibody levels in the ID areas are related to long-term iodised salt consumption. Nevertheless, the results of this survey are still worthy of our attention. Although the mechanisms by which iodide induces abnormal thyroid antibodies are still unclear, some scholars believe the following: first, excess iodine induces the production of cytokines and chemokines that can recruit immunocompetent cells to the thyroid; second, processing excess iodine in thyroid epithelial cells may result in elevated levels of oxidative stress, leading to harmful lipid oxidation and thyroid tissue injuries; and finally, iodine incorporation in the protein chain of thyroglobulin may augment the antigenicity of this molecule(Reference Luo, Kawashima and Ishido7). In addition, in this study, we found that the prevalence of subclinical hypothyroidism in IE area was the highest among the three areas studied, which is consistent with the Shan’s study and the previous research results of our study team(Reference Liu, Wang and Liu6,Reference Jin, Jiang and Liu25,Reference Shan, Teng and Li41) . No statistical difference was detected among the three groups of other types of thyroid diseases. Our findings provide a reference on the initial dose of iodine used in the implementation of the USI policy in ID areas as an overdose can result in AITD.
According to previous studies, different thyroid antibody status may be related to sex and thyroid hormone levels(Reference McLeod and Cooper42,Reference Wang, Guo and Tian43) . Therefore, we selected the thyroid antibody double-negative subjects as an internal control of corresponding areas in each area. The findings showed that in each of the three areas, the percentage of AITD in the females was higher than the males (ID, 27·6 v. 6·9 %; IA, 14·7 v. 6·2 %; IE, 17·3 v. 8·1 %), which suggest that independent of the area, women are more likely to have abnormal thyroid antibody levels. This finding is in agreement with a previous study(Reference McLeod and Cooper42). In this study, the level of FT3 in the patients with AITD in the ID and IE areas was significantly lower than the subjects with double-negative antibodies. The level of FT4 in patients with AITD was significantly lower than the patients with double-negative antibodies in the IA areas. The TSH levels in the AITD group in each of the three areas were higher than the level in the TPOAb (−) & TGAb (−) group, although these differences were only significant among the ID and IE areas. This finding indicates that abnormal thyroid function is more likely to prevail among people with abnormal antibodies than people with double-negative antibodies.
In view of the above results, what is the distribution of thyroid disease in people with different thyroid antibody status in the three areas? We analysed the prevalence of thyroid disease in people with different thyroid antibody status in the three areas to answer this question. The results showed that there was no statistical difference in the total prevalence of thyroid disease among the people with AITD in the three areas and no statistical difference in the prevalence of any form of thyroid disease, suggesting that different water iodine areas had no influence on the types of diseases among the people with AITD. Compared with the TPOAb (−) & TGAb (−) group of the three areas, the prevalence of hypothyroidism (14·6 %) in the IE area was higher than the IA (10·2 %) and ID areas (3·8 %), suggesting that IE is closely related to hypothyroidism, which is consistent with a previous study(Reference Teng44). In addition, although the prevalence of goitre in the ID areas (1·9 %) was higher than the IA (0·3 %) and IE areas (0·0 %), the proportion was <5 % and, therefore, of little practical significance. In this study, we also noted that the total prevalence of thyroid disease in people with AITD was higher than people with TPOAb (−) & TGAb (−) in the three areas, and there were more types of thyroid diseases in the people with abnormal thyroid antibodies in the three areas. Li reported that although some antibody-positive people have normal thyroid function, the probability of thyroid dysfunction is significantly increased(Reference Li, Shan and Guan45). At the same time, people with autoimmune genetic background or positive thyroid autoantibodies are more likely to develop thyroid disease when iodine is sufficient or in excess. The population susceptible to thyroid diseases is appreciable in the general population, which is estimated to account for approximately 15 % of the total population(Reference Wang, Guo and Tian43). Therefore, regular follow-up evaluations are needed for people with abnormal thyroid antibodies, such as finding problems and taking corresponding treatment measures.
Serum iodine is a sensitive indicator used to evaluate individual iodine nutrition status(Reference Jin, Jiang and Liu25). Therefore, the level of individual serum iodine may be of great significance for the follow-up evaluation of subclinical and clinical thyroid diseases in people with abnormal thyroid antibody levels. Our results showed that there was a strong non-linear correlation between serum iodine, urine iodine and thyroid function in adults with AITD. We found that urine iodine increased with an increase in serum iodine. Generally, dietary iodine can be detected serially in circulating blood within a few minutes after eating, after which a large proportion of the unabsorbed iodide in blood will be excreted by the kidneys, while the remainder will be taken up by the thyroid and other tissues(Reference McConahey, Keating and Power37). Therefore, iodine concentration rapidly increases in the urine as iodine in the diet is absorbed into the blood(Reference Keating and Albert36). In addition, some researchers have compared the disappearance rates of iodine from blood with the values obtained from simultaneous observations in urine, finding that the difference was not significant(Reference Zhang, Yan and Liu31). These results show a strong correlation between serum and urine iodine. Theoretically, both indicators can be used to evaluate the iodine nutrition status of individuals. However, urinary iodine is greatly affected by diet, urine volume, collection time, as well as other factors. Therefore, urine iodine is not an ideal indicator for individual iodine nutrition evaluation. Serum iodine is an important biomarker reflecting recent iodine intake(Reference Keating and Albert36). To maintain balance, excess iodine can be excreted through the urine(Reference Pedersen, Knudsen and Carle46). Finally, the iodine deposited in the blood tends to be stable. Therefore, in the evaluation of individual iodine nutrition status, serum iodine may be more sensitive and relatively stable than urinary iodine. It was also found in this study that TSH levels decreased with an increase in serum iodine levels, then increased with an increase in serum iodine levels. The TSH level of AITD adults exhibited a positive ‘U’ curve relationship with serum iodine levels, suggesting that both high and low serum iodine levels affect thyroid health. In addition, this study also showed that FT3 and FT4 levels increase with an increase in serum iodine levels in adults with AITD. We think that thyroid hormone production in the body is insufficient when the serum iodine level is low (<50 μg/l); however, to maintain a normal level of thyroid hormones, the body will promote an increase in the TSH level by a negative feedback mechanism of the thyroid. Subsequently, with an increase in serum iodine levels, thyroid hormone in the body gradually stabilises to meet the normal physiological needs of the human body. Nevertheless, a higher level of serum iodine (≥110 μg/l) may trigger thyroid oxidative stress mechanisms in people who are susceptible to thyroid disease, and the harmful lipid oxidation will cause thyroid tissue damage(Reference Luo, Kawashima and Ishido7), such as thyroid follicular cell destruction. In addition, FT3 and FT4 are released into the blood, causing serum FT3 and FT4 levels to rise. The above findings suggest that people with abnormal thyroid antibodies are more likely to have abnormal thyroid function when serum iodine levels are very low or very high. Serum iodine does hold promise as a biomarker in the follow-up evaluation of subclinical and clinical thyroid diseases in people with abnormal thyroid antibody levels.
Finally, we also found some evidence related to the risk of AITD in adults. Living in areas where the MWI ≤ 10 µg/l with iodised salt fortification increased the risk of AITD, and females were at greater risk than males. This finding is in congruence with a previous study(Reference Keating and Albert36). Pedersen et al. (Reference Pedersen, Knudsen and Carle46) confirmed that Denmark used to be an area of mild-to-moderate iodine deficiency (median UIC = 61 µg/l). However, after 5 years of iodine fortification with iodised salt, the iodine status significantly improved (median UIC = 101 µg/l), but the prevalence of thyroid antibodies increased. TPOAb >30 U/ml increased from 14 to 24 % and TGAb >20 U/ml increased from 14 to 20 %. In addition, we found that a SIC > 110 µg/l increased the risk of AITD compared with the normal reference range (52–109 µg/l) of serum iodine by inductively coupled plasma-MS (Quest Diagnostics)(Reference Han, Wu and Yu32). Notably, this finding was similar to the Jin et al. study(Reference Jin, Jiang and Liu25). Jin et al. believe that SIC < 50 µg/l and >100 µg/l increases the risk of AITD. However, we found no statistical association between serum iodine <50 µg/l and AITD. According to our analysis, the iodine nutrition of adults in the areas we investigated was sufficient or above the recommended level, the level of SIC was basically in the range of the normal reference values, and there were few participants in whom the serum iodine level was < 50 μg/l. TSH is also considered to be a risk factor for AITD. TSH increases the risk of AITD if too high or too low. Abnormal TSH levels were divided into two categories in this study: hypothyroidism and subclinical hypothyroidism (>4·2 µIU/ml); and hyperthyroidism and subclinical hyperthyroidism (<0·27 µIU/ml). Therefore, our results suggest that adults with any of the above thyroid disorders may increase the risk of AITD. Finally, goitre is positively associated with the risk of AITD. Goitre is likely to be accompanied by the occurrence of AITD and should therefore receive attention.
Strengths and limitations
The main advantages of this study are as follows. First, we investigated three different water iodine areas, which provided a better representation of the general population. Second, we found that the thyroid immune response of people in ID areas (iodised salt fortification) was more apparent than IE area, even though many epidemiological investigations have reported that thyroid autoimmune response was more obvious in IE areas(Reference Du, Gao and Meng20,Reference Li, Teng and Shan47) . We strongly believe that our findings are strengthened by the inclusion of areas with different water iodine levels. Third, we discussed the distribution of thyroid disease in people with AITD and people with double-negative thyroid antibodies in different water iodine levels and clarified the relationship between serum and urine iodine in people with AITD, as well as the relationship between serum iodine and thyroid function in the present study, which were lacking in the other epidemiological surveys. Finally, we discussed the risk factors for AITD in adults. This study also had some limitations. First, due to the limited population with AITD, the study may be biased to some extent; thus, additional cases should be collected for further discussion. Second, adjustments need to be made in matching the sex ratio. In addition, a small number of participants filled out the questionnaire information with omissions. In the future, field investigations should be strictly controlled.
Conclusion
USI has improved the iodine nutritional status of the population in ID areas in China. However, non-step-by-step iodine fortification may induce the transformation of thyroid autoimmune diseases from recessive-to-dominant in susceptible people. We suggest that a USI policy should be implemented, starting with small doses of iodine in ID areas and gradually reaching the optimal fortification dose of iodine over several stages. In the AITD population of all areas, UIC, FT3, FT4 and TSH have a non-linear correlation with serum iodine. The TSH level has a positive ‘U’ curve relationship with the serum iodine level. An abnormal TSH level, SIC > 110 µg/l and goitre were risk factors for AITD in adults, especially women. People with abnormal thyroid antibodies should have regular follow-up of thyroid function, regular check-ups, and if problems are found, appropriate treatment measures should be offered.
Acknowledgements
The authors are grateful for the assistance provided by the Institute for Prevention and Treatment of Endemic Disease of Shandong Province for collecting epidemiological data and samples, and contributions and support from all participants.
This work was supported by the National Natural Science Foundation of China (grant no. 81872561) and Scientific Research and Practice Innovation Fund for Postgraduate of Harbin Medical University (YJSKYCX2019-16HYD).
The contribution of each author is as follows: H. S. and L. L. designed the study; H. S., L. L., S. W., M. Q., H. W., B. R., W. J. and X. W. conducted the research; S. W. analysed the data; S. W. wrote the paper; H. S. and L. L. took primary responsibility for the final content of the manuscript. All authors read and approved the final version of the manuscript.
There are no conflicts of interest to declare.
The authors have nothing to disclose.











