INTRODUCTION
Salmonella spp. are an important cause of human illness. In 2007, there were 13 213 laboratory-confirmed cases of Salmonella in humans in the UK, although the true number of cases is considered to be four times higher [1]. Consumption of poultry products is well documented as a major source of infection, with the public health risk arising from accidental undercooking of meat or cross-contamination of other foods [1–Reference Palmer3]. The contribution of turkey meat is, however, unknown. Each year about 17 million turkeys are raised in the UK, 10 million of these for the Christmas market [4]. Currently, most major turkey companies undertake voluntary monitoring for Salmonella and most Salmonella isolations are not associated with clinical disease. In 2007, there were 112 reports of Salmonella in turkey flocks in Great Britain [1].
Salmonella in poultry traditionally arouses much political interest, with pressure to reduce contamination at the farm level. October 2006 marked the start of a European Union (EU)-wide baseline survey over one year, to determine the prevalence of Salmonella spp. in breeding and fattening turkey (Meleagris gallopavo) flocks [5]. Results of the survey have been used to set targets to reduce the prevalence of Salmonella in both breeding and fattening turkey flocks, as part of EU-wide National Control Plans that come into force from 2010.
The pathways of Salmonella dissemination on poultry units are dynamic and complex, and researchers have identified numerous potential sources and risk factors for Salmonella infection on poultry farms [Reference Hoover6–Reference Henken10]. Only a few studies have looked at the risk factors in turkey flocks specifically, which differ from chicken broiler flocks in their seasonality of production and longer growing period. Arsenault and others [Reference Arsenault11] reported that turkey flocks were more likely to be infected if they originated from a particular hatchery, or were raised in houses where two or more persons had access. They did not detect association between Salmonella infection at flock level and variables related to pest control, downtime period, manure disposal, and poultry-house cleaning and disinfection practices. Analysis of data collected during the EU baseline survey in 2006–2007 revealed increased risk of Salmonella in fattening turkey flocks with a larger number of turkeys on the holding. The presence of breeding turkeys on the same unit and having a free-range flock also increased the risk. While the risk of Salmonella infection varied throughout the survey period, Salmonella vaccination and subdividing the birds on a holding into a relatively large number of flocks was found to decrease the risk [12].
A greater understanding of farm husbandry and management practices which are associated with Salmonella infection in turkeys would assist in the challenge of reducing Salmonella prevalence, as required by the EU, in a realistic, practical, and cost-effective way. The baseline survey provided the opportunity to gather information on turkey fattening farms to investigate potential risk factors for Salmonella infection, as recommended to member states by the European Food Safety Authority (EFSA). This paper describes the results of the UK investigation.
METHODS
Baseline survey
The survey in the UK was carried out between October 2006 and September 2007 as part of the EU survey to establish the baseline prevalence of Salmonella in commercial turkey flocks. A detailed description of the survey design, the sampling and bacteriological testing is given in the document of European Commission, Directorate General for Health and Consumer Affairs (DG SANCO) [13]. A total of 317 holdings were selected at random from a list of commercial fattening turkey holdings in the UK, stratified by region (England, Wales, Scotland, Northern Ireland) and number of turkeys on the holding (holding size). Holdings with <500 fattening birds were excluded from the sampling frame. From each holding one flock was sampled within 3 weeks of slaughter/depopulation. Five pooled environmental faecal samples were collected from each sampled flock, using boot swabs pre-moistened with sterile water and worn over disposable plastic over-boots. Samples were despatched to the laboratory on the day of collection, and examined on the day of arrival. Samples from England, Wales and Scotland were submitted to the Veterinary Laboratories Agency (VLA), Weybridge and samples from Northern Ireland to the Agri-Food and Biosciences Institute (AFBI), Belfast. The isolation method used a modified semi-solid Rappaport–Vassiliadis medium (MSRV) as the single selective enrichment medium, as described in Annex D of ISO 6579 [14].
Data collection
Basic farm-level information was required by the EU and gathered for all member states. This included details of the production type and size of holding, as well as data relating to the sampled house (number of birds, age, crops per year, expected age at slaughter, Salmonella vaccine and recent medicine use). In addition, flock owners in the UK were requested to complete an additional voluntary questionnaire, by interview with the animal health officer taking the samples. This questionnaire was designed to collect information on additional flock, house and farm-level factors that may be related to the risk of Salmonella (Fig. 1). All data from the two questionnaires and test results from the sampled holdings in the UK were collated, entered and validated by trained data entry staff into a Microsoft Access 2000 database at the Centre for Epidemiology and Risk Analysis, VLA, Weybridge.

Fig. 1. Farm and house factors on which data were gathered using the two questionnaires.
All statistical analyses were conducted using Stata/IC version 10.0 for Windows (Stata Statistical Software Release version 10.0, StataCorp LP, USA) using the survey commands for analysing complex survey design data [Reference Levy and Lemeshow15, 16].
Statistical methods
A holding was classified as Salmonella-positive if Salmonella spp. was cultured from at least one of the five pairs of boot-swab samples. Similarly, a holding was classified as positive for Salmonella Typhimurium (ST) if ST was cultured. All observations were weighted by the inverse of the selection probability in each of four holding size strata [Reference Levy and Lemeshow15, Reference Dargatz and Hill17] and a finite population correction was also applied to adjust standard errors for sampling a large proportion of each strata. Two separate models were developed. For the first model, the outcome was the detection of Salmonella (any serovar). In the second model the outcome was detection of ST vs. farms where no ST was detected. These two outcomes were considered of most interest to the turkey industry in terms of realistic farm-level interventions.
The questions that formed the questionnaires were critically evaluated and a total of 420 variables were created. Univariate analysis was carried out to examine the potential association of each variable with Salmonella status based on Pearson's χ2 statistic with the Rao & Scott second-order correction [16]. Any biologically plausible variables significant at P⩽0·10 for the Salmonella spp. model, or P⩽0·20 for the ST model, were then assessed for inclusion in the multivariate models to estimate the association of holding-level Salmonella status with various risk factors. Variables that showed collinearlity (determined using the _rmcoll command in Stata) were not assessed in the model at the same time and variables with insufficient observation numbers, which would have compromised the power of the model, were excluded. Holding size, flock type, belonging to one of the 10 largest UK turkey-producing companies and seasonal production (defined as fattening a single crop of turkeys per year) were included in both models as a priori potential confounders because it was considered that these four variables may be associated independently with the outcomes and potential risk factors. Due to the large number of variables being considered, a forwards stepwise approach introducing variables in order of significance was used. Variables were included or excluded from the model based on the adjusted Wald test statistic and only variables with P Wald<0·05 were retained in the final model [Reference Hosmer and Lemeshow18]. As a final step, variables that were not selected initially were added back individually and retained if significant at P Wald<0·05. Model fit was assessed using the Hosmer–Lemeshow goodness-of-fit test after removing the weighting [Reference Hosmer and Lemeshow18]. Where more than one combination of variables was possible, the best-fit model which demonstrated the most useful combination regarding farm interventions for Salmonella control was selected.
RESULTS
Response to voluntary questionnaire
A total of 252 (79·5%) of the 317 fattening turkey holdings enrolled in the EU survey completed the additional questionnaire on risk factors. Analysis of the farms which responded, compared to those that did not respond, indicated no significant difference in terms of production type, Salmonella status, Salmonella vaccination status of the flock, or whether the farm was a seasonal producer. Respondents included representation from all four regions of the UK, with no significant difference in response between region (response rate between 76% and 100%). Smaller farms were less likely to respond to the additional questionnaire, with a response rate of 72% in farms with <5000 birds; this rate of response increased as holding size increased, with a 100% response rate from the largest holdings of >50 000 birds. This trend was statistically significant (Pearson's χ2=18·9, P<0·001), but was not considered to present unacceptable bias.
Prevalence of Salmonella spp. and ST
Salmonella was isolated from 86 of the 252 holdings giving a Salmonella spp. prevalence of 34·1%. Table 1 shows the serovars of Salmonella isolated. The most common serovar isolated was S. Kottbus, occurring in 45 (52%) of the 86 positive holdings. The second most common serovar was ST, isolated from 15 (17%) of the 86 positive holdings, giving an overall ST prevalence of 6·0%.
Table 1. Frequency of isolation of serovars of Salmonella

* Some holdings had more than one serovar.
Factors associated with farm-level Salmonella status
Univariate analysis
Of the variables initially considered, 54 variables significant at P⩽0·10 (Salmonella spp. model) and 50 variables significant at P⩽0·20 (ST model) were considered for inclusion in the multivariate models. Tables 2 and 3 show the variables significantly associated (P<0·05) with Salmonella spp. and ST in the univariate analysis, in addition to the univariate results of those variables which were included in the multivariate models.
Table 2. Results of univariate analysis of factors significantly associated (P<0·05) with Salmonella (all serovars)
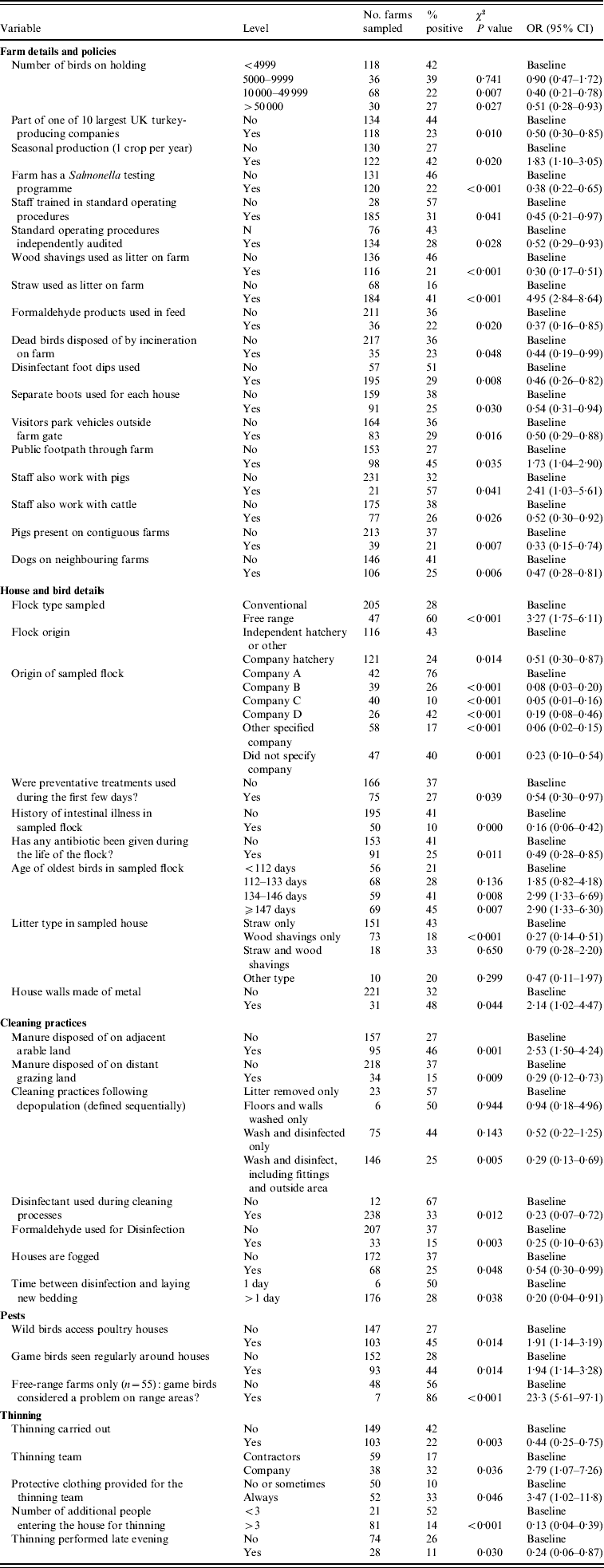
OR, Odds ratio (weighted by sampling probability of the holding); CI, confidence interval.
Table 3. Results of univariate analysis of factors significantly associated (P<0·05) with Salmonella Typhimurium and those included in the multivariate model
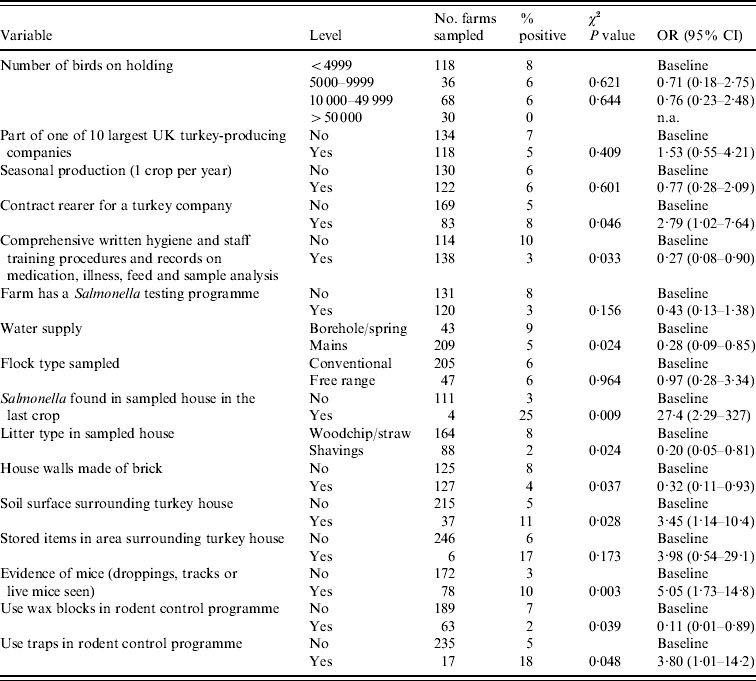
OR, Odds ratio (weighted by sampling probability of each holding); CI, confidence interval.
Multivariate results
Table 4 shows the final multivariate model with adjusted odds ratios (OR) and 95% confidence intervals (CI) for factors associated with Salmonella spp. at the farm level. Table 5 shows a similar model, but specific to ST.
Table 4. Multivariate analysis of factors associated with holding level Salmonella (all serovars) status (n=243)
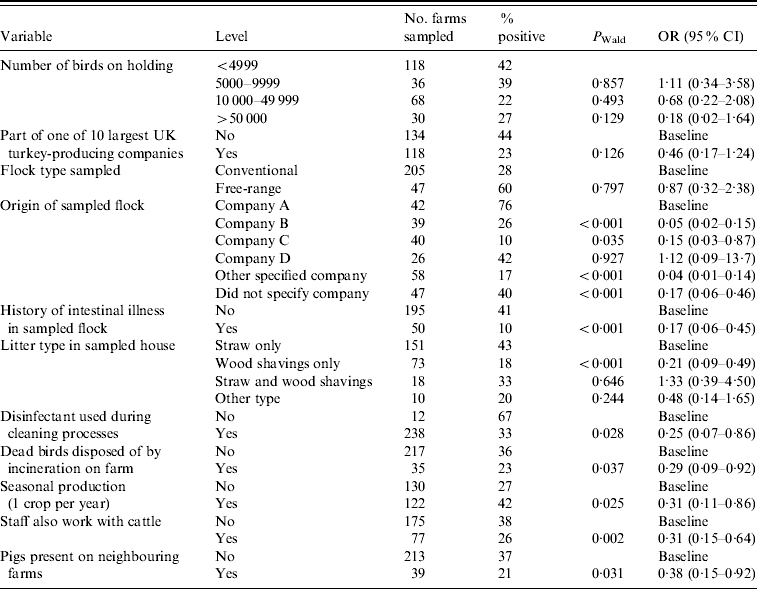
OR, Odds ratio; CI, confidence interval.
Hosmer–Lemeshow test: χ2 (8 d.f.)=5·57, P=0·6948.
Table 5. Multivariate analysis of variables associated with holding level Salmonella Typhimurium status (n=249)
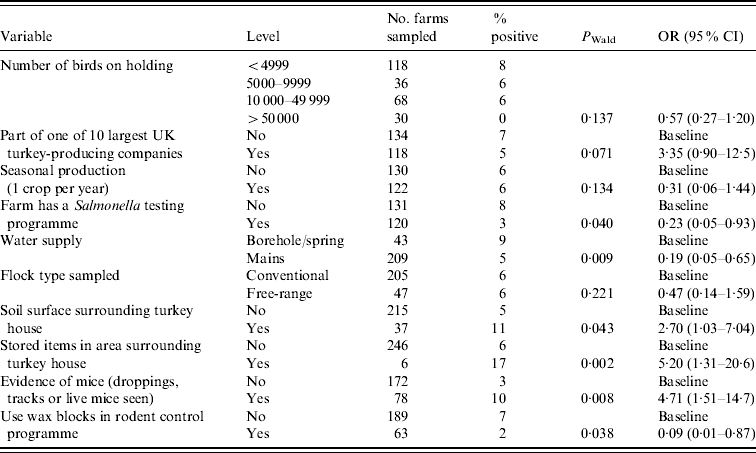
OR, Odds ratio, CI, confidence interval.
Hosmer–Lemeshow test: χ2 (8 d.f.)=11·28, P=0·1863.
Factors associated with farm-level Salmonella status (all serovars)
Of the a priori confounding variables, only on farms with seasonal production was there a significantly reduced risk of Salmonella (OR 0·31), compared to those which rear multiple crops of turkeys per year.
The majority (58%) of turkey flocks in this study were sourced from four companies. The risk of isolating Salmonella varied between these companies, as shown in Table 4. For example, in comparison to company A there was a significant decrease in risk for farms which sourced their poults from companies B or C (OR 0·05 and 0·15, respectively), but there was no significant difference between companies A and D.
The use of wood shavings as the sole type of litter had a protective effect for Salmonella (OR 0·21) compared to using straw only, but this effect was lost when wood shavings were used in combination with straw as bedding. The use of disinfectant in cleaning following depopulation also significantly reduced the likelihood of finding Salmonella on a holding (OR 0·25) compared to those farms which did not use disinfectant.
If a flock had a history of intestinal illness prior to sampling, the likelihood of isolating Salmonella was significantly reduced (OR 0·17) compared to those which had no such history. While birds which had suffered intestinal illness prior to sampling were significantly more likely to have received antibiotic treatment (OR 20·8, 95% CI 8·75–48·7, P<0·001), the use of antibiotic medications itself did not show any significant association with Salmonella in the final model. There was significantly less risk of isolating Salmonella from a holding if dead birds were disposed of by incineration on farm (OR 0·29) compared to farms which used off-site carcase disposal methods.
The presence of pigs on neighbouring farms was shown to confer a significant protective effect (OR 0·38) compared to holdings without a contiguous pig farm. Having staff that also worked with cattle was shown to reduce the likelihood of isolating Salmonella from a holding (OR 0·31) compared to those farms whose staff did not work with cattle.
Factors associated with farm-level ST status
Size of holding, production type, being part of one of the 10 major UK turkey-producing companies and seasonal production were included in the final model as before, although none were significantly associated with presence of ST on the holding compared to flocks which were either positive for other Salmonella serovars or negative.
Those farms which had a Salmonella testing programme in place were significantly less likely to test positive for ST (OR 0·23) compared to those farms which had no Salmonella test programme. Use of mains water also significantly reduced the likelihood of testing positive for ST (OR 0·19), the source of water for the majority (85%) of non-mains water supplied farms was a borehole. A soil surface and storage of items in the area surrounding the turkey house increased the risk of isolating ST from a flock (OR 2·70 and 5·20, respectively) compared to those flocks which had no soil surface or stored items surrounding the turkey house.
The presence of mice (either live mice, mice droppings or tracks observed) on a holding resulted in more than a fourfold increase (OR 4·71) in the probability of isolating ST compared to those farms which had no evidence of mice. The use of wax blocks in the farm rodent control programme as opposed relying solely on other means (e.g. other bait types, contact rodenticides or trapping) was protective against ST (OR 0·09).
DISCUSSION
This study has identified a number of risk factors for Salmonella infection on fattening turkey units. As a cross-sectional study, the analysis was not able to differentiate factors related to introduction of Salmonella onto a holding from factors that facilitate the persistence of Salmonella infection on a holding. The large number of variables analysed may increase the potential of type I error and measures were therefore taken to reduce the likelihood of this occurrence. These included consideration of the biological importance and plausibility of variables prior to inclusion in the models, and their significance at the univariate level.
Seasonally produced flocks were shown to be around three times less likely to have Salmonella than those flocks on holdings rearing more than one crop of birds per year. To our knowledge, this has not been previously demonstrated in turkey meat production. An extended period between batches of birds is conducive to comprehensive cleaning and disinfection, a reduction in environmental contamination and environmental reservoirs of infection and it interrupts the carry-over of infection that may occur between successive batches.
The risk of isolating Salmonella varied significantly between the companies from which the poults originated. Other studies have also identified the source company or hatchery of the flock as a risk factor for Salmonella in poultry [Reference Arsenault11, Reference Chriél, Stryhn and Dauphin19–Reference Byrd21]. This reinforces the importance of Salmonella control at each level in the production pyramid. It was not possible to perform separate analyses for each Salmonella serovar due to the small numbers of farms with some of these serovars. However, in the UK, at the time of this study, S. Kottbus was the predominant isolate from both breeding and fattening flocks [12].
The use of wood shavings alone as litter was found to have a significant protective effect on Salmonella infection in this study, yet this effect was lost when wood shavings were used in combination with straw. This is an interesting finding as there has been little research to substantiate the persistence of Salmonella in different types of poultry litter. It is possible that properties intrinsic to wood shavings may inhibit Salmonella organisms, or that superior drainage offered by shavings as opposed to straw may encourage Salmonella persistence on the latter. It is also possible, given its favourability by rodents as harbourage, that straw may become contaminated in the field and during storage.
Incineration of dead birds on farm is a marker for good practice for disease control. On-farm incineration is likely to reduce environmental contamination and attraction of pests that can be associated with carcases, especially if carcases are stored insecurely on the farm for any length of time prior to removal. A history of intestinal illness in the sampled flock was an unexpected protective factor, but the variable showed moderate correlation with the use of antibiotics in the flock. The use of such agents, targeted at coliform-induced intestinal disease, is likely to have reduced excretion of Salmonella.
It is widely reported that pigs may act as a source of Salmonella infection [22, 23]. It is therefore surprising that this study identified the presence of pigs on neighbouring farms as a protective factor. It is possible that turkey farms situated close to pig units may employ stricter biosecurity measures although no correlation could be determined from our data. Farms where staff also work with cattle were less likely to have Salmonella, but there does not appear to be a ready biologically plausible explanation for this. It may, however, be linked with other unidentified protective farm management factors. It is also possible that both these variables are negatively correlated with poultry density and further analysis of location data and population distribution may be of use.
The UK Code of Practice for the Control of Salmonella in Commercial Turkey Flocks [24] underlines the importance of thorough cleaning and disinfection between flocks and this study confirmed the value of disinfection in reducing the risk of Salmonella infection. This is in accord with other studies, although many workers have emphasized the importance of thorough cleaning prior to disinfection as most disinfectants are readily inactivated by residual organic matter [Reference Martinez, Berchieri and Paulillo25–Reference Snow27]. Persistent contamination of the environment with Salmonella is a major problem on poultry units and a source of infection to new batches of birds [Reference Henzler and Opitz28–Reference Wales, Breslin and Davies30].
Holding size, while significant at the univariate level, did not retain association with farm Salmonella status in the final models. This is contrary to risk factor studies in laying hens [Reference Snow27] and to the overall EU baseline turkey survey, where larger holdings of birds were more likely to be Salmonella positive [12]. Similarly in contrast to the EU survey, our study did not identify free-range production as a particular risk factor for Salmonella infection on fattening turkey holdings. Being part of one of the 10 major turkey-producing companies in the UK was not significant in the multivariate models, but retained as a potential confounder.
ST accounted for 13·4% of the cases of salmonellosis in humans in the UK in 2007 and is considered to be one of the priority Salmonella serovars by EFSA [1]. The EU has set a reduction target for the priority serovars, S. Enteritidis and ST of no more than 1% of flocks to test positive by 31 December 2012 [31]. It is therefore of particular importance to consider specific risk factors for ST. ST is also one of the main serovars that can sometimes cause clinical disease in turkeys [Reference Hafez, Jodas, Wray and Wray32] and is often associated with multiple antibiotic resistance [Reference Davies33, Reference Jones34].
Having a Salmonella testing programme, using mains water and using wax blocks to control rodents were all shown to reduce the risk of testing positive for ST. A testing programme should not only enable units to promptly detect and respond to Salmonella infection, before it spreads and becomes persistent, but also help in developing action plans to eliminate infection. Mains water will have undergone statutory purification and testing to ensure it complies with certain health and safety standards and therefore have a much lower risk of being contaminated with Salmonella – although water is unlikely to be a source of Salmonella, the lack of mains water may be an indication for other reduced husbandry standards. The provision of rodenticide via wax blocks is currently considered one of the most effective methods of mouse control, compared to whole wheat bait which is normally only suitable for rats.
The role of mice in Salmonella epidemiology on poultry farms is well documented in the literature [Reference Henzler and Opitz28, Reference Davies and Wray29, Reference Garber35]. In our study the presence of mice on a holding was significantly associated with ST infection specifically. Infected mice can act as a vector of Salmonella, amplifying and spreading the organism between houses, consecutive flocks and possibly even to neighbouring units [Reference Davies and Wray29]. In our study, rats were not found to pose a statistically significant risk for Salmonella infection on turkey farms, but rats are more likely to be observed and effectively controlled, minimizing breeding populations.
Both the storage of items around the turkey accommodation and a soil surround were identified as risk factors for ST. Soil, vegetation and clutter around houses are likely to increase the risk of infestation of houses by rodents. Such surfaces and items are also difficult to disinfect and while not significant in the final model, disinfection of areas surrounding the house was shown to reduce the risk of Salmonella (all serovars) in the univariate analysis.
The comprehensive questionnaire examined a very wide variety of issues relating to turkey production and farm management. A number of variables that were significant in the univariate analysis and provided biologically plausible explanations of farm-level Salmonella status were not confirmed in the multivariate models, although the power of detection may have been compromised by the small sample. Variables specific to free-range farms or those farms which operate thinning policies had to be excluded from the multivariate models for this reason. Similarly, the EU survey demonstrated that vaccination conferred significant protection against Salmonella [12]. Of the respondents in this study, only one farm indicated that the sampled flock was vaccinated against Salmonella and therefore it could not be used as a study variable, although it is an intervention step which may be considered as part of the package of control measures to limit ST infection in turkeys.
ACKNOWLEDGEMENTS
This study was funded by Defra. The assistance of Animal Health staff in collection of samples and administration of the questionnaire and the cooperation of the farmers contributing to the survey is gratefully acknowledged. The authors are grateful for the assistance of the data entry and scientific staff at VLA Weybridge and AFBI and appreciate the support of the library staff at VLA Weybridge and colleagues in the veterinary surveillance division at VLA Thirsk.
DECLARATION OF INTEREST
None.








